Abstract
Background
Angiogenic dysfunction and abnormalities in psychopathology and brain structure have been reported in schizophrenia, but their relationships are mostly unknown. We recently demonstrated that sFlt-1, anti-angiogenic factor, was significantly elevated in patients at familial high-risk for psychosis (FHR). We hypothesized that elevated sFlt-1 correlates with baseline and longitudinal changes in psychopathology, cognition, and brain structure.
Methods
Plasma sFlt-1 in FHR (n=35) and HC (n=39) was obtained at baseline. Schizotypal, cognitive, soft neurologic signs, and structural brain imaging (1.5T T1-weighted MRI, FreeSurfer software) measures were obtained in both groups. Longitudinal clinical and brain structural measures were obtained in a subgroup of FHR patients. Baseline data analysis used correlations between sFlt-1 and clinical/imaging measures and adjusted for multiple corrections. Linear mixed-effects models described differences in trajectories between high sFlt-1 and low sFlt-1.
Results
Baseline sFlt-1 was significantly correlated with soft neurologic signs (r=0.27, p=0.02) and right entorhinal volume (r=0.50, p=0.02), but not other baseline clinical/brain structural measures. Longitudinal examination of the FHR group (sFlt-1 high, n=14; sFlt-1 low, n=14) demonstrated that high sFlt-1 was significantly associated with worsening schizotypal symptoms (t=2.4, p=0.018). Reduced right hippocampal/parahippocampal volume/thickness trajectories were observed in high versus low sFlt-1 groups.
Conclusions
The findings from this FHR study demonstrate that peripheral markers of angiogenic dysfunction can predict longitudinal clinical and brain structural changes. Also, these findings further support the hypothesis of altered microvascular circulation in schizophrenia and those at risk.
Introduction
The neurobiological basis of schizophrenia (SZ) has several proposed hypotheses implicating mechanisms (Schizophrenia Working Group of the Psychiatric Genomics, 2014), but its pathophysiology remains to be elucidated. Microvascular abnormality may contribute to the pathophysiology of psychotic disorders, as evidenced by capillary ultra-structural damage, reduced blood flow, altered glucose metabolism, retinal microvascular abnormalities, uncoupling of cerebral blood flow/volume, and arteriolar cerebral blood volume reductions in SZ (Hua et al., 2016; Meier et al., 2013; Talati et al., 2015; Uranova et al., 2010; Uranova, 2013), and investigating angiogenic pathways may provide a novel biological framework for understanding these phenotypes (Lopes et al., 2015). Angiogenesis involves a balance between pro- and anti-angiogenic factors and can result in various pathological conditions (Wu et al., 2010). We recently reported a significant increase of the anti-angiogenesis factor, soluble fms-like tyrosine kinase-1 (sFlt-1) in antipsychotic-naïve familial high-risk for psychosis (FHR) patients that was not present in healthy controls (Lizano et al., 2016). Two small studies have demonstrated sFlt-1 elevations in chronic schizophrenia (Kim T.H., 2007) and severe autism (Emanuele et al., 2010).
sFlt-1 is a splice variant of the vascular endothelial growth factor (VEGF) receptor with two proposed mechanisms; 1) VEGF trapping and 2) hetero-dimerization with the VEGF receptor (Kendall et al., 1996), altering VEGF receptor mediated signaling (Cindrova-Davies et al., 2011). Little is known about sFlt-1 in SZ, but much is known about VEGF in SZ and neurodevelopment (Dumpich et al., 2015). For instance, a VEGF knockout model resulted in growth-cone path-finding errors and altered optic chiasm development (Erskine et al., 2011). In SZ, post-mortem brain analyses demonstrated reduced VEGF in the prefrontal cortex (PFC) (Fulzele and Pillai, 2009), thalamic VEGF dysregulation (Chu et al., 2009), and increased circulatory VEGF (Pillai et al., 2015). Medications targeting VEGF signaling (sorafenib, sunitinib, and pazopanib) have shown to result in psychosis (Demirci et al., 2015; Kunene and Porfiri, 2011; Kuo et al., 2014) and cognitive (Cao et al., 2004) symptoms, domains affected in psychotic disorders (Tamminga et al., 2014).
In FHR, abnormalities affecting brain structure and function have been identified. A review showed that accelerated volume reductions over time were associated with symptom and cognitive deficits (Thermenos et al., 2013). Also, PFC and hippocampal volume alterations were consistently reported in FHR neuroimaging studies (Thermenos et al., 2013). Investigations correlating angiogenesis biomarkers to brain structure and cognition has been described. VEGF polymorphisms in healthy patients have been associated with hippocampal volume (Blumberg et al., 2008). Elevations in serum VEGF levels were associated with decreased PFC volume (Pillai et al., 2015). An animal model investigating offspring from pre-eclamptic mothers, demonstrated that elevated sFlt-1 had a reduction in brain volume that was prevented with prenatal pravastatin treatment (Carver et al., 2014). Taken together, these findings point to the importance of examining the role sFlt-1 on psychosis risk.
The current study assessed the impact of circulating sFlt-1 levels in antipsychotic-naïve FHR patients on baseline and longitudinal measures of psychopathology, cognition, and brain structure. We hypothesized that elevated sFlt-1 correlates with baseline and longitudinal changes in psychopathology, cognition, neurological function, and brain structure.
Methods and Materials
Participants
The study protocol, consent form, and in the case of minors, assent, with a guardian providing informed consent, were reviewed and approved by the IRB at the University of Pittsburgh and VA Pittsburgh Healthcare System (VAPHS). The FHR participants had either a first- or second-degree relative diagnosed with schizophrenia or schizoaffective disorder. The parental diagnosis was confirmed by Structural Clinical Interview for DSM-IV Axis I (SCID) interviews (First, 2012). Healthy controls (HC) were recruited by advertisements in the same geographic area. The FHR and HC participants were evaluated using the Schedule for Affective Disorders and Schizophrenia for Children (K-SADS), diagnoses were confirmed by consensus (Chambers et al., 1985; First, 2012); participants diagnosed with DSM-IV mental retardation, lifetime psychotic disorder, prior antipsychotic exposure, significant neurological/medical conditions, or IQ <75 were excluded (Keshavan et al., 2008).
Clinical assessments and MRI scans were conducted at several time points. The planned follow-up time was 1, 2 and 3 years, unless onset of psychosis occurred earlier, at which point assessments were conducted soon after transition.
Thirty-five FHR participants had plasma sFlt-1 collected at baseline. Three of 35 participants converted to psychosis (schizophrenia, n=1; schizoaffective, n=2) during the 3 year follow up period. At follow-up, non-converters had either no psychiatric diagnosis (n = 9) or non-psychotic psychiatric disorder (n = 23). For the longitudinal examination of clinical and imaging measures, we median split sFlt-1 in the FHR group.
Clinical assessments
Outcome was assessed by interim medical/psychiatric histories and annual interviews by the same clinicians who assessed the participant at baseline. The Structured Interview for Prodromal Symptoms (SIPS) and the Chapman Schizotypy Scales (CHAP) were obtained at baseline and follow up. (Chapman et al., 1994). The SIPS scale evaluates positive, negative, disorganized, and general symptoms and rates severity on the Scale of Prodromal Symptoms (SOPS). The CHAP scale includes true-false self-report questions that measure positive (magical ideation and perceptual aberration) and negative schizotypy (Chapman et al., 1994), and the former is predictive for future conversion to psychosis (Diwadkar et al., 2006; Keshavan et al., 2008). The modified neurological evaluation scale (NES) was performed by trained raters and yields two subscale scores (repetitive motor and cognitive-perceptual) (Heinrichs and Buchanan, 1988; Keshavan et al., 2003). Percentage of perseverative errors (PERSERR) committed on the Wisconsin Card Sort Test (WCST) was used to assess executive function (Robinson et al., 1980). In the longitudinal analysis, the FHR group had 28 patients with one or more follow up clinical measures. We performed a median split by sFlt-1 level to create two groups with 14 participants in each group.
Image Processing, Quality Assurance, and Reliability
All magnetic resonance imaging (MRI) scans were conducted at the University of Pittsburgh Medical Center (1.5 T Signa Whole Body Scanner, GE Medical Systems, Milwaukee, WI). At baseline there were 23 FHR and 12 healthy comparison patients with available MRI data. For the longitudinal analysis the FHR group had 13 patients with one or more MRI scans. We performed a median split by sFlt-1 level to create two groups with 7 participants in the high sFlt-1 and 6 in the low sFlt-1 group. All images underwent rigorous quality control, checked for scanner artifacts, and performed blind to participant identity. Images were converted to Neuroimaging Informatics Technology Initiative format and checked for scanner artifacts by trained raters. Images were run through a first-level auto-reconstruction in Free-Surfer 5.1 software. The skull- stripped brains were checked for remaining dura or sinuses that could interfere with accurate segmentation. When non-brain tissue was found, trained raters edited images manually. All raters had inter-rater reliabilities and intra-rater reliability greater than 95%. When deemed sufficiently clean for segmentation by an independent rater, images were run through second- and third-level auto-reconstruction, during which gray matter thickness and volume measures were extracted.
sFlt-1 assay
Plasma samples were collected at baseline after overnight fasting from a total of 74 patients (39 controls, 35 FHR). Samples were de-identified and plasma aliquots frozen at −80 °C until use to avoid freeze/thaw cycles. Laboratory analysis was conducted in Dr. Yao’s laboratory at VAPHS. Plasma samples were processed with MESO SCALE DISCOVERY’S (MSD) MULTI-ARRAY® Technology (Maryland, DE). The MSD human growth factor panel I assay kit provided quantifications for sFlt-1. MSD is a multiplex immunoassay system with specific capture antibodies for analytes that are coated in arrays, within each well of a 96-well carbon electrode plate. The detection system uses patented SULFO-TAG labels. The electrical stimulation is decoupled from the output signal, which is light, to generate the assays with minimal background. MSD labels can be conveniently conjugated to biological molecules, are stable and non-radioactive. Assays were developed, validated, and raw intensities converted to absolute concentrations after comparison with a standard curve. Technicians ran assays without knowledge of clinical status of the participants.
Statistical Analysis
All statistical analyses were performed using the R statistical analysis software (version 3.1.1). Demographics of HCs and FHR participants were analyzed with independent t-tests or Fisher’s exact. sFlt-1 values were normalized through logarithmic transformation due to non-normal distribution. Mean log sFlt-1 values were compared between FHR and HCs using an independent sample t-test. Pearson’s correlations were performed among log sFlt-1 and baseline age, CHAP, SOPS, NES, and PERSERR scores. Pearson’s correlations were followed by Benjamini and Hochberg adjustment for multiple comparisons (Benjamini, 1995).
The MTL (hippocampal, parahippocampal, and entorhinal) and dorsolateral PFC (DLPFC) grey matter volumes and thickness were compared between HCs and FHR patients using separate univariate analysis of covariances, with age and sex as covariates. Partial Pearson’s correlations with age and sex as covariates (ICV for volumetric analysis) were tested on these brain volume/thickness measures and log sFlt-1 in HC and patients separately. The group of analysis of covariance tests and the group of partial correlations were each corrected for multiple comparisons with the Benjamini–Hochberg’s correction (Benjamini, 1995). Outliers with more than 4 SDs from the mean were removed (FHR, n = 1).
Longitudinal analysis was performed on CHAP, NES and PERSERR by calculating the change in-group trajectories of participants using the lmer function for mixed- effects modeling in the lme4 R statistical package. Mixed effects modeling accounts for nested random effects such as varying time-dependent covariates like ‘age at assessment’ and availability of subjects at assessment. To determine group differences we setup a conditional growth model with the effects of sex, race and age at assessment*group status as fixed effects. Random effects included an intercept per person to account for within person dependence and random slopes to account for varying assessment age per subject. We also performed mixed effects models for structural MRI constructs (volume/thickness), but the models failed to attain convergence and we attribute it to patient attrition.
Model 1: lmer (CHAP ~time point age + race + sex + (1+time point age| subject)
Model 2: lmer (CHAP ~ time point age x sFlt-1 high-low + race + sex + (1+time point age| subject))
Results
Participant characteristics
The demographic, symptom and log sFlt-1 values for HCs and patients are listed in Table 1. There were significant differences in age between the two groups, with the FHR being younger. There was no significant difference for sex or race between FHR and HC patients. Mean plasma sFlt-1 levels demonstrated a significant increase in the FHR group relative to HCs, p = 0.002 (Lizano et al., 2016). Mean NES (repetitive motor, cognitive-perceptual, total) and PERSERR scores revealed a significant increase in the FHR group relative to HC (Table 1). All FHR participants were antipsychotic-naïve, two patients in the FHR group had either a comorbid alcohol or cannabis use disorder. CHAP and SOPS sub-scores in the FHR group were below the attenuated psychosis threshold (Miller et al., 2002).
Table 1.
Demographic information for HC and FHR
| HC
|
FHR
|
T-test/Fisher’s exact test
|
|||
|---|---|---|---|---|---|
| N | Mean (s.d.) | N | Mean (s.d.) | p-value | |
| Age (years) | 39 | 25.1±1.0 | 35 | 16.5±0.6 | <0.001 |
| Gender (M/F) | 39 | 25/14 | 35 | 15/20 | 0.08 |
| Race (Cau/AA/other) | 39 | 24/10/5 | 35 | 15/20/0 | 0.10 |
|
| |||||
| Anti-angiogenesis marker | |||||
|
| |||||
| Plasma sFlt-1 (pg/ml) | 39 | 106±7.7 | 35 | 182±19 | 0.002 |
|
| |||||
| Psychopathology | |||||
|
| |||||
| CHAP Magical Ideation | - | 32 | 3.6±0.5 | ||
| CHAP Perceptual Aberration | - | 32 | 2.1±0.4 | ||
| SOPS Positive Symptoms | - | 20 | 0.3±0.1 | ||
| SOPS Disorganized Symptoms | - | 20 | 0.7±0.3 | ||
|
| |||||
| NES | |||||
|
| |||||
| Repetitive-Motor | 35 | 0.19±0.05 | 33 | 0.32±0.08 | <0.001 |
| Cognitive-Perceptual | 35 | 0.18±0.04 | 33 | 0.29±0.08 | 0.002 |
| Total | 35 | 0.24±0.03 | 33 | 0.32±0.05 | <0.001 |
|
| |||||
| WCST | |||||
|
| |||||
| Preservative Error | 36 | 10.7±1.3 | 33 | 14.2±1.5 | 0.002 |
Abbreviations: HC, healthy control; FHR, familial high risk for psychosis; s.d., standard deviation; M, Male; F, Female; Cau, Caucasian; AA, African American; sFlt-1, soluble fms-like tyrosine kinase; CHAP, Chapman schizotypy scale; SOPS, Scale of Prodromal Symptoms; NES, Buchanan and Heinrichs Neurological Evaluation Scale; WCST, Wisconsin card sorting test. Bold p-values indicate significant results.
Baseline correlations between plasma sFlt-1 levels and symptom or cognition
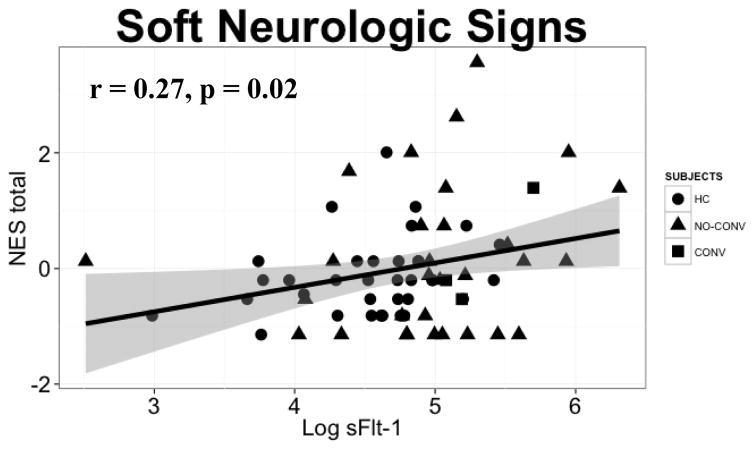
Plasma sFlt-1 levels did not show significant association with age, CHAP, SOPS, NES, and WCST in either HCs or FHR patients (Supplementary Table 1). There was a significant positive association between NES total (repetitive motor plus cognitive-perceptual) and plasma sFlt-1 in the combined groups (r = 0.27, p = 0.02, p-adj = 0.12), indicating worsening symptomatology (Supplementary Table 1, Figure 1). Three converters to psychosis have higher sFlt-1 levels and worse NES total scores (Figure 1).
Figure 1.
At baseline, plasma sFlt-1 levels show a significant positive correlation with neurologic evaluation scale (NES) total score in healthy control (HC) and familial high-risk (FHR) subjects (n = 68). Shapes depict group distributions; HC (●) and FHR [32 non-converters (no-conv, ▲) and 3 converters to psychosis (conv, ■)].
Baseline correlations between plasma sFlt-1 levels and brain volume/thickness
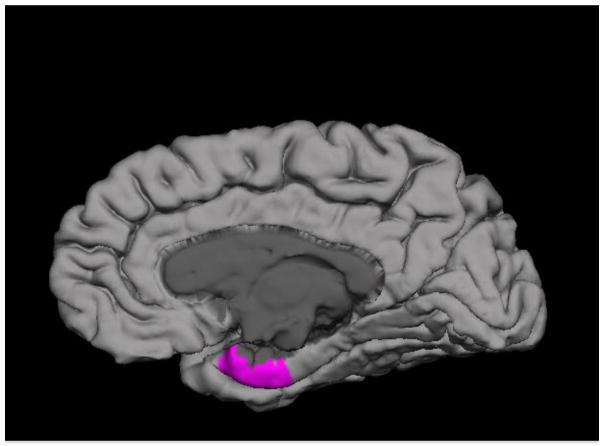
There were no significant group differences in grey matter volume or thickness between FHR and HC patients after co-varying for age and sex. At baseline, partial correlations with age and sex as covariates revealed a significant positive correlation between plasma sFlt-1 levels and right entorhinal volume (r = 0.50, p = 0.02) in FHR patients (Supplementary Table 2, Figure 2). A trend was noted for a positive association between sFlt-1 levels and right hippocampal and parahippocampal volume in FHR patients (Supplementary Table 2). Plasma sFlt-1 did not correlate with either grey matter volume or thickness in HCs.
Figure 2.
At baseline, plasma sFlt-1 levels show a significant positive correlation with the right entorhinal cortical gray matter volume in familial high-risk (FHR) subjects compared to healthy controls.
Symptomatology trajectories in FHR
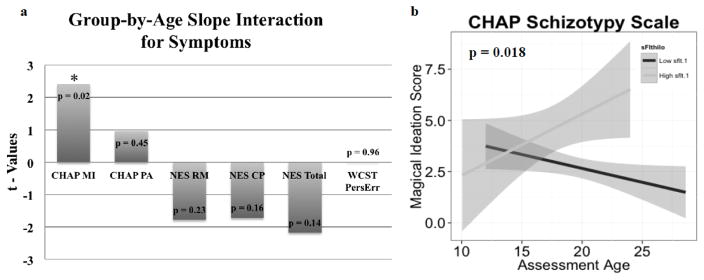
A subsample of the FHR group containing both baseline plasma sFlt-1 levels and longitudinal measures for CHAP, NES, and PERSERR were utilized for the trajectory analysis. No significant difference was observed for age, sex, or race in the subsample. Positive t-values for CHAP and WCST indicate worsening symptoms in high sFlt-1 (n = 14) compared to the low group (n =14). Negative t-values for NES indicate improving scores. Relative to low sFlt-1, high sFlt-1 exhibited greater worsening of the CHAP magical ideation score (p = 0.02), while a trend towards improvement was noted in the NES total score (Figure 3).
Figure 3.
a) Values from t-tests for group-by-age slope interactions. Groups are sFlt-1 high (n = 14) versus low (n = 14). Positive t-values for CHAP MI (magical ideation), PA (perceptual aberration), and WCST (perseverative error) indicate worsening symptoms in sFlt-1 high compared to low subjects, with CHAP MI showing a significant difference (*, p = 0.02). Negative t-values for NES RM (repetitive-motor), CP (cognitive-perceptual) and Total indicate improving scores, with a trend towards significance for NES Total. b) Plot of individual (high versus low sFlt-1) CHAP magical ideation scores, by assessment age, showing a worsening symptom trajectory for high sFlt-1 subjects. Abbreviations: CHAP, Chapman schizotypy scale; NES, Neurological Evaluation Scale; WCST, Wisconsin card sorting test.
Brain volume/thickness trajectories in the FHR group
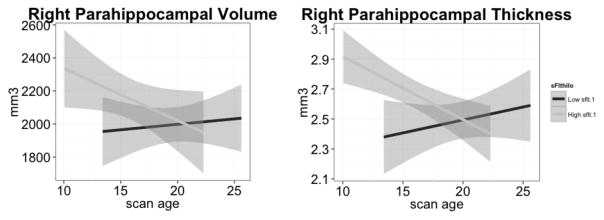
A subsample of the FHR group containing plasma sFlt-1 levels and grey matter volume/thickness measures was median split into 7 high and 6 low sFlt-1 patients. There was no significant difference in age, sex or race, but a trend was noted for age and race. The sample size was too small to perform statistical analysis due to patient attrition. Descriptively, high sFlt-1 mostly started off with higher volume and thickness measures at baseline that either “caught up” with those in the low sFlt-1 group or showed an ongoing decline. For example, the high sFlt-1 group showed a downward trajectory for the right parahippocampal volume and thickness in comparison to the low sFlt-1 group (Figure 4).
Figure 4.
Plots of individual (sFlt-1 high n = 7 versus low n = 6), right parahippocampal grey matter volume and thickness (mm3), by scan age, demonstrating progressive structural loss of left parahippocampal volume and thickness for high sFlt-1 subjects.
Discussion
We previously described an abnormal angiogenic signature early in the course of SZ (Lizano et al., 2016), which may have important implications for diagnostic and predictive markers. In the present study, we sought to integrate the anti-angiogenic marker, sFlt-1 with clinical findings to further understand the role of angiogenesis in the pathophysiology of SZ. We evaluated the effect of plasma sFlt-1 on symptom and brain structure in a longitudinal FHR sample. Our findings demonstrate a longitudinal association between sFlt-1 and worsening clinical and brain structural trajectories. Elevated plasma sFlt-1 was correlated with worse baseline NES scores. High sFlt-1 levels were significantly associated with progressive worsening of CHAP magical ideation scores. sFlt-1 was significantly positively correlated with right entorhinal volume and high sFlt-1 demonstrated a trend for right hippocampal volume and parahippocampal thickness loss over time. While the sample size is small, it’s strengths include a unique sample of FHR relatives that are medication naïve, limited substance use, and below attenuated psychotic symptom level at study inclusion, all of which are commonly confounded in other studies. The longitudinal approach may help resolve the debate regarding the central brain effects of peripheral indices, by demonstrating that peripheral markers predicted clinical and brain structural changes.
The non-finding that sFlt-1 was not correlated with baseline psychopathology was expected (Supplementary Table 1), given that this FHR sample had symptomatology scores below the attenuated psychosis threshold (Miller et al., 2002). We expected elevated baseline levels of sFlt-1 to predict worsening of psychopathology and this was demonstrated utilizing linear mixed effects modeling (Figure 3). No prospective studies examining the role of sFlt-1 with psychopathology exist, but one cross-sectional SZ study didn’t find a correlation between serum VEGF and psychopathology (Pillai et al., 2015). A post-mortem study in SZ showed that VEGFR-2 expression was reduced in the PFC and associated with positive symptoms (Hino et al., 2016). Also, there are case reports of psychosis and hallucinations resulting from angiogenesis inhibitor treatment (Demirci et al., 2015; Kunene and Porfiri, 2011; Kuo et al., 2014). A small study of offspring from pre-eclamptic mothers with elevated sFlt-1 (mean levels twice that of HCs), showed an increase in the rate of psychopathology in the offspring from pre-eclamptic mothers compared to controls (Ratsep et al., 2016). These data demonstrate that angiogenic disruption may play a role in worsening psychopathology via sFlt-1 by VEGF trapping, hetero-dimerization with the VEGF receptor (Kendall et al., 1996) or alteration of VEGF receptor mediated signaling (Cindrova-Davies et al., 2011). Future studies could look at the effect of sFlt-1 on psychopathology in first episode psychosis and chronic schizophrenia samples. It would also be noteworthy to determine if the increased rate of schizophrenia in pre-eclamptic mothers is due to elevations of sFlt-1 during pregnancy.
Soft neurologic signs were significantly different between HC and FHR (Table 1), and has been previously demonstrated (Neelam et al., 2011; Prasad et al., 2009). sFlt-1 correlated with soft neurologic signs at baseline (Figure 1) and trended towards improving trajectories (Figure 3), suggesting that sFlt-1 might be a state marker for neurologic dysfunction. Soft neurologic signs are an important endophenotype in SZ with strong familial association (Neelam et al., 2011) and brain structural correlates (Zhao et al., 2014). This is the first study examining the role of sFlt-1 on soft neurologic signs. Our data is in agreement with a mouse model of pre-eclampsia induced by pregnancy-specific overexpression of sFlt-1, where offspring displayed impairments in balance and coordination, and was prevented with maternal pravastatin treatment (Carver et al., 2014). These findings may transform our understanding of the pathophysiologic role of angiogenesis in altering the soft neurologic signs endophenotype, with the potential for novel therapeutic avenues. Future studies should look at the effects of sFlt-1 on soft neurologic signs in first episode and chronic schizophrenia patients, as well as offspring from pre-eclamptic mothers.
We identified significant positive correlations between sFlt-1 and right entorhinal volume (Figure 2) in FHR patients with a positive trend noted for the right hippocampal and parahippocampal volumes (Supplementary Table 2). This finding demonstrates that normal circulatory levels of sFlt-1 do not have an effect on brain structure, while elevated levels may be utilized as a target for brain structural trajectories. This finding may suggest that attenuation of angiogenesis could explain the shift towards a more pathologic process that initially results in brain structural enhancement followed by accelerated grey matter loss. The longitudinal analysis showed two important findings: 1) high sFlt-1 had mostly larger baseline volume/thickness measures compared to the low sFlt-1 group; 2) high sFlt-1 mostly showed downward trajectories compared to an upward trend in the low sFlt-1 group (Figure 4). While these findings are interesting, the sample size was too small to determine significance. This is the first longitudinal study evaluating the effects of sFlt-1 on brain structure in psychotic disorders. Only a few studies have looked at the effects of angiogenesis on brain structure. A mouse model of pre-eclampsia with sFlt-1 overexpression demonstrated brain structural impairments in offspring, an effect that was attenuated with maternal pravastatin treatment (Carver et al., 2014). A similar study in humans, offspring of pre-eclamptic mothers with elevated sFlt-1 showed brain structural and vascular changes compared to controls (Ratsep et al., 2016). In a cross-sectional study of schizophrenia, an inverse correlation between serum VEGF and frontal pole volume was identified (Pillai et al., 2015). A longitudinal Alzheimer’s study demonstrated that elevated CSF VEGF was associated with optimal brain aging (Hohman et al., 2015), suggesting that anti-angiogenesis factors could have a contrasting effect. Another study measured the effects of cytokines in clinical high risk for psychosis patients and showed that a pro-inflammatory aggregate (TNF-α, IL-2, and IFN-γ) at baseline was strongly predictive of steeper rates of gray matter reduction in the right PFC of cases transitioned to psychosis (Cannon et al., 2015). Taken together this demonstrates that baseline sFlt-1 could be used to 1) determine brain structural trajectories, 2) that dysregulation of angiogenesis could be detrimental to brain structure, and 3) that VEGF studies can provide a conceptual framework for understanding our current findings. Future studies will need to examine the effects of sFlt-1 on brain structure in a first episode and chronic schizophrenia sample, as well as the effects of maternal preeclampsia on the neurodevelopment of their offspring.
The mechanism for increased sFlt-1 in the schizophrenia population is currently unknown. There are several hypothesized mechanisms by which sFlt-1 could be increased in this population. A functional genomic analysis of available schizophrenia-associated genes from candidate gene, genome-wide association and postmortem expression studies found an overrepresentation of genes involved in vascular function that support both the neurodevelopmental and adult vascular-ischemic hypothesis (Moises et al., 2015). In a study of pregnant mothers and their offspring, the FLT1 genotype has been associated with sFlt-1 levels (Muehlenbachs et al., 2008). The gene-environment interaction of vascular genes and obstetrical complications is of particular interest in schizophrenia (Schmidt-Kastner et al., 2012). Additionally, sFlt-1 could be directly regulated by VEGF (Fan et al., 2014), microglia activity (Ryu et al., 2009), or inflammation (Muehlenbachs et al., 2008). We hypothesis that sFlt-1 and other genes related to angiogenesis contribute to schizophrenia risk from a neurodevelopmental standpoint with environmental influences.
Angiogenic perturbations due to increased sFlt-1 expression might attenuate VEGF-mediated arterial differentiation (Mukouyama et al., 2005), vasoregulation (Rosenstein et al., 2010), venular development (Stalmans et al., 2002), and capillary density (DiPietro, 2016) via the mechanisms discussed above. Due to advances in retinal and neuroimaging, it is now possible to study arteriolar, venular, and vasoregulatory mechanisms in vivo and there are a few studies implicating microvasculature disruption in patients with schizophrenia (Allen et al., 2016; Hua et al., 2016; Meier et al., 2013). Developmentally, a large longitudinal study showed that second trimester maternal angiogenesis levels were associated with narrower childhood retinal arteriolar caliber, but not with retinal venular caliber (Gishti et al., 2015). Thus, elevations in sFlt-1 could have lasting effects on microvascular structure, fluid dynamics, and neurodevelopmental processes and have the potential to inform diagnosis, prognosis, and treatment.
Limitations of this study include the small sample size and sample attrition, which resulted in nearly 20% loss to follow up. This level of attrition had the greatest impact on the longitudinal structural imaging analysis that limited statistical analysis.
Supplementary Material
Acknowledgments
Funding source
This work was supported in part by Department of Veterans Affairs [Merit Reviews (JKY) and Senior Research Career Scientist Award (JKY)] and the VA Pittsburgh Healthcare System, National Institute of Health [MH58141 (JKY), MH64023 (MSK), KO2 MH 01180 (MSK), MH45156 (MSK), c UL1 RR024153, and NIH/NCRR/GCRC Grant M01 RR00056].
The authors are grateful to P. Cheng, C. Korbanic and J. Haflett for their technical assistance and to Diana Mermon for her help in clinical assessments of FHR subjects, and Jean Miewald for her help in data management. We thank the clinical core staff of the Center for the Neuroscience of Mental Disorders (MH45156, MH084053, David Lewis MD, Director) for their assistance in diagnostic and psychopathological assessments. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The contents of this article do not represent the views of the Department of Veterans Affairs, the United States Government, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Contributions
Paulo Lizano contributed to study design, data analysis and interpretation, drafting of the article, revising the article, and final approval.
Jeffrey Yao contributed to study design, laboratory assays, data analysis and interpretation, revising the article, and final approval.
Neeraj Tandon conducted data analysis, data interpretation, and revised the article.
Suraj Sarvode Mothi conducted data analysis and revised the article.
Debra Montrose contributed to study design.
Matcheri Keshavan contributed to study design, revising, and final approval.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen P, Chaddock CA, Egerton A, Howes OD, Bonoldi I, Zelaya F, Bhattacharyya S, Murray R, McGuire P. Resting Hyperperfusion of the Hippocampus, Midbrain, and Basal Ganglia in People at High Risk for Psychosis. The American journal of psychiatry. 2016;173(4):392–399. doi: 10.1176/appi.ajp.2015.15040485. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995;(57):289–300. [Google Scholar]
- Blumberg HP, Wang F, Chepenik LG, Kalmar JH, Edmiston E, Duman RS, Gelernter J. Influence of vascular endothelial growth factor variation on human hippocampus morphology. Biological psychiatry. 2008;64(10):901–903. doi: 10.1016/j.biopsych.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R North American Prodrome Longitudinal Study C. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biological psychiatry. 2015;77(2):147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nature genetics. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carver AR, Andrikopoulou M, Lei J, Tamayo E, Gamble P, Hou Z, Zhang J, Mori S, Saade GR, Costantine MM, Burd I. Maternal pravastatin prevents altered fetal brain development in a preeclamptic CD-1 mouse model. PloS one. 2014;9(6):e100873. doi: 10.1371/journal.pone.0100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Archives of general psychiatry. 1985;42(7):696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. Journal of abnormal psychology. 1994;103(2):171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- Chu TT, Liu Y, Kemether E. Thalamic transcriptome screening in three psychiatric states. Journal of human genetics. 2009;54(11):665–675. doi: 10.1038/jhg.2009.93. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovascular research. 2011;89(3):671–679. doi: 10.1093/cvr/cvq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci NS, Erdem GU, Dogan M, Ozdemir NY, Zengin N. A rare case: Hallucination associated with pazopanib. J Cancer Res Ther. 2015;11(4):961–962. doi: 10.4103/0973-1482.160919. [DOI] [PubMed] [Google Scholar]
- DiPietro LA. Angiogenesis and wound repair: when enough is enough. Journal of leukocyte biology. 2016;100(5):979–984. doi: 10.1189/jlb.4MR0316-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(2):230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Dumpich M, Mannherz HG, Theiss C. VEGF Signaling Regulates Cofilin and the Arp2/3-complex within the Axonal Growth Cone. Curr Neurovasc Res. 2015;12(3):293–307. doi: 10.2174/1567202612666150603141144. [DOI] [PubMed] [Google Scholar]
- Emanuele E, Orsi P, Barale F, di Nemi SU, Bertona M, Politi P. Serum levels of vascular endothelial growth factor and its receptors in patients with severe autism. Clin Biochem. 2010;43(3):317–319. doi: 10.1016/j.clinbiochem.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, Alakakone B, Shewan D, Ruhrberg C. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70(5):951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, Gambhir SS, Ambati BK, Cross JC, Nayak NR. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. The Journal of clinical investigation. 2014;124(11):4941–4952. doi: 10.1172/JCI76864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2012. [Google Scholar]
- Fulzele S, Pillai A. Decreased VEGF mRNA expression in the dorsolateral prefrontal cortex of schizophrenia subjects. Schizophrenia research. 2009;115(2–3):372–373. doi: 10.1016/j.schres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Gishti O, Jaddoe VW, Felix JF, Reiss I, Hofman A, Ikram MK, Steegers EA, Gaillard R. Influence of maternal angiogenic factors during pregnancy on microvascular structure in school-age children. Hypertension. 2015;65(4):722–728. doi: 10.1161/HYPERTENSIONAHA.114.05008. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. The American journal of psychiatry. 1988;145(1):11–18. doi: 10.1176/ajp.145.1.11. [DOI] [PubMed] [Google Scholar]
- Hino M, Kunii Y, Matsumoto J, Wada A, Nagaoka A, Niwa SI, Takahashi H, Kakita A, Akatsu H, Hashizume Y, Yamamoto S, Yabe H. Decreased VEGFR2 expression and increased phosphorylated Akt1 in the prefrontal cortex of individuals with schizophrenia. J Psychiatr Res. 2016;82:100–108. doi: 10.1016/j.jpsychires.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Hohman TJ, Bell SP, Jefferson AL Alzheimer’s Disease Neuroimaging I. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 2015;72(5):520–529. doi: 10.1001/jamaneurol.2014.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Brandt AS, Lee S, Blair NI, Wu Y, Lui S, Patel J, Faria AV, Lim IA, Unschuld PG, Pekar JJ, van Zijl PC, Ross CA, Margolis RL. Abnormal Grey Matter Arteriolar Cerebral Blood Volume in Schizophrenia Measured With 3D Inflow-Based Vascular-Space-Occupancy MRI at 7T. Schizophrenia bulletin. 2016 doi: 10.1093/schbul/sbw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochemical and biophysical research communications. 1996;226(2):324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophrenia research. 2008;103(1–3):114–120. doi: 10.1016/j.schres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Sanders RD, Sweeney JA, Diwadkar VA, Goldstein G, Pettegrew JW, Schooler NR. Diagnostic specificity and neuroanatomical validity of neurological abnormalities in first-episode psychoses. The American journal of psychiatry. 2003;160(7):1298–1304. doi: 10.1176/appi.ajp.160.7.1298. [DOI] [PubMed] [Google Scholar]
- Kim TH, KDH, Lee SK, Son BK, Jung JS. The Effect of Antipsychotic Drug Treatment on Serum VEGF, sVEGFR-1, and sVEGFR-2 level in Schizophrenia - A Preliminary Study. Korean Journal of Biological Psychiatry. 2007;14:232–240. [Google Scholar]
- Kunene V, Porfiri E. Sunitinib-induced acute psychosis: case report. Clinical genitourinary cancer. 2011;9(1):70–72. doi: 10.1016/j.clgc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Kuo YL, Yang YK, Cheng HC, Yen CJ, Chen PS. Psychotic disorder induced by a combination of sorafenib and BAY86-9766. General hospital psychiatry. 2014;36(4):450, e455–457. doi: 10.1016/j.genhosppsych.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Lizano PL, Keshavan MS, Tandon N, Mathew IT, Mothi SS, Montrose DM, Yao JK. Angiogenic and immune signatures in plasma of young relatives at familial high-risk for psychosis and first-episode patients: A preliminary study. Schizophrenia research. 2016;170(1):115–122. doi: 10.1016/j.schres.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes R, Soares R, Coelho R, Figueiredo-Braga M. Angiogenesis in the pathophysiology of schizophrenia - A comprehensive review and a conceptual hypothesis. Life sciences. 2015;128:79–93. doi: 10.1016/j.lfs.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Meier MH, Shalev I, Moffitt TE, Kapur S, Keefe RS, Wong TY, Belsky DW, Harrington H, Hogan S, Houts R, Caspi A, Poulton R. Microvascular abnormality in schizophrenia as shown by retinal imaging. The American journal of psychiatry. 2013;170(12):1451–1459. doi: 10.1176/appi.ajp.2013.13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. The American journal of psychiatry. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Moises HW, Wollschlager D, Binder H. Functional genomics indicate that schizophrenia may be an adult vascular-ischemic disorder. Transl Psychiatry. 2015;5:e616. doi: 10.1038/tp.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Natural selection of FLT1 alleles and their association with malaria resistance in utero. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(38):14488–14491. doi: 10.1073/pnas.0803657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132(5):941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Neelam K, Garg D, Marshall M. A systematic review and meta-analysis of neurological soft signs in relatives of people with schizophrenia. BMC Psychiatry. 2011;11:139. doi: 10.1186/1471-244X-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Howell KR, Ahmed AO, Weinberg D, Allen KM, Bruggemann J, Lenroot R, Liu D, Galletly C, Weickert CS, Weickert TW. Association of serum VEGF levels with prefrontal cortex volume in schizophrenia. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.96. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Sanders R, Sweeney J, Montrose D, Diwadkar V, Dworakowski D, Miewald J, Keshavan M. Neurological abnormalities among offspring of persons with schizophrenia: relation to premorbid psychopathology. Schizophrenia research. 2009;108(1–3):163–169. doi: 10.1016/j.schres.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratsep MT, Paolozza A, Hickman AF, Maser B, Kay VR, Mohammad S, Pudwell J, Smith GN, Brien D, Stroman PW, Adams MA, Reynolds JN, Croy BA, Forkert ND. Brain Structural and Vascular Anatomy Is Altered in Offspring of Pre-Eclamptic Pregnancies: A Pilot Study. AJNR Am J Neuroradiol. 2016;37(5):939–945. doi: 10.3174/ajnr.A4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AL, Heaton RK, Lehman RA, Stilson DW. The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions. J Consult Clin Psychol. 1980;48(5):605–614. doi: 10.1037//0022-006x.48.5.605. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Krum JM, Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6(2):107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Cho T, Choi HB, Wang YT, McLarnon JG. Microglial VEGF receptor response is an integral chemotactic component in Alzheimer’s disease pathology. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(1):3–13. doi: 10.1523/JNEUROSCI.2888-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HW, Rutten BP. An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Molecular psychiatry. 2012;17(12):1194–1205. doi: 10.1038/mp.2011.183. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. The Journal of clinical investigation. 2002;109(3):327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati P, Rane S, Skinner J, Gore J, Heckers S. Increased hippocampal blood volume and normal blood flow in schizophrenia. Psychiatry research. 2015;232(3):219–225. doi: 10.1016/j.pscychresns.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophrenia bulletin. 2014;40(Suppl 2):S131–137. doi: 10.1093/schbul/sbt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos HW, Keshavan MS, Juelich RJ, Molokotos E, Whitfield-Gabrieli S, Brent BK, Makris N, Seidman LJ. A review of neuroimaging studies of young relatives of individuals with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(7):604–635. doi: 10.1002/ajmg.b.32170. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Zimina IS, Vikhreva OV, Krukov NO, Rachmanova VI, Orlovskaya DD. Ultrastructural damage of capillaries in the neocortex in schizophrenia. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2010;11(3):567–578. doi: 10.3109/15622970903414188. [DOI] [PubMed] [Google Scholar]
- Uranova NZI, Vikhreva O, Rachmanova V, Klintsova A, Orlovskaya D. Reduced Capillary Density in the Prefrontal Cortex in Schizophrenia. American Journal of Medical Sciences and Medicine. 2013;1(3):45–51. [Google Scholar]
- Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. Journal of cellular and molecular medicine. 2010;14(3):528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Li Z, Huang J, Yan C, Dazzan P, Pantelis C, Cheung EF, Lui SS, Chan RC. Neurological soft signs are not “soft” in brain structure and functional networks: evidence from ALE meta-analysis. Schizophrenia bulletin. 2014;40(3):626–641. doi: 10.1093/schbul/sbt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.