Abstract
Background
Use of electronic cigarettes (e-cigarettes) is increasing rapidly. Chinese pharmacist Hon Lik is frequently cited as inventing the modern e-cigarette in 2003. However, tobacco companies have developed electronic nicotine delivery systems since at least 1963.
Methods
We searched the University of California San Francisco Truth (formerly Legacy) Tobacco Industry Documents beginning with the terms ‘electric cigarette’ and ‘electronic cigarettes’, ‘e-cigarette’, ‘smokeless cigarettes’, ‘nicotine aerosol’, ‘tobacco aerosol’, and ‘vaping’ and then expanded the search using snowball sampling. We focused our analysis on Philip Morris (PM) documents discussing technology that aerosolised a nicotine solution because these devices resembled modern e-cigarettes. Over 1000 documents were reviewed; 40 were included in the final analysis.
Results
PM started developing a nicotine aerosol device in 1990 to address the health concerns and decreased social acceptability of smoking that were leading smokers to switch to nicotine replacement therapy. PM had developed a capillary aerosol generator that embodied basic e-cigarette technology in 1994, but in the mid-to-late 1990s focused on applying its aerosol technology to pharmaceutical applications because of uncertainty of how such products might affect potential Food and Drug Administration regulation of tobacco products. In 2001, PM resumed its work on a nicotine aerosol device, and in 2013, NuMark (a division of Altria, PM's parent company) released the MarkTen, a nicotine aerosol device.
Conclusions
Rather than a disruptive technology, PM developed e-cigarette technology to complement, not compete with, conventional cigarettes and evade tobacco control regulations.
INTRODUCTION
Electronic cigarettes (e-cigarettes or electronic nicotine delivery systems (ENDS)) have been promoted as a healthier alternative to cigarettes because they deliver nicotine aerosol without burning tobacco.1 The invention of the e-cigarette is often credited to Chinese pharmacist Hon Lik in 20032–4 and considered a ‘disruptive technology’ that could compete with cigarettes.5–7
Since at least 1963, cigarette companies have been working on ENDS that did not burn tobacco in an effort to develop ‘reduced harm’ or ‘socially acceptable’ cigarette alternatives,8,9 including products that heated tobacco instead of burning it, such as British American Tobacco's (BAT) 1960s Ariel cigarette,10 RJ Reynolds’ (RJR) 1980s Premier,11 RJR's 1990s Eclipse12 and Philip Morris’ (PM's) 1990s/2000s Accord.9 None of these products heated a liquid nicotine solution, so they bore little resemblance to modern e-cigarettes, and none achieved commercial success. In 2013, NuMark, a PM subsidiary, released the MarkTen e-cigarette based on nicotine aerosol technology that PM had been developing since 1990, 13 years before Hon Lik developed his e-cigarette.
METHODS
Between October 2010 and October 2015, we searched the University of California, San Francisco Truth (formerly Legacy) Tobacco Documents Library (http://industrydocuments.library.ucsf.edu/tobacco) beginning with ‘electric cigarette’, ‘electronic cigarettes’, ‘e-cigarette’, ‘smokeless cigarettes’, ‘nicotine aerosol’, ‘tobacco aerosol’ and ‘vaping’. We applied standard snowball sampling techniques13 to refine subsequent searches, which revealed that PM, RJR and BAT all attempted to create reduced risk cigarettes and cigarette alternatives. This paper focuses on PM because only PM documents described a device resembling the modern e-cigarette. We identified several PM projects that we used as search terms, including ‘Ideal Smoke’, ‘Project Leap’ (a component of the Ideal Smoke programme), ‘Project Beta’ (a subproject of Project Leap), ‘Tobacco Aerosol Research Project’ (TARP) and ‘Vision Technologies’. These searches revealed that PM used a capillary aerosol generator (CAG) to develop a nicotine aerosol device and a pharmaceutical drug delivery device. Searches for ‘capillary aerosol generator’ and ‘aerosol generator’ yielded additional documents. We then searched using product names including Accord, an electrically heated cigarette sold by PM in the late 1990s, Aria (a pharmaceutical inhaler developed by Chrysalis, a division of PM USA), MarkTen, NuMark's e-cigarette released in 2010 and Ruyan (one of the earliest e-cigarettes, developed by Chinese pharmacist Hon Lik). (Both NuMark and PM USA are subsidiaries of Altria; PM International became its own company in 2008.) We also searched key individuals and examined documents with adjacent Bates numbers. We screened over 1000 documents and included 40 of these in this analysis. Between October 2010 and April 2016, we used Google Patents (https://patents.google.com) to locate relevant patent applications for devices referenced in the documents. In April 2016, we gathered information on the MarkTen e-cigarette from the NuMark website (http://www.nu-mark.com).
RESULTS
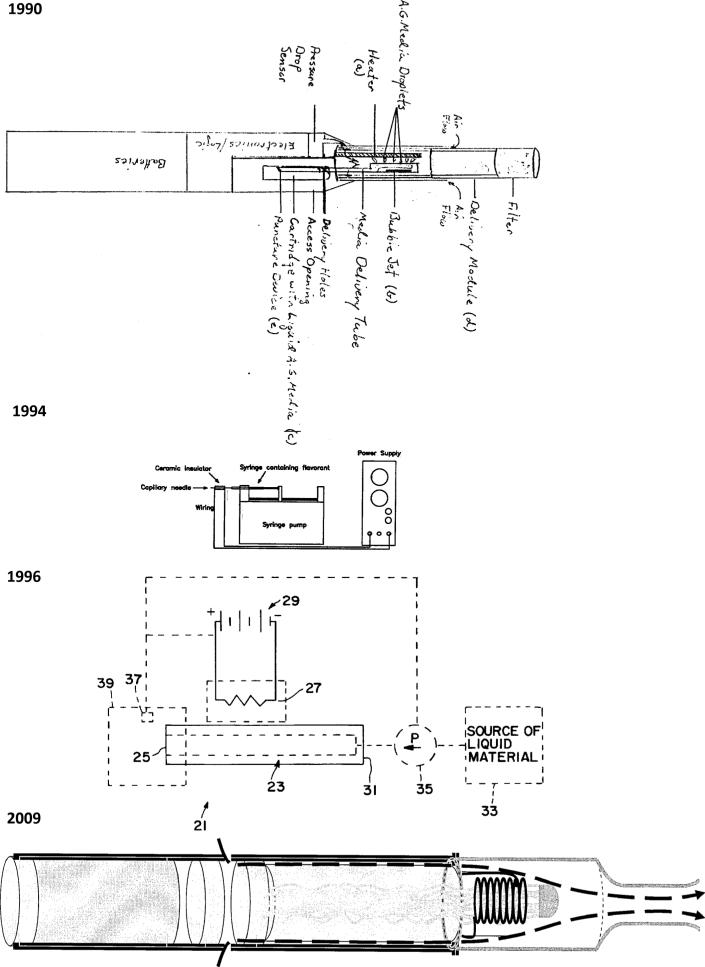
The first evidence of an e-cigarette-like device in the Truth Tobacco Documents Library is a 1990 PM invention record (figure 1) describing:
An electric smoking article comprising (1) a single permanent heater, (2) a bubble jet or inkjet printhead or comparable controlled liquid application device, (3) a liquid aerosol generating medium contained in a disposable cartridge...(4) a battery-powered, puff (pressure drop) activated, programmable logic-controlled circuit to...power...the printhead and heater, and (5) a disposable delivery module providing desirable pressure drop, air-flow and filtration for aerosol delivery to the smoker.14
Figure 1.
Evolution of PM capillary aerosol generators to deliver a nicotine aerosol. 1990: Spray jet/heater electric smoking device from PM invention record,14 1994: capillary aerosol generator from PM invention record,23 1996: US patent application for aerosol and a method and apparatus for generating an aerosol,27 2009: PM US patent application for electrically heated smoking system having a liquid storage portion.59 Philip Morris.
Puffing on the device activated the heater, the printhead sprayed liquid on the heater and the heated liquid became an aerosol to be inhaled by the user. The cartridge was ‘analogous to fountain pen cartridge, may contain liquid aerosol generation media equivalent to any number of individual cigarettes, a single pack or a single carton’.14
Internal documents from 1992 discuss the motivation behind pursuing a nicotine aerosol device. A document that appears to be part of PM's 1991–1992 research and development (R&D) report expressed concern about:
A declining US market, the growth of smoking restrictions, the animosity of a growing anti-smoking movement which is attempting to establish itself worldwide, and a marked decline in the social acceptability of the smoking experience. Faced with this situation, there are only three possible choices that companies within our industry can take...pursue business as usual...take the path of diversification and milking the tobacco business...and to determine what new products can solve current and anticipated problems.15
PM was also concerned that smokers were using nicotine replacement therapy (NRT) to stop smoking. According to the 1992–1996 PM Operations Plan,16 PM was also concerned that, in addition to Nicorette gum and transdermal nicotine patches, a pharmaceutical nicotine inhaler was being developed:
Products of this type pose a dual threat to PM USA...they make it easier for smokers to quit, and smokers who quit are the major cause of loss of sales of our products. We lose far more customers to quitting than we do to competitive brands. Secondly, studies have shown that some individuals use Nicorette not simply as an aid to quitting, but as a method of enjoying the effects of nicotine. Consequently, Nicorette and other alternative products must be regarded as competitive products.16
PM's 1992–1996 R&D plan identified nicotine aerosol devices as one way to counteract these threats to the cigarette business:
There are at least four types of products which address such [health] ‘concerns;’ namely, 1) products which deliver tobacco flavor through heating rather than burning tobacco; 2) ‘zero tar’ conventional cigarettes; 3) tobacco flavor and impact delivered in aerosol form; and 4) products which significantly reduce nicotine delivery which have impact comparable to a conventional cigarette...a product which can provide tobacco flavor and impact in aerosol form, is of considerable significance.17
In an appendix to the R&D plan, PM hypothesised that consumers would purchase a nicotine aerosol device instead of NRT if it provided more efficient nicotine delivery than NRT:
Since smokers smoke in order to obtain nicotine...any method which introduces nicotine through a non-cigarette product will assist a smoker in giving up cigarettes...the two types of nicotine-containing smoking cessation aids currently or about to be marketed in the US do not introduce nicotine in an efficient manner. A device which would introduce nicotine efficiently would be an aerosol delivery device.18
The appendix also predicted that:
It will not be long before nicotine-delivery devices which are both effective and appealing are introduced into the market with endorsement [by medical authorities]. Should this happen, it will clearly become the next wave in the ‘cigarette’ market driven by health concerns. If PM is not prepared to participate in this new market, it is possible that the repercussions would be severe.18
The appendix quoted a Lancet article19 as evidence of potential medical endorsement: “Although these nicotine replacement products are marketed as aids to stopping smoking, with further refinement some may also have the potential for long-term use and, if permitted, might actually replace tobacco on the open market.”
According to the R&D plan, in addition to potentially addressing smokers’ health concerns and desire for nicotine, an aerosol product might help deal with anticipated regulation of tobacco products:
They [the US antismoking movement] will advocate laws and regulations which either make it difficult for our consumers to smoke (e.g., smoking restrictions, restricted marketing) or which make smokers less likely to smoke (e.g., increased taxes, reduced social acceptability)...Many of the attributes of those products discussed above [tobacco aerosol products, heated tobacco products, ‘zero tar’ cigarettes, and reduced nicotine cigarettes] already address many present and anticipated government regulations. These attributes include the significant reduction or elimination of ETS as well as zero ignition propensity...Consequently, these products address both ‘consumer health concerns’ and government regulations.17
According to the plan, PM planned “feasibility studies...in 1992 to determine what technology PM US R&D needs to develop or license for the manufacture of [tobacco flavor] aerosol type devices...[and to] continue aerosol research with direction towards novel methods of generation and flavor and impact enhancement.”17
Project Ideal Smoke
In 1993, PM created the Ideal Smoke programme to (1) develop satisfying products (in terms of sensory and nicotine delivery aspects) that addressed consumers’ concerns about the health effects of cigarettes, and (2) to design products that would be accepted (or even endorsed) by the medical community as less harmful than traditional cigarettes without being considered therapeutic drug delivery devices subject to regulation by the Food and Drug Administration (FDA).20,21
According to a 1993 report authored by Howell (senior research scientist in aerosol physics in PM's Product Research Division) that describes a project entitled Aerosol Physics led by Lipowicz (senior research scientist at PM),22 PM was conducting aerosol physics research to:
Formulate a flavor system for a water aerosol...Dr. Frank Gullotta [a scientist at PM's INBIFO [(Institut für Biologische Forschung) in Germany] has agreed to compare the physiological response of nicotine contained in an aqueous aerosol to the response obtained from selected cigarettes...the information he obtains will give us a ball park concentration for nicotine in the aerosol that equates to a specific cigarette delivery.22
According to the report, Institut für Biologische Forschung (INBIFO) was testing a nicotine solution using a DeVilbiss chamber, which is similar to a humidifier. The report reveals two other consultants on the project:
Contact has been made with George Patskan [senior associate scientist at PM] and we are presently reviewing material on inhalation therapy which may give precedence for some ingredients that can be used to flavor the aqueous aerosol. Patent and secrecy agreements are also being updated for Peter Byron [a Virginia Commonwealth University professor who did research for PM] at MCV [Medical College of Virginia] so that we can begin a dialog with him on non-toxic inhalable flavorants and modifiers.22
PM develops the CAG
In 1994, Howell developed the CAG, which advanced the Ideal Smoke programme23 (figure 1). It generated aerosol by pumping liquid into a small heated tube (capillary). PM scientists described this device as ‘correlating the sensory response of nicotine as delivered in a cigarette to the sensory response of nicotine delivered in an aqueous aerosol’.24 PM also recognised it as a way to deliver aerosolised pharmaceuticals.25
An R&D report entitled ‘Internal Report 1994 Annual Report for the Leap Activity’, authored by Cox (PM principal research scientist) and Whidby25 referred to the CAG as Project Leap (project number 1A04). The objective of Project Leap, which was a component of the Ideal Smoke programme, was to ‘develop a smoking article which satisfies the mission of the Ideal Smoke programme and delivers a minimal set of well-defined compounds’ and develop ‘the technical means for generating a fine aerosol from a liquid’.25 In 1994, Leap was:
Define[ing] the regulatory requirements for both product introduction and project development in several key countries... Develop[ing] safe, acceptable procedures for inhalation testing of aerosols...Monitor[ing] and summarize[ing] the competitive situation with respect to the introduction of similar products...[and] Develop[ing] and evaluate[ing] an optimized ultrasonic aerosol generator.25
A 1995 internal PM report, ‘Leap Research Capillary Aerosol Generator: State of Development’, describes how the CAG and its components were developed.26 In ‘a search for a mental model for how a cigarette might produce an aerosol during the smoking process’, PM scientists filled a syringe with peanut oil and heated the needle of the syringe. “Highly visible aerosol was produced when the oil was expelled from the syringe body through the hot needle. Canola oil, propylene glycol and glycerin all behaved in a similar manner while water and ethanol vaporized and disappeared into the air.”26
The 1995 CAG device heated liquid that flowed through a ‘bundle of capillary tubes which act as the tobacco matrix’, forming an aerosol ‘when the hot vapourised fluid exits the capillary tube and condenses to small liquid droplets’.26 The aerosol could be produced in a continuous or pulsed mode (in short bursts). Heating wire was wrapped around a small tube to evenly heat the liquid in the tube; the wire-wrapped tube was placed in an “outer quartz or ceramic [tubing that] is required for insulating the heater and as a support for the capillary.”
In May 1996, Howell and Sweeney27 (a principal engineer for PM/Altria Client Services) submitted a patent application for the CAG entitled ‘Aerosol generator and a method and apparatus for generating an aerosol’. The application described the CAG as a drug delivery device composed of a liquid-filled wire-wrapped tube heated via the wire (figure 1). The heat source was preferably a resistance heater powered by a battery. The inventors recommended using a puff sensor attached to the mouthpiece to regulate aerosol delivery. The patent noted that:
In drug delivery applications, it is typically desirable to provide an aerosol having average mass median particle diameters of less than 2 microns to facilitate deep lung penetration...It has been observed that liquid materials such as propylene glycol and glycerol can be formed into aerosols having mass median particle diameters and temperatures in the preferred ranges.27
Obstacles to development of a nicotine aerosol device
Although PM hoped a nicotine aerosol device would evade FDA regulation, PM also expressed reluctance about marketing such a device before FDA established its role in tobacco product regulation. According to the ‘Internal Report 1994 Annual Report for the Leap Activity’:
The FDA initiated and currently continues in its attempt to define a possibly larger role in this [tobacco industry] regulation. Given the long development period required for a Leap product and the uncertainty in the changes to relevant regulations which might occur in the interim, it was concluded that a detailed study of worldwide regulations be delayed until a later date. No further action was taken beyond the identification of potential consultants.25
The report stated that “Inhalation studies have yet to be carried out. The research has not advanced beyond the identification and characterization of acceptable means for aerosol generation. Much additional work remains before inhalation studies which comply with internal procedures can be given approval.”25A draft speech on Project Leap in Cox's files proposed:
The development of a smoking device based upon the capillary generator...We are not, now pursuing this strategy for two reasons. First and foremost, it [Project Leap] complicates our [PM's] efforts to resist the FDA's attempts to regulate the tobacco industry, and, second, such a product may erode the support of tobacco farmers...Let's suppose that 3-5 years from [now] we have the following situation...The FDA has clarified its regulatory role with respect to the tobacco industry...We would be well along our way if at this time, the CAG had already been commercialized and accepted as a medical inhalation device in the pharmaceutical industry.28
A 1998 draft report (no author) in Cox's files entitled ‘Message Points Aerosol Patent’ stated that “Philip Morris U.S. A. scientists have patented a new device for generating highly concentrated fine aerosols from liquids.”29 However, “Philip Morris determined it was not in our business interests to continue to pursue research on the device, however, we recognized the potential advantages this invention could have to the pharmaceutical and medical community.”29
PM applies aerosol technology to a pharmaceutical inhaler (Aria)
In 1998, while placing development of a nicotine aerosol device on hold, PM was ‘supporting research at Virginia Commonwealth University to further the development and evaluation of the potential of the aerosol generator for the therapeutic treatment of asthma’.29,30 In 2000, Altria Group (PM's new name)31 created a pharmaceutical subsidiary, Chrysalis Technologies,32 to diversify the PM USA product portfolio, generate income and “create...products that have the potential to reduce the harm caused by cigarette smoking.”33
In 2000, the CAG laboratories, documents and employees moved from PM offices to Chrysalis34 and Chrysalis submitted at least two patent applications for the aerosol generator.35,36
After 2000, the number of available documents on application of aerosol research to a smoking device declined. A 2005 PowerPoint presentation from Altria Federal Tobacco Team Planning Meeting37 reported that Chrysalis had used its CAG to create Aria, an inhaler that was a ‘functional prototype currently configured as pharmaceutical drug delivery device’. However, PM encountered barriers to promoting Aria as a drug delivery device. For example, the American Thoracic Society denied PM efforts to promote Aria at a medical conference because of its ties with the tobacco company.38 In 2008, PM sold the Aria technology to Discovery Laboratories39 (which became Windtree Therapeutics in 2016)40 and paid Discovery Laboratories $4.5 million to continue developing Aria under an agreement that allowed PM to profit from future sales.
PM resumes application of aerosol technology to nicotine delivery
In 2001, the year after the US Supreme Court ruled that FDA did not have authority to regulate tobacco products41 and several years after PM shifted the focus of its CAG to pharmaceutical applications,25,28,29 interest in developing a nicotine aerosol device re-emerged. A PowerPoint presentation from a 2001 PM Research, Development, and Engineering Strategic Planning Meeting stated that PM's goals for aerosol generation were to ‘find methods to deliver nicotine without burning it’ and ‘develop a portfolio of flavoured aerosol products which deliver, for example, nicotine, caffeine, antioxidants and vita-mins’.42 PM wanted to ‘develop an acceptable aerosol generator smoking product’ while establishing its ‘harm reduction’ implications and determining the likelihood of ‘consumer acceptance of an aerosol generator’.42
Ruyan electronic cigarette developed in China
In 2003, Beijing SBT Ruyan Technology & Development Co. released an electronic cigarette, Ruyan,43 which reached the USA and Europe by 2007.44 In 2004, PM created a new product focus team45 to investigate the device,43 which was patented in 2003 in China by pharmacist Hon Lik.4,43,46 According to the Ruyan patent, it pumps a nicotine solution into a cavity where ultrasonic waves (generated by a piezoelectric element) heat and vapourise the liquid.4
According to a 1994 presentation by Lipowicz47 entitled ‘Aerosol Generation Using Ultrasonic Waves Generated by Piezoelectric Transducers’, in the early 1990s, PM had investigated using a piezoelectric transducer to create an aerosol generator. However, according to an unauthored 1990s document obtained from Cox's files entitled ‘Leap (1A04)’, PM abandoned its efforts to develop an aerosol generator based on the piezoelectric transducer after Howell invented the CAG in 1994.28
PM nicotine aerosol technology advances
In 2004, nicotine aerosol technology continued to advance at PM, but with a shift in terminology. A 2004 presentation to PM's Product Assessment Scientific Advisory Board by PM's Director of Product Assessment on future plans of PM's Health Sciences Research described three projects,48 including PM's nicotine aerosol research under the name ‘vision 2 non-tobacco’ (V2N).48,49 (The other projects were V1, a ‘reduced harm, great tasting cigarette’; and V2T [tobacco], a “minimal or de minimus harm” product “comparable to cigarette smoking: enjoyable [consumable].”) The goal of V2N was to create a “de minimus harm [product] comparable to cigarette smoking [that did] not utilize tobacco and would be a stand-alone product category.” V2N was only one of three projects that resembled the modern e-cigarette; therefore, we searched for additional documents that used this terminology. A 2005 PM Research and Technology Project report50 indicated that PM, through Project 1650 (TARP), was using CAG technology to pursue V2N by developing ‘flavour systems to replace toxic components associated with smoke and tobacco which will be incorporated into a developed aerosol delivery system’.50 TARP's product would provide a sensory experience similar to smoking, reduce harmful chemical constituents to address consumers’ health concerns and make consumers feel comfortable using the product around non-smokers. TARP used the CAG to:
Create flavor formulations from food approved FEMA GRAS [Generally Recognized as Safe by the Flavor & Extract Manufacturers Association for ingestion, not inhalation51] ingredients that simulate the aromatics of cigarette and cigar smoke to create as safe smoke-like aerosol from food approved ingredients.50
In a 2005 email entitled ‘RE: ETD Project Check-in’, Werley52 (PM associate principal scientist conducting aerosol research) and a staff engineer at PM discussed plans to conduct animal testing for a nicotine aerosol device since “we know that no one has been exposed to these [tobacco] extracts before.” The email stated:
We are doing...[a]cute inhalation study in the rat—to assess acute toxic effects from single high doses...[a] 14-day repeated inhalation study—to determine the No-observed-effect level, and repeated exposure effects (local to the lung and systemic)...[and a] Single dose tolerance/ dose escalation study in man. All of this will take about 6 months...we can then conduct 2-week sensory panel studies in man at doses to be determined based on the results from the animal studies.52
The list also included characterising the biochemical makeup of the aerosol, Ames tests to determine mutagenic potential and cytotoxicity testing to assess the irritant potential of the ‘smoke’.52 An attachment to an email53 sent from Werley to the Manager of External Studies at PM, titled ‘Test Request for Aerosol Device and Formulation’,54 reveals the contents of the ‘test material—tobacco extract in PG/glycerol and 3–4% nicotine’.
In January 2006, a senior principal scientist at PM's Clinical Evaluation department discussed the need for further clinical study of aerosol technology at a meeting entitled ‘Aerosol Project Single Dose/Dose Escalation Safety & Tolerance Study’.55 He outlined the results of animal exposure studies to the “Tobacco-based aerosol formulation [with] -Intended use: like a cigarette [that] -Currently exists as a bench-top generator [that uses a] Formulation [that] contains -Tobacco extract -Humectants -Water [and] –Solvent.” Ten rats were exposed to a 1 mg/L (1 mg tobacco extract liquid per litre of air) solution, and 10 were exposed to 2 mg/L (nicotine concentration not specified). The only results presented were a lack of DNA mutations in the rats, although 1 of the 10 rats exposed to the 2 mg/L solution via nose-only inhalation died during the 2-week period (cause of death undisclosed). The presentation included future plans to “Assess the acute safety and tolerance of the TE-[tobacco extract] aerosol in human subjects before testing in a [human] sensory panel [emphasis in original]...36 healthy adult smokers...[at]...MDS [laboratories] Lincoln, Nebraska... [in] March/April 2006 [with] Study costs: $400 K (estimated).” We did not find any documentation or results of human testing of a tobacco extract aerosol.
One month later, in February 2006, an email exchange between the Illinois Institute of Technology Research Institute (IITRI), which regularly performed inhalation toxicology research for PM, and a PM scientist discussed plans to repeat the 14-day inhalation toxicology study at IITRI on rats using a tobacco extract aerosol nicotine solution of 4% nicotine and 96% solvent (40% glycerin, 30% propylene glycol, 20% ethanol and 10% water). The studies were completed by May 2006,56 but we were unable to locate the results or any other mention of a nicotine aerosol device in the documents after 2006.
Altria patents an electronic cigarette
In 2007, PM filed an international patent application for an indirectly heated CAG57 designed to remedy the overheating and clogging of capillary tubes encountered in directly heated CAG technology.58 It was not clear whether this device was intended to be a nicotine aerosol device or to deliver pharmaceuticals.
In 2009, PM filed a patent application for an ‘electrically heated smoking system having a liquid storage portion’.59 The device's:
Shell includes an electric power supply and electric circuitry. The [replaceable] mouthpiece includes a liquid storage portion and capillary wick...the first end [of the wick] extending into the liquid storage portion for contact with liquid therein...[and] at least one heating element for heating the second end of the capillary wick, an air outlet, and an aerosol forming chamber between the second end of the capillary wick and the air outlet...The supersaturated vapor [created] is mixed and carried in the air flow from the...air inlet to the aerosol forming chamber... [where] the vapor condenses to form an aerosol...The liquid preferably comprises a tobacco-containing material comprising volatile tobacco flavor compounds which are released from the liquid upon heating. Alternatively, or in addition, the liquid may comprise a non-tobacco material. The liquid may include water, solvents, ethanol, plant extracts and natural or artificial flavors. Preferably, the liquid further comprises an aerosol former. Examples of suitable aerosol formers are glycerine and propylene glycol...Preferably, the electrically heated smoking system is portable...may have a size comparable to a conventional cigar or cigarette.59
Altria releases the MarkTen e-cigarette
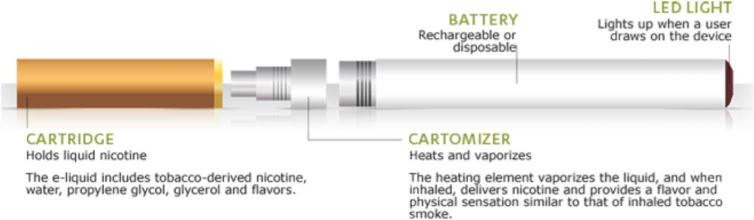
In 2013, Nu Mark, a division of Altria,60 released the MarkTen e-cigarette, a ciga-like e-cigarette, in test markets in Indiana and Arizona.61 The 2013 Altria shareholder's report states that Altria formed the NuMark company to deliver ‘new, innovative products’, such as ‘electronic cigarettes’, or ‘e-vapour products to meet the evolving preferences of adult tobacco consumers’.61 In April 2016, the NuMark website described the second MarkTen model, MarkTen XL e-vapour, a rechargeable product ‘to give adult vapers a more consistent experience, puff-to-puff and day-to-day’.60 All MarkTen XL models (Classic, Fusion and Menthol) contain tobacco-derived nicotine, glycerol, propylene glycol and flavours. The website describes ‘e-vapour products’ as ‘a battery-powered device that contains a heating element and a cartridge filled with a liquid solution’. The diagram included on the website (figure 2) states that e-cigarettes like MarkTen include a cartridge that ‘holds liquid nicotine’, a cartomiser, ‘the heating element [that] vaporizes the liquid, and when inhaled, delivers nicotine and provides a flavor and physical sensation similar to that of inhaled tobacco smoke’.
Figure 2.
Image of MarkTen e-cigarette from the NuMark website.62
DISCUSSION
Since at least 1990, PM and its affiliates have been pursuing a device that was visually and experientially similar to smoking and would deliver a nicotine aerosol.14 We found strong parallels between descriptions of Howell's CAG in Project Leap documents,26 the CAG patent application27 and PM's 2009 electronic cigarette patent59 (table 1). Both the CAG and e-cigarette consist of an inner tube that contains a liquid and a protective outer tube. A heater heats the inner tube through wire wound around the tube. The heater heats the liquid to the point of vapourisation and it exits the device as an aerosol through a mouthpiece. A puff sensor is used to regulate the release of the aerosol.
Table 1.
Device specification for PM capillary aerosol generator and PM's 2009 electrically heated smoking system having a liquid storage portion
| CAG | Electrically heated smoking system |
|---|---|
| Project Leap CAG (1995)26 | PM 2009 patent59 |
| “The aerosol is formed when the hot vaporized fluid exists the capillary tube.” | “In the aerosol forming chamber, the vapor condenses to form an aerosol, which is carried towards the air outlet.” |
| Pump forces liquid into small tube (capillary) | “liquid is transferred from the liquid storage towards the heating element by capillary action of the wick.” |
| Small tube, or capillary is heated | “a heating element for heating the second end of the capillary wick.” |
| Capillary contains liquid | “The mouthpiece includes a liquid storage portion.” |
| Liquid in tube is aerosolised | “Liquid at the second end of the capillary wick is vaporized by the heating element.” |
| Heating wire wrapped around a small tube to evenly heat liquid in tube | “at least one heating element comprises a coil of wire surrounding the capillary wick.” |
| “Nichrome heating wire...was found to be useful for supplying heat to the capillary tube evenly.” | “Other alternatives include a heating wire or filament, for examine a Ni—Cr, platinum, tungsten or alloy wire.” |
| “Two power sources may be needed to operate the aerosol generator in a pulse mode if the unit is to be puff actuated.” | “The electric circuitry is arranged to provide an electric current pulse to the at least one heating element when the user initiates a puff.” |
| “Mouthpiece was placed on one end of the outer tube.” | “An electrically heated smoking system includes a shell and a replaceable mouthpiece.” |
| Aerosol and method and apparatus for generating aerosol patent (1996)27 | PM 2009 patent (contd)59 |
| Ideal electric source for heater was a battery. | “The battery supplies a current pulse...to the heating coil.” |
| “For portable applications. replaceable, rechargeable battery, such as. nickel-cadmium battery pack.” | “An example of suitable circuitry is one or more capacitors or rechargeable batteries.” |
| “puff-actuated sensor” | “Preferably, the electric circuitry comprises a sensor to detect air flow indicative of a user taking a puff.” |
| “propylene glycol and glycerol. preferred” | “Examples of suitable aerosol formers are glycerine and propylene glycol.” |
CAG, capillary aerosol generator; PM, Philip Morris.
Important similarities exist between the description of the design and intentions of nicotine aerosol devices described in PM documents and product information for the MarkTen e-cigarette on the NuMark website60,62 (table 2). Both devices contain liquid tobacco-derived nicotine that is aerosolised, use the solvents propylene glycol and/or glycerin, do not use combustion, do not emit smoke, and are designed to mimic the sensation of smoking.
Table 2.
Intentions for products between PM's tobacco aerosol research in the 1990s and 2000s and the MarkTen e-cigarette, produced by NuMark (a subsidiary of Altria)
| Early versions of PM's nicotine aerosol device | MarkTen |
|---|---|
| Truth Tobacco Industry Documents | Nu Mark website (20 1 6)60,62 |
| Ingredients: “tobacco extract aerosol nicotine solution formulation”63 | Ingredients: “tobacco-derived nicotine” |
| One goal of Ideal Smoke was to deliver nicotine without combustion.64 | E-vapour products like MarkTen “do not burn tobacco” |
| Tobacco Aerosol Research Project designed to “replace toxic components associated with smoke and tobacco”50 | E-vapour products like MarkTen “do not generate or emit smoke” |
| “Highly visible aerosol was produced”22 from CAG | E-vapour product “users inhale and exhale a vapor” |
| CAG designed for “nicotine delivered in an aqueous aerosol”65 | E-cigarettes like MarkTen have a cartridge that holds “liquid nicotine” |
| One goal of Ideal Smoke was to produce a ‘heat generated aerosol’64 | E-cigarettes like MarkTen have a cartomiser, “the heating element [that] vaporizes the liquid” |
| CAG was a method of “correlating the sensory response of nicotine as delivered in a cigarette to the sensory response of nicotine delivered in an aqueous aerosol.”65 | E-vapour products like MarkTen provide a “physical sensation similar to that of inhaled tobacco smoke.” |
| Vision 2 non-tobacco products produce a ‘smoke-like aerosol’ that “simulate[s] the aromatics of cigarette and cigar smoke.”50 | E-vapour devices like MarkTen “delivers nicotine and provides a flavor and physical sensation similar to that of inhaled tobacco smoke.” |
| Ideal Smoke product “satisfies the smoking ritual.”21 | |
| 1996: PM wanted to develop a device that provided “tobacco flavor and impact delivered in aerosol form.”17 | MarkTen e-cigarette “doesn't have any burned tobacco...Part of the solution that it delivers and aerosolizes is nicotine.”66 |
| PM's goals for aerosol generation in 2001: “find methods to deliver nicotine without burning it”42 |
CAG, capillary aerosol generator; PM, Philip Morris.
Political and legal concerns appear to have played an important role in PM's decisions to conduct (and suspend) research on nicotine aerosol devices. In the 1990s, concern arose around avoiding FDA regulation25,28,29 and the potential impact of a nicotine aerosol device on PM's relationship with tobacco farmers28 who had historically allied with tobacco companies to oppose tobacco control policies.67 The development of what would become e-cigarette technology was also motivated by an attempt to evade smoke-free laws,68,69 an important reason that smokers use e-cigarettes.70–73
Limitations
It is unclear what prevented other large tobacco companies from producing electronic cigarettes earlier. Although the documents provide information about earlier products produced by tobacco companies other than PM, such as RJR’ Eclipse heated cigarette,12 the documents provide little insight on these companies’ e-cigarettes. We were unable to locate any documents discussing technology related to RJR's Vuse e-cigarette, released in Colorado in July 201374 and nationwide in June 2014;75 blu e-cigarettes, purchased by Lorillard in 2012;76 or BAT's Vype e-cigarette released in 2013 and refillable Vype eTank released in July 2015.77
Documents research has a number of limitations, including occasional missing information on document authors and dates, and the fact that the documents are not complete archives of company activities. Another limitation is the lack of documents from 2006 to 2012 that included our search terms. Since e-cigarette litigation is more recent than much of cigarette litigation, documents have not yet been released about e-cigarettes as a result of litigation.
CONCLUSION
The technology for the modern e-cigarette did not originate solely in China in 2003; the idea that Hon Lik was the first person to develop a device that aerosolised a nicotine solution is an oversimplification of the history of the e-cigarette. PM began exploring nicotine aerosol generation technology in 1990 to create a product that would serve as an alternative to NRT therapy for health-concerned smokers and deal with smoke-free environments. PM did not market an e-cigarette until 2013 (the MarkTen) because of concern about triggering FDA regulation of conventional cigarettes and the potential impact of the product on their political relationship with farmers. As PM anticipated, many e-cigarette users report using e-cigarettes to try to quit smoking (but smokers who use e-cigarettes are significantly less likely to quit smoking cigarettes than smokers who do not use e-cigarettes)78 and in place of conventional cigarettes in smoke-free environments.70–73 PM developed e-cigarettes to provide a product for health-concerned smokers and smokers facing increased restrictions on smoking in public. Researchers and policymakers should recognise that PM developed e-cigarette technology to evade tobacco control regulations.
What this paper adds
▶ Hon Lik, a Chinese pharmacist, is often cited as, in 2003, inventing the modern e-cigarette, which some see as a disruptive technology to compete with conventional cigarette companies.
▶ Philip Morris has researched nicotine aerosol technology similar to the modern e-cigarette since 1990.
▶ Philip Morris developed nicotine aerosol technology to attempt to keep health conscious smokers from using nicotine replacement while circumventing regulation and restrictions on cigarettes.
▶ In the 1990s, concerns about triggering Food and Drug Administration regulation of cigarettes led Philip Morris to shift its focus towards pharmaceutical applications of aerosol technology.
▶ Rather than a disruptive technology, Philip Morris developed e-cigarette technology to complement, not compete with, conventional cigarettes and evade tobacco control regulations.
Acknowledgments
Funding This work was supported by National Cancer Institute Grants CA-087472, CA-113710 and 1P50CA180890 from the National Cancer Institute and FDA Center for Tobacco Products.
Footnotes
To cite: Dutra LM, Grana R, Glantz SA. Tob Control Published Online First: [please include Day Month Year] doi:10.1136/tobaccocontrol-2016-053406
Contributors LMD and RG did the documents searches and drafted the manuscript. All three authors (LMD, RG, SAG) contributed to editing the manuscript and revising the manuscript in response to the reviewers’ and editors’ feedback.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US FDA.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement All source materials are available in the UCSF Truth Tobacco Industry Documents Library, available at http://industrydocuments.library.ucsf.edu/tobacco or on other publicly available websites.
REFERENCES
- 1.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–86. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boseley S. Hon Lik Invented the E-Cigarette to Quit Smoking—but Now He's a Dual User. The Guardian. 2015 Jun 9; [Google Scholar]
- 3.Lik H. [24 Jul 2015];Wikipedia. 2015 https://en.wikipedia.org/wiki/Hon_Lik.
- 4.Lik H. Electronic Atomization Cigarette. United States: [26 Apr 2016]. Google Patents, assignee. http://www.google.com/patents/US20070267031. [Google Scholar]
- 5.Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311:135–6. doi: 10.1001/jama.2013.285347. [DOI] [PubMed] [Google Scholar]
- 6.Fagerstrom K, Etter JF, Unger JB. E-cigarettes: a disruptive technology that revolutionizes our field? Nicotine Tob Res. 2015;17:125–6. doi: 10.1093/ntr/ntu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stimson GV, Thom B, Costall P. Disruptive innovations: the rise of the electronic cigarette. Int J Drug Policy. 2014;25:653–5. doi: 10.1016/j.drugpo.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Glantz SA. The cigarette papers. University of California Press; Berkeley, CA: 1996. [Google Scholar]
- 9.Ling PM, Glantz SA. Tobacco industry consumer research on socially acceptable cigarettes. Tob Control. 2005;14:e3. doi: 10.1136/tc.2005.011239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draft: “Safer” Cigarette. [12 Apr 2016];Tobacco Products Liability Project. https://industrydocuments.library.ucsf.edu/tobacco/docs/xskn0050.
- 11.Fishel D, Reynolds RJ. Announced in September 1987 (870900) that the company was developing a new cigarette that heats rather than burns tobacco. [27 Feb 2014];RJ Reynolds. 1988 http://legacy.library.ucsf.edu/tid/mln44d00.
- 12.Eclipse Cigarette. RJ Reynolds; Feb 11, 2004. [12 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/xrmv0190. [Google Scholar]
- 13.Malone RE, Balbach ED. Tobacco industry documents: treasure trove or quagmire? Tob Control. 2000;9:334–8. doi: 10.1136/tc.9.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Philip Morris. Raymond W. Philip Morris; Nov 15, 1990. [9 August 2016]. Invention record preliminary disclosure for consideration of patentability. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/tgcc0114. [Google Scholar]
- 15.Philip Morris [3 Aug 2016];1992 https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/mjhm0130.
- 16.Philip Morris . Philip Morris USA 92–96 operations. Philip Morris; Jul 27, 1992. [3 Aug 2016]. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/llch0116. [Google Scholar]
- 17.Philip Morris . 1992–1996 Philip Morris USA R&D strategic plan. Philip Morris; 1992. [29 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/jyyx0128. [Google Scholar]
- 18. [8 Aug 2013]; Unknown. Appendix J Smoking Cessation- Nicotine Delivery Devices. Unknown. Philip Morris. https://industrydocuments.library.ucsf.edu/tobacco/docs/hkhw0064.
- 19.Nicotine use after the year 2000. Lancet. 1991;337:1191–2. [PubMed] [Google Scholar]
- 20.1993 R&D initiatives. Philip Morris; 1993. [11 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/mjhw0110. [Google Scholar]
- 21.Problem how do we get to Ideal Smoke? Philip Morris; 1993. [21 Mar 2014]. http://legacy.library.ucsf.edu/tid/ciu18d00. [Google Scholar]
- 22.Howell T, Lipowicz P. Aerosol physics. Philip Morris; Jun, 1993. [9 Aug 2016]. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/gzvw0092. [Google Scholar]
- 23.Howell A, Philip Morris . Invention record (preliminary disclosure for consideration of patentability) Philip Morris; Jan 14, 1994. [13 Oct 2013]. http://legacy.library.ucsf.edu/tid/yel58e00. [Google Scholar]
- 24.Philip Morris. Howell T. Aerosol generator—system procedure. Philip Morris; Aug 16, 1993. [14 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/jtxc0083. [Google Scholar]
- 25.Philip Morris. Cox K, Whidby J. Internal report 1994 annual report for the Leap Activity (1a04) Philip Morris; Mar 15, 1995. [12 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/stvw0025. [Google Scholar]
- 26.Leap research capillary aerosol generator: state of development. Philip Morris; Feb 25, 1995. [25 Apr 2014]. http://legacy.library.ucsf.edu/tid/qwi06c00. [Google Scholar]
- 27.Howell T, Sweeney W. Philip Morris USA Inc, assignee. Aerosol and Method and Apparatus for Generating an Aerosol. USA: May 15, 1996. [Google Scholar]
- 28.Leap (1a04) Philip Morris; 1998. [25 Apr 2014]. http://legacy.library.ucsf.edu/tid/tkw89h00. [Google Scholar]
- 29.Message points aerosol patent. Philip Morris; 1998. [4 Aug 2016]. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/pynd0064. [Google Scholar]
- 30.Capillary aerosol generator. Philip Morris; May 11, 1998. [4 August 2016]. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/mynd0064. [Google Scholar]
- 31.Smith EA, Malone RE. Altria means tobacco: Philip Morris's identity crisis. Am J Public Health. 2003;93:553–6. doi: 10.2105/ajph.93.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BioPortfolio. Chrysalis Technologies Company Profile; 2016. [6 Apr 2016]. http://www.bioportfolio.com/corporate/company/23110/Chrysalis-Technologies.html. [Google Scholar]
- 33.Chrysalis Role—062705.Doc. Philip Morris; Jun 28, 2005. [6 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/kynv0221. [Google Scholar]
- 34.Reale A, Philip Morris Management Corp . Chrysalis Technologies—monthly report. Philip Morris; Jun 14, 2000. [25 Oct 2013]. https://industrydocuments.library.ucsf.edu/tobacco/docs/#id=pkbv0071. [Google Scholar]
- 35.Cox K, Beane T, Sweeney W, Chrysalis Technologies Inc . Aerosol generator and methods of making and using an aerosol generator. Philip Morris; Mar 29, 2012. [8 Aug 2016]. US Patent, https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/gmyn0189. [Google Scholar]
- 36.Sprinkel F, Nichols W, Cox K, et al. Chrysalis Technologies Inc . Aerosol generator having heater in multilayered composite and method of use thereof. Philip Morris; Mar 29, 2012. [8 Aug 2016]. US Patent, https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/jmyn0189. [Google Scholar]
- 37.Altria Corporate Service Inc, PMUSA . New product and technology developments. Philip Morris; Jan 12, 2005. [8 Aug 2016]. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/fpyw0219. [Google Scholar]
- 38.O'Connell V. Rx from Marlboro Man: device that delivers drugs, not smoke. [6 Apr 2016];Wall Street J. 2005 http://www.wsj.com/articles/SB113037865225180846.
- 39.Warrington Discovery Labs and Chrysalis Technologies Modify Collaboration for Future Development of Aerosolized Drug Device Products. [2 Jul 2014];Health IT Outcomes. 2008 http://www.healthitoutcomes.com/doc/discovery-labs-and-chrysalis-technologies-mod-0001.
- 40.PR Newswire [4 Aug 2016];Discovery Labs Changes Name to Windtree Therapeutics, Inc. (Nasdaq: Wint) 2016 http://www.prnewswire.com/news-releases/discovery-labs-changes-name-to-windtree-therapeutics-inc-nasdaq-wint-300252562.html.
- 41.Food and Drug Administration. Brown V, Williamson Tobacco Corp et al. Certiorari to the United States Court of Appeals for the Fourth Circuit. US Supreme Court; 2000. pp. 1–75. No. 98–1152. [Google Scholar]
- 42.Philip Morris . Philip Morris USA & Philip Morris International Rd&E Strategic Planning Meeting 20010605–20010608 Chantilly, Va. Philip Morris; Jun 8, 2001. [6 Oct 2015]. https://industrydocuments.library.ucsf.edu/tobacco/docs/yyjf0218. [Google Scholar]
- 43. [23 Feb 2009];Ruyan Asserts Patent Rights to E-Cigarette in Key China Court Ruling. 2009 http://www.prnewswire.com/news-releases/ruyan-asserts-patent-rights-to-e-cigarette-inkey-china-court-ruling-65760637.html.
- 44.LaMotte S. [4 Aug 2016];Where We Stand Now: E-Cigarettes. 2016 http://www.cnn.com/2015/12/31/health/where-we-stand-now-e-cigarettes/
- 45.Becker U, Callicutt C, Jaccard G, et al. New product focus team test plan & status report for Beijing Saybolt Ruyan Technologies Product. Philip Morris; Jun 22, 2004. [7 Apr 2016]. https://idl.ucsf.edu/tobacco/docs/zzcx0150. [Google Scholar]
- 46.Lik H. Noncombustible Electronic Atomized Cigarette. China: [26 Apr 2016]. https://patents.google.com/patent/CN1530041A/en?q=atomizing&q=electronic+cigarette&q=2003. [Google Scholar]
- 47.Lipowicz P. Aerosol generation using ultrasonic waves generated by piezoelectric transducers. Philip Morris; 1994. [4 May 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/pgbw0092. [Google Scholar]
- 48.Sab Mtg Oct 2004 Draft Patskan.Ppt. Philip Morris; Oct 18, 2004. [7 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/kslh0218. [Google Scholar]
- 49.Vision 2n years 7–10 product ideas. Philip Morris; Jun 16, 2004. [5 May 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/tkxc0219. [Google Scholar]
- 50.Research & technology project sheet. Philip Morris; Jan 20, 2005. [16 Dec 2013]. http://legacy.library.ucsf.edu/tid/pkk92g00. [Google Scholar]
- 51.Flavor & Extract Manufacturers Association [22 Apr 2016];Safety Assessment and Regulatory Authority to Use Flavors: Focus on E-Cigarettes. 2013 http://www.femaflavor.org/safety-assessment-and-regulatory-authority-use-flavors-focus-e-cigarettes.
- 52.Werley M. Fw: Etd Project Check-In. Philip Morris; Aug 29, 2005. [18 Mar 2015]. http://legacy.library.ucsf.edu/tid/mlm29j00. [Google Scholar]
- 53.Werley M. Philip Morris; Aug 29, 2005. [19 Aug 2016]. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/jspk0150. [Google Scholar]
- 54.Philip Morris; Jan 25, 2005. [10 Aug 2016]. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/ztxd0219. [Google Scholar]
- 55.Mendes P. Philip Morris; Jan 9, 2006. [6 Oct 2015]. No Title, https://industrydocuments.library.ucsf.edu/tobacco/docs/hmny0218. [Google Scholar]
- 56.Jerome AM. Re: report—Re: draft report—14 day inhalation toxicity study of extract formulation in rats. Philip Morris; May 25, 2006. [7 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/llhh0219. [Google Scholar]
- 57.Belcastro M, Swepston J, Philip Morris Products S.A . Indirectly Heated Capillary Aerosol Generator. World Intellectual Property Organization; [26 Apr 2016]. assignee. https://patents.google.com/patent/WO2007141668A2/en?q=e&q=cigarette. [Google Scholar]
- 58.Belcastro M. Re: novel Ag project. Philip Morris; Nov 17, 2004. [8 Apr 2016]. https://idl.ucsf.edu/tobacco/docs/gtxl0151. [Google Scholar]
- 59.Thorens M, Flick JM, Cochand OY. assignee. Electrically Heated Smoking System Having a Liquid Storage Portion. 2009 Google Patents.
- 60.NuMark L. [25 Apr 2016];Markten E-Vapor. 2016 http://www.nu-mark.com/our-products/mark-ten/Pages/default.aspx.
- 61.Altria Group, Inc. [11 Apr 2016];2013 Annual report. Philip Morris. 2013 https://industrydocuments.library.ucsf.edu/tobacco/docs/xlhy0219.
- 62.NuMark L. [11 Apr 2016];Product overview. 2016 http://www.nu-mark.com/our-products/products-overview/Pages/default.aspx.
- 63.Hu SC. Re: upcoming 14-day inhalation toxicity study. Philip Morris; Feb 1, 2006. [7 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/zjwl0219. [Google Scholar]
- 64.Mission of the Ideal Smoke Program. Philip Morris; 1998. [6 Oct 2015]. https://industrydocuments.library.ucsf.edu/tobacco/docs/xrwg0175. [Google Scholar]
- 65.Howell T. Philip Morris; 1993. [13 May 2014]. No Title, http://legacy.library.ucsf.edu/tid/qdl58e00. [Google Scholar]
- 66.United Reporting Inc. Videotaped deposition of Richard Jupe. Philip Morris; Apr 10, 2014. [11 Apr 2016]. https://industrydocuments.library.ucsf.edu/tobacco/docs/zykx0217. [Google Scholar]
- 67.Fallin A, Glantz SA. Tobacco-control policies in tobacco-growing states: where tobacco was king. Milbank Q. 2015;93:319–58. doi: 10.1111/1468-0009.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lempert LK, Grana R, Glantz SA. The importance of product definitions in US e-cigarette laws and regulations. Tob Control. 2016;25:e44–51. doi: 10.1136/tobaccocontrol-2014-051913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cox E, Barry RA, Glantz S. E-cigarette policymaking by local and state governments: 2009–2014. Milbank Q. 2016;94:520–96. doi: 10.1111/1468-0009.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pepper JK, Ribisl KM, Emery SL, et al. Reasons for starting and stopping electronic cigarette use. Int J Environ Res Public Health. 2014;11:10345–61. doi: 10.3390/ijerph111010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt L, Reidmohr A, Harwell TS, et al. Prevalence and reasons for initiating use of electronic cigarettes among adults in Montana, 2013. Prev Chronic Dis. 2014;11:E204. doi: 10.5888/pcd11.140283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biener L, Song E, Sutfin EL, et al. Electronic cigarette trial and use among young adults: reasons for trial and cessation of vaping. Int J Environ Res Public Health. 2015;12:16019–26. doi: 10.3390/ijerph121215039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baggett TP, Campbell EG, Chang Y, et al. Other tobacco product and electronic cigarette use among homeless cigarette smokers. Addict Behav. 2016;60:124–30. doi: 10.1016/j.addbeh.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mangan D. Reynolds American sees e-cigarette launch as a “game changer”. [5 May 2016];CNBC. 2013 http://www.cnbc.com/id/100796458.
- 75.Reynolds RJ, Vapor RJ. [5 May 2016];Reynolds Vapor Company to Begin National Distribution of Vuse Digital Vapor Cigarette on 23 June 2014. http://files.shareholder.com/downloads/RAI/3257786716x0x762402/1eb3fe53-7cc1-49d2-a763-3890144a7186/
- 76.Esterl M. The Wall Street Journal Got a Light—Er Charger? [5 May 2016];Big Tobacco's Latest Buzz. 2012 http://www.wsj.com/articles/SB10001424052702304723304577365723851497152.
- 77.British American Tobacco [5 May 2016];E-cigarettes and other next generation products: a growing market with huge potential. 2016 http://www.bat.com/ecigarettes.
- 78.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4:116–28. doi: 10.1016/S2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]