Abstract
During adolescence, individuals experience a broad range of dynamic environments as they strive to establish independence. Learning to respond appropriately in both new and previously encountered environments requires that an individual identify and learn the meaning of cues indicating that a behavior is appropriate, or alternatively, that it should be altered or inhibited. Although the ability to regulate goal-directed behavior continues to develop across adolescence, the specific circumstances under which adolescents experience difficulty with inhibitory control remain unclear. Here we review recent findings in our laboratory that address how adolescents learn to proactively inhibit a response. Much of our research has utilized a negative occasion setting paradigm, in which one cue (a feature) gates the meaning of a second cue (a target). The feature provides information that resolves the ambiguity of the target and indicates the appropriate behavioral response to the target. As such, we have been able to determine how adolescents learn about ambiguous stimuli, such as those whose meaning changes in accordance with other features of the surrounding environment. We consider why adolescents in particular exhibit difficulty in negative occasion setting compared to either pre-adolescents or adults. In addition, we review findings indicating that a balance between orbitofrontal cortex and nucleus accumbens is necessary to support normal negative occasion setting. Finally, we consider aspects of associative learning that may contribute to adolescent inhibitory control, as well as provide insight into adolescent behavior as a whole.
Keywords: Adolescence, Inhibition, Learning
1. Introduction
Adolescence is marked by dramatic changes in brain function as well as behavior. For instance, adolescent humans and other animals typically exhibit heightened exploration and novelty-seeking behavior compared to other age groups [1–3]. These behavioral tendencies serve a number of adaptive functions and promote independence, making them essential for the successful transition into adulthood [4, 5]. At the same time, they can also lead to risky and impulsive behaviors that increase the vulnerability to substance abuse and the risk of injury or even premature death [6–9]. Accordingly, there is significant interest in understanding the interplay between the specific neurobiological and behavioral factors that characterize adolescence and identifying why particular individuals are susceptible to negative outcomes.
One factor that has been the focus of substantial research on risk-taking and impulsivity during adolescence is the capacity to inhibit a response [e.g., 10]. Indeed, there is substantial evidence that adolescent humans and laboratory rodents experience difficulty inhibiting maladaptive or inappropriate behavioral responses. In particular, adolescents are often less able, compared to other age groups, to successfully use environmental cues that signal that a response should be withheld [11–20]. Notably, these difficulties are particularly apparent in experiments that involve increases in the appetitive [21–24] or aversive [25] nature of task-related stimuli. This is consistent with an emerging theory that age-related differences in behavioral control are correlated with cognitive load, which increases alongside the emotional salience of the surrounding environment [26, 27].
To date, most research on inhibitory processes has focused on the ability to abort a response that has already been initiated, a process referred to as ‘reactive’ inhibition [28–30]. Reactive inhibition is most commonly assessed using the Stop-Signal Task (SST) [31, 32] in which a ‘stop-signal’ (e.g., a tone) indicates that the pre-potent response to a previously-presented ‘go-signal’ (e.g., a visual stimulus presented on a computer screen) should be aborted. Yet, perhaps surprisingly, most studies indicate that reactive inhibition is similar in adolescents and adults [14, 23, 33]. In comparison, significantly less research (particularly in adolescents) has focused instead on the ability to use environmental cues that signal that a response should not be initiated in the first place, a process termed ‘proactive’ inhibition [28–30, 34]. To address this gap in the literature, our laboratory has recently conducted a set of studies to examine proactive inhibitory processes during development and to determine the neural systems that support proactive inhibition.
2. Negative occasion setting
An essential feature of adaptive behavior and a key developmental milestone is the ability to detect and use cues that signal the changing demands of complex environments. For example, some environmental cues indicate the conditions under which a response to another stimulus should be emitted or omitted. To illustrate, in the daily life of an adolescent the stimulus of seeing a friend will in many cases elicit the response of engaging in conversation, with the anticipated outcome of a gratifying social exchange. However, if the friend is encountered in a classroom setting, environmental cues such as the teacher standing in front of the class indicates that this behavior is inappropriate and will likely not result in the desired outcome. Cues such as the teacher are commonly referred to as occasion setters, as they ‘set the occasion’ for the meaning of an otherwise ambiguous ‘target’ stimulus and modulate behavior that is directed to it [35–37]. In this way, occasion setters enable an individual to categorize and retain multiple incongruent experiences with stimuli in the environment [38]. Moreover, this hierarchical organization facilitates the representation of events that an individual has experienced and helps to ensure that expectations and responses are appropriate to the present environment.
In the specific case of negative occasion setting, the cue indicates that the response to an upcoming stimulus should be withheld. Thus, negative occasion setting has direct bearing on inhibitory control. Moreover, presentation of an occasion setting cue prior to the onset of the ‘target’ stimulus models a key aspect of proactive inhibition in that the cue indicates that a response should not be initiated in the first place. Despite the importance of negative occasion setters for guiding adaptive behavior, very little prior research has considered the developmental course of this form of learning. As described below, we recently addressed this by conducting a series of experiments using laboratory rats to test for age differences in the ability to use negative occasion setters to withhold behavior.
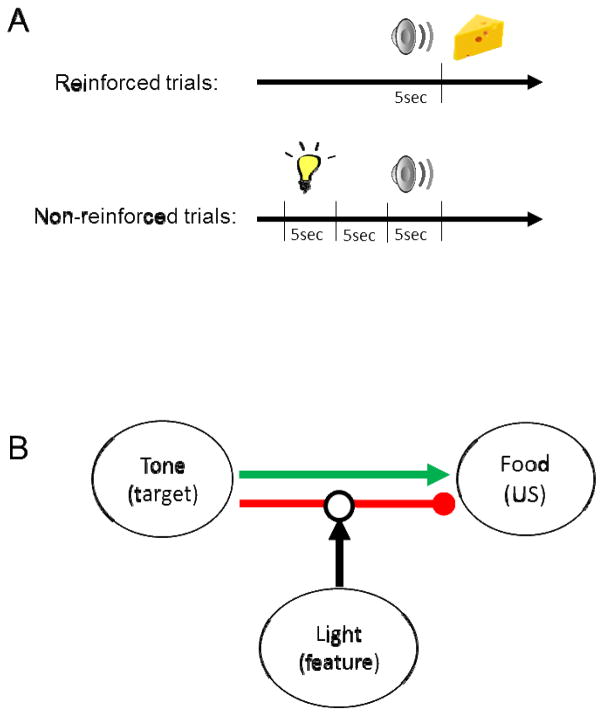
In the negative occasion setting procedure used in our studies (Figure 1A), a target stimulus (e.g., a tone) is presented by itself on some trials and followed immediately by reinforcement (reinforced trials). On other trials, a ‘feature’ stimulus (e.g., a light) is presented just before the target and no reinforcement occurs on those trials (non-reinforced trials). Thus, the feature stimulus acts to set the occasion, or the context, for the meaning of the target stimulus [39–41]. Over the course of training, rats learn to approach the food cup (anticipatory response) during presentation of the target when it is presented by itself, and to withhold responding during the target when it is preceded by the feature [42, 43, 19]. Using the training parameters defined by Holland et al. [42], normal adult rats typically require about ~10 training sessions to exhibit significantly more responding during the target on reinforced versus non-reinforced trials [19, 20, 42–44].
Figure 1.
A) Illustration of the two types of training trials used in our standard negative occasion setting procedure with rats. The feature (light) and target (tone) are each presented for 5 sec and the interval between them on non-reinforced trials is also 5 sec. On reinforced trials, food is delivered immediately after the tone is terminated. B) Model of the associations that are thought to be formed during negative occasion setting [40]. Red and green lines indicate inhibitory and excitatory relationships in the behavioral procedure, respectively (US = unconditioned stimulus). The feature stimulus acts to gate, or ‘set the occasion’ for the meaning of the target stimulus and indicates that a response should be withheld during the subsequent presentation of the target.
Using negative occasion setting to model proactive inhibition is particularly amenable to studies of adolescent rats since the training procedures can be completed within 2–3 weeks. In addition, the negative occasion setting paradigm can be used to study how individuals inhibit behavior when the appropriate course of action is ambiguous, an aspect of behavioral control that has receive scant attention in adolescents.
3. Negative occasion setting in adolescence
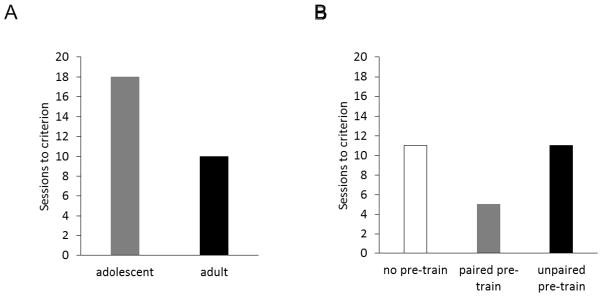
In an initial experiment [19], we tested the hypothesis that adolescent rats would require more training than adults to exhibit negative occasion setting. We based this prediction on our prior findings that negative occasion setting is dependent on regions within prefrontal cortex [43], which are not fully developed in adolescents. Consistent with this notion, we found that adolescent rats that began training on post-natal day (PND) 35 required 18 training sessions to consistently exhibit greater responding to the target on reinforced compared to non-reinforced trials, while adult rats (began training on PND 70) required only 10 sessions (Figure 2A). Although these findings indicated that adolescents experience difficulty using negative occasion setters to regulate behavior, it was unclear from the study design whether the additional training required by adolescents was indicative of difficulty acquiring the task contingencies, or instead reflected an inability to express what was learned until a certain age.
Figure 2.
A) Number of daily training sessions required by adolescent (started training on PND 35) and adult (PND 70) rats to consistently exhibit significantly more responding to the target on reinforced versus non-reinforced trials (adapted from [19]). Criterion was 3 consecutive sessions in which the group mean difference in responding during reinforced and non-reinforced trials was significantly greater than zero (i.e., Z-score of at least 2.325, p<0.01). B) Results of an experiment to test whether adolescent rats were impaired in acquiring the contingencies during negative occasion setting, or in expressing that learning. Rats in the ‘paired pre-train’ group received six sessions of negative occasion setting training starting on PND 35. Rats in the ‘unpaired pre-train’ group were treated similarly but the stimuli were presented randomly and in an unpaired fashion. Both groups remained in their home cages for 9 days after the pre-training sessions. Then when they were 50 days only, they resumed negative occasion setting training. The no pre-train group did not receive any pre-training and had its first experience with the negative occasion setting procedures starting on PND 50.
To address this, a group of PND 35 rats was trained for just six sessions and then remained in their home cages until they reached PND 50, which was the approximate age when the adolescent group in the initial study first exhibited significantly more responding during the target on reinforced versus non-reinforced trials. Interestingly, the group that received the six sessions of pre-training discriminated between the trials types in fewer sessions once training resumed compared to a group that received no pre-training and began the experiment on PND 50 (Figure 2B) [19]. These results suggested that adolescents are able to acquire (encode) information about the contingencies of the procedure early in training, but are unable to express that learning until they reach ~50 days of age. Importantly, the results could not be attributed to changes in motivation to consume food, or to difficulties with motoric inhibition. Indeed, prior research indicates that adolescents do not experience difficulties implementing response inhibition in reaction to a stop signal [33]. Moreover, another group of PND 35 rats that received unpaired-presentations of the stimuli during the 6 pre-training sessions to control for stimulus pre-exposure effects did not benefit from the pre-training (Figure 2B). Thus, we conclude that relevant information about the excitatory and inhibitory nature of the cues was acquired by adolescent rats, but processed in such a way that it was not readily integrated into response patterns until closer to adulthood.
In adult rats, the feature cue is believed to modulate, or ‘gate’ an inhibitory association between the target stimulus and the US, rather than becoming associated with the US itself (Figure 1B; [45]). Adolescents, however, may have difficulty accessing or deploying the gating properties of the feature cue, or alternatively, may encode the inhibitory properties of the feature in a different way. We recently addressed this by parsing out the associations formed between the target stimulus, feature cues, and the unconditioned stimulus (US) over the course of training guided by the theory that the inhibitory properties of an occasion setter are specific for its relationship with the target stimulus [37, 46]. In other words, the inhibitory properties of a true occasion setter should not affect conditioning to other stimuli.
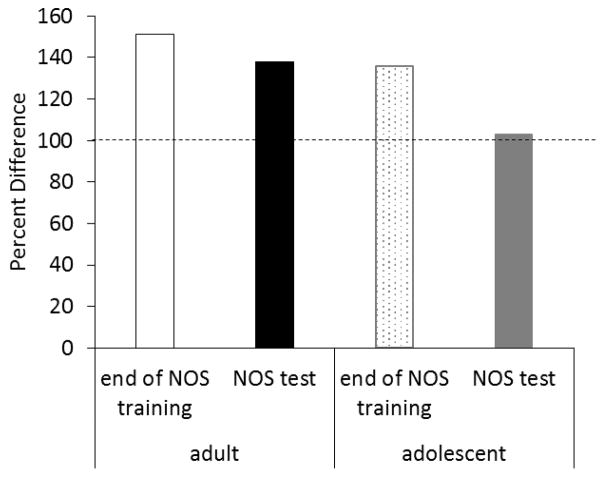
With this in mind, we exposed adolescent and adult rats to training sessions consisting of paired presentations of the feature stimulus and the US after they had learned to discriminate between reinforced and non-reinforced presentations of the target during negative occasion setting (i.e., counterconditioning). Following several sessions of counterconditioning, rats received a test session in which they were re-exposed to the negative occasion setting procedures. In adult rats, the differential levels of responding to the target were still apparent, despite having learned an excitatory association between the light and reinforcer during counterconditioning (Figure 3). In contrast, adolescents did not differentiate responding during the negative occasion setting test session, instead exhibiting comparable food cup responding during both trial types, and importantly, an increase in responding during non-reinforced trials relative to the end of the initial negative occasion setting training. These findings suggest that adolescents encode the inhibitory properties of the feature differently than adults. In particular, adolescents may encode the feature as an inhibitory cue, but fail to encode the ability of the feature to gate the meaning of the target. As a result, any inhibitory properties of the feature that are acquired must directly compete with the excitatory properties of the target during presentations of the latter. Thus, failures to exhibit behavioral inhibition under these conditions may be attributed to a bias towards the excitatory properties of the target, consistent with literature linking inhibitory control failures with the presence of emotionally arousal cues, including those that predict a reward [47].
Figure 3.
Effects of counterconditioning (light paired with food) on conditioned responding to the target when rats were returned to the negative occasion setting procedures (NOS test). Percent difference reflects the amount of responding to the target on reinforced trials divided by responding to the target on non-reinforced trials, multiplied by 100. Thus, a value of 100% (dotted line) indicates that responding during reinforced trials was the same as responding on non-reinforced trials. Adult rats exhibited little change in responding to the target during the NOS test session compared to the end of NOS training. In contrast, counterconditioning reduced responding to the target in adolescent rats.
Differences in encoding the negative occasion setting properties of the feature cue could also be related to the amount of attention directed towards the cue during training. Consistent with this, we have shown that adolescents exhibit less orienting behavior directed to the feature cue compared to adults [19]. Orienting behavior, in this case rearing up on the hind legs when the light is presented, is an often used measure of attentional processing [48, 49]. Additionally, pharmacological interventions that enhance attentional processes, such as nicotine, have been linked to improved performance in negative occasion setting, including in adolescents [44, 50, 51]. Further research is necessary to establish the ways in which the salience of environmental cues differs during adolescence and in turn how this influences inhibitory control.
4. Non-linear development of negative occasion setting
Since prefrontal regions develop linearly over time [52–58], it might be expected that rats trained prior to adolescence would experience even greater difficulties with negative occasion setting than adolescents since their prefrontal cortex is even less well-developed. Indeed, mounting evidence supports a link between the attainment of adult-like inhibitory behavior and maturation of the prefrontal cortex [14, 15, 23, 59–62, 72], specifically the ventral lateral prefrontal cortex and the orbitofrontal cortex (OFC) in humans [14, 23, 72], and the medial prefrontal cortex and the OFC in rodents [15, 59–62]. This is in line with our finding that the ability to use a negative occasion setter coincided with the age at which the rodent prefrontal cortex is thought to reach functional maturity. Indeed, the maturation of the rodent medial prefrontal cortex is followed by the maturation of OFC between ~PND 50–53 [61, 127].
At the same time, emerging evidence suggests that the full behavioral profile of adolescents cannot be completely explained by the maturational status of prefrontal cortical regions alone. For example, behavioral tendencies indicative of attenuated cognitive control, such as risk-taking behavior, are substantially less apparent in age groups both younger and older than adolescence [63]. One explanation for this comes from contemporary research that considers in parallel the developmental trajectories of multiple brain regions as well as the refinement of inter-regional interactions [64–66]. Aptly named, the ‘imbalance model’ framework takes into account evidence from neurochemical, structural, and functional measures in both humans and animals suggesting that subcortical limbic regions mature earlier in development than cortical control areas [58, 67–70]. The result is a functional imbalance specifically during the adolescent period, when limbic regions may exert stronger influence on behavior than younger ages, where all regions are still developing, and adulthood, where all regions are fully mature [64, 71–74], as illustrated in Figure 4.
Figure 4.
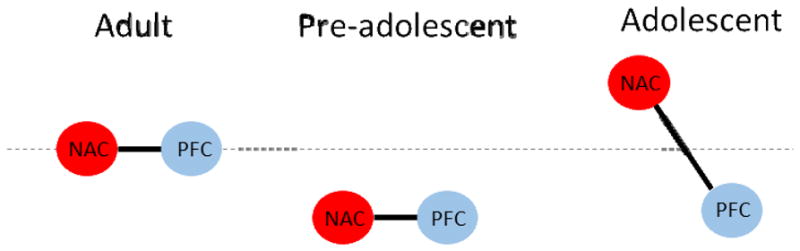
‘Imbalance model’ of adolescent brain development [64]. NAC = nucleus accumbens, PFC=prefrontal cortex. Dotted line refers to activity levels in adults. During adulthood, both NAC and PFC have reached functional maturity and their activity levels are in balance. Similarly, during pre-adolescence, both regions are immature and activity levels are lower than in adults, but still balanced in relationship to each other. In contrast, there is an imbalance that arises in adolescents because maturation of PFC lags behind that of NAC, and NAC activity is increased.
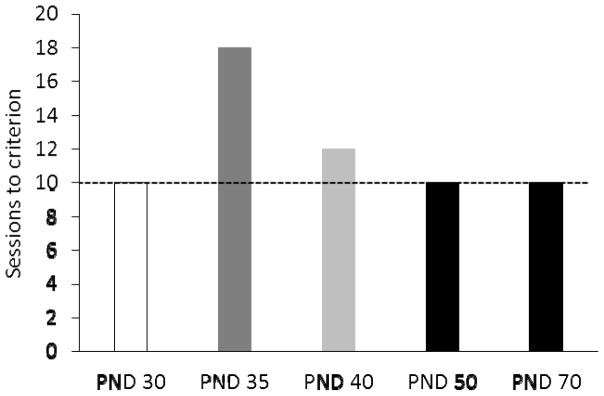
In light of this model, we recently trained additional age groups in negative occasion setting [20]. One group began training as pre-adolescents (PND 30), another began training on PND 40 (adolescents), and a late adolescent/early adult group began on PND 50. Including the PND 40 adolescent group provided an opportunity to test the prediction that discrimination between the trials types would emerge sooner than in the PND 35 adolescent group since the PND 40 group began training closer to adulthood than the PND 35 group. As shown in Figure 5, rats that began training at PND 50 or PND 30 each required 10 training sessions to exhibit differential responding during the target on reinforced and non-reinforced trials, like full-adults that began training on PND 70. In contrast, rats that began training on PND 35 required 18 sessions and the rats that began on PND 40 required 12 sessions. Three important observations emerge from these findings: First, the comparable performance of the PND 30 and PND 50 groups indicates that the development of negative occasion setting is non-linear, and thus cannot depend solely on the maturation of prefrontal cortical regions. Second, the PND 40 group began training when they were 5 days older than the PND 35 group, and accordingly, required 6 fewer days to exhibit negative occasion setting. This suggests that within the period of adolescence there is a relationship between brain maturation and the ability to use negative occasion setters. Third, despite the fact that the latter part of training occurred during adolescence for the PND 30 group, they still exhibited adult-like performance. This finding harkens back to the notion that adolescent rats may encode the task contingencies early in training in a different way than non-adolescents.
Figure 5.
Number of daily training sessions required to exhibit negative occasion setting by rats that started training prior to the onset of adolescence (PND 30), during adolescence (PND 35, 40), late adolescence/early adulthood (PND 50), and full adulthood (PND 70). Adapted from [20].
In summary, the pattern of findings across the different age groups indicates that difficulty using negative occasion setters is unique to adolescence, consistent with the imbalance model of adolescence behavior and brain maturation [64–66]. Thus, like increased risk-taking behavior and impulsivity, differences in proactive inhibitory processes (at least as they are reflected in negative occasion setting) appear to be characteristic of adolescence but not early or later developmental stages. This suggests that difficulty using inhibitory signals to withhold behavior may rely on the imbalance of activity in prefrontal control areas and subcortical reward-related areas, as postulated by the imbalance model [64, 72–74].
5. Relating behavioral control to brain development
As alluded to above, prefrontal control regions and subcortical limbic structures exhibit differential developmental trajectories, resulting in a functional imbalance between these regions that may contribute to behavioral patterns during adolescence [64–66, 75]. Indeed, areas of prefrontal cortex continue to develop throughout adolescence [76], which contrasts markedly with evidence of both structural and functional maturation in subcortical regions, particularly the nucleus accumbens (NAC) and amygdala [72, 77]. Moreover, additional evidence indicates that neural activity is higher in NAC and amygdala in adolescents compared to any other age, resulting in a disproportionate contribution to behavior [72, 78–81]. Taken together, functional measures indicate that during adolescence an imbalance results from simultaneously low activity in prefrontal regions and over-activity in NAC.
Nevertheless, support for the imbalance model has been derived primarily from correlational studies. This is largely due to the lack of viable means to simultaneously manipulate neural activity in two brain regions in different directions over an extended period of training. However, the advent of chemogenetic techniques [82] has provided a means to directly test the effects of the combination of excessive activity in NAC and hypo-activity in prefrontal cortex that exist during adolescence. Using an approach known as DREADDs (Designer Receptors Exclusively Activated by Design Drugs), a recent study in our laboratory causally tested the imbalance model by simultaneously decreasing neural activity in the OFC and increasing activity in NAC in adult rats [83]. We specifically targeted the OFC region of prefrontal cortex because prior work has linked changes in behavioral control across development to the relative functional activation between OFC and NAC [72, 88]. Moreover, OFC is known to be involved in representing contingencies between predictive stimuli and reward outcomes and in updating response strategies after outcome value has changed [122–124].
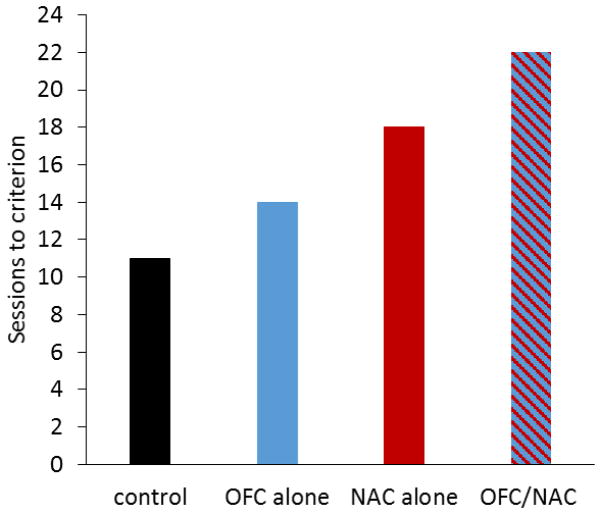
In our study [83], two different viral constructs encoding synthetic G-protein coupled receptors were surgically infused into the OFC and NAC. A construct containing the DNA for an inhibitory receptor was infused into OFC. Once expressed, activation of the inhibitory receptor for the activating agent, clozapine-N-oxide (CNO) hyperpolarizes neurons and silences neural activity [84–86]. A second construct, containing the DNA for an excitatory receptor, was infused into NAC, which when activated by CNO, depolarizes neurons and increases activity [82, 84, 87]. We tested the effects of this simultaneous manipulation on negative occasion setting by treating rats with CNO (systemically) just prior to each training session. We found that rats with a simultaneous decrease in OFC activity and increase in NAC activity took longer to exhibit negative occasion setting (22 training sessions; Figure 6) than various control groups (~11 sessions) [83]. Moreover, compared to rats in which either region was targeted independently, the combined manipulation produced a greater effect. Thus, by chemogenetically manipulating the adult brain to reproduce the functional imbalance observed during adolescence, we were able to recapitulate the delay in negative occasion setting exhibited by normal adolescent rats (Figures 2 and 5). Our findings provide direct evidence that the simultaneous under-activity in OFC and over-activity in NAC present during adolescence [72, 88] can disrupt behavioral control processes. Moreover, by taking into consideration the functional roles of OFC and NAC we may begin to elucidate the underlying cognitive behavioral factors that contribute to the delays in negative occasion setting that result from disruption of normal activity in these regions. Specifically, our data supports the notion that these regions work together in the process of integrating cues in the environment, in this case the feature and target, into a cohesive representation that can be used to guide behavior. This is supported by evidence of extensive connectivity between OFC and limbic areas of the brain including NAC that has previously been implicated in the integration and updating of the physical aspects of rewarding and aversive outcomes with emotional information [89–92].
Figure 6.
Number of daily training sessions required to exhibit negative occasion setting in adult rats with artificially reduced levels of activity in orbitofrontal cortex (OFC), or increased activity in NAC, or a combination of both manipulations. Adapted from [83].
In light of our findings regarding the balance between OFC and NAC that is necessary to support normal negative occasion setting, it may be that attenuated activity in the adolescent OFC is a key contributing factor to difficulties using the feature to disambiguate whether or not the target will be reinforced. In addition, over-excitation of NAC may exacerbate the delay in exhibited negative occasion setting by disproportionately increasing the excitatory properties of the target stimulus relative to the inhibitory properties of the feature. Indeed, NAC plays a key role in attributing incentive-salience to reward-related cues, which has been shown to invigorate approach behavior [92, 93]. Such excitation may result in difficulty overcoming NAC-mediated approach behaviors even in the presence of the feature, which would otherwise trigger inhibitory control processes. This parallels a number of studies suggesting that failures to regulate behavior during adolescence emerge as a result of increased motivation to pursue rewards [47, 63].
6. Implications for understanding adolescent behavior
Our findings to date add to the literature by indicating some of the specific circumstances under which adolescents experience difficulty with inhibitory control. The overarching theme of our findings in this regard is that adolescents experience difficulties effectively using cues in the environment that indicate a behavior is inappropriate and should be withheld. This appears to be particularly evident when adolescents are required to learn and maintain information about relationships between cues in the environment, as is required for proactive inhibitory control. In the following sections, we consider two aspects of associative learning in adolescence that may contribute to this effect, as well as provide insight into adolescent behavior as a whole. First, adolescents represent cues with ambiguous meanings differently than adults, contributing to the ineffective encoding of information important for disambiguating features of the environment. Second, adolescent decision making processes that mediate the emission of a response are biased towards individual cues rather than the integrated network of cues used to inform adult behavior.
6.1 Responding to ambiguity
Emerging evidence suggests that adolescent behavior differs from other ages particularly when the meaning of a stimulus, or an anticipated outcome of a response, is ambiguous. In particular, adolescents exhibit a greater willingness to engage in behaviors for which the outcome is uncertain relative to adults [94, 95]. Encountering an ambiguous stimulus indicates the need to consider the information at hand and potentially acquire more information before making a decision [94, 96]. Whereas adults may be highly motivated to attain disambiguating information, adolescents may be less likely to do so and thus react more ‘impulsively’ (i.e. without all of the information that could help guide appropriate decision making). Along the same lines, this predisposition has been associated with engagement in reckless behaviors during adolescence [94].
With regard to negative occasion setting, an increased tolerance of the ambiguity of the target stimulus might decrease the negative prediction error that should occur during non-reinforced trials. In turn, this would delay learning about the extent to which the reinforcement contingencies of the target are dependent on other factors (in this case, the feature). Moreover, a smaller prediction error may also minimize the motivation to attend to other aspects of the environment in search of information to disambiguate the meaning of the target. This could provide an explanation for the attenuated attentional processes associated with the inhibitory feature during adolescence
Consistent with this, our laboratory has recently found age-related delays in inhibitory control during negative occasion setting to be particularly robust as the uncertainty regarding the potential for reinforcement following the target cue increases. During each daily training session the two trial types are intermixed. However, the protocol is such that no two reinforced trials will ever be presented consecutively. As a result, when considering only the target, the ambiguity surrounding whether or not the target will be followed by reinforcement is greatly reduced during non-reinforced trials that immediately follow reinforced trials. Conversely, during non-reinforced trials that do not immediately follow a reinforced trial, the target regains a one-in-four chance of predicting reinforcement. When we isolated each category of non-reinforced trials for analysis, no age differences were observed in the number of training sessions required to exhibit significantly less responding during target on the former subset of non-reinforced trials than on reinforced trials. However, age differences were apparent when we isolated non-reinforced trials that did not immediately follow a reinforced trial. Indeed, the number of sessions required to exhibit lower responding during this subset of non-reinforced trials relative to reinforced trials was very similar to the data in Figure 2A. Thus, the negative occasion setting delays we observe may in part be driven by differences in how adolescents respond in the face of uncertainty. Specifically, adolescents are more likely to approach when presented with ambiguous cues.
It has previously been shown in humans that children, like adolescents, are more tolerant of ambiguity than adults [97], and furthermore, that ambiguity tolerance decreases linearly across development into adulthood [95]. Thus, additional factors must be contributing to the ontogeny of negative occasion setting to account for the non-linearity of behavioral performance. One explanation may be that prior to adolescence, individuals experience less motivation to approach reward-predictive cues. Indeed, the ongoing development of regions of the brain that mediate reward processing, including NAC, has been shown to contribute to a reduced neural and behavioral response to reward-related cues [23, 72].
The contribution of heightened reward sensitivity to adolescent behavior, particularly a reduced ability to exert behavioral control in the face of environmentally salient cues, has been the topic of a number of comprehensive reviews [47, 74, 98, 99] and thus will not be discussed in detail here. However, it is of note that the salience of a cue determines how it will be weighted relative to other cues during the process of developing an integrated representation for the environment as a whole. Thus, attenuated learning about the meaning of a negative occasion setter may be exacerbated by the heightened sensitivity to rewards observed specifically during adolescence. Notably, excitatory cues appear to be highly salient for adolescents whether they are excitatory or aversive. Indeed, research discussed elsewhere in this issue has suggested that adolescents differ relative to both juveniles and adults with regard to learning about aversive cues as well [74, 100–102].
6.2 Response heuristic formation
As a whole, our findings with negative occasion setting suggest that adolescents experience difficulty learning about the relationships between cues in the environment. As a result, adolescents may preferentially generate response patterns based on isolated cues (e.g. responding elicited by the target) rather than responding based on the environment as a whole (i.e. incorporating the feature). This is consistent with previous data suggesting that the ability to mediate behavior based on context, consisting of an amalgamation of olfactory, tactile and visual information, is attenuated specifically during adolescence [100].
It is feasible that behavioral proclivities during adolescence may result from differences in the computations underlying the development of a response heuristic [103–107]. A response heuristic is a cognitive device based largely on previous experiences that also incorporates information from the environment at hand. Utilizing such a device provides an informed basis for what outcome to expect after making a response and thus can be used in the service of achieving both the current and the long term goals of an organism [e.g., 108, 109]. Adult patterns of responding are believed to be based on heuristic level representations of potential outcomes that capture the essence of a potential outcome without the details [110]. Because of this, adults may avoid potentially aversive outcomes by default without assessing the actual probabilities at hand. In contrast, adolescent patterns of responding reflect explicit computations that incorporate the anticipated outcome of different options [104]. In other words, adolescents may less readily generalize the predictive nature of environmental stimuli that have previously been experienced.
With respect to negative occasion setting, adolescents may calculate the probability of reinforcement following the target and be less deterred from responding. Specifically, one of every four presentations of the target will be followed by reinforcement and thus approaching the food cup non-discriminately during the target will allow the adolescent to immediately obtain the food when it is delivered. In contrast, adults are more likely to encode the partial reinforcement schedule as a general feature of the environment and limit responding to conserve energy. Along these same lines, we have recently shown that during an excitatory conditioning paradigm adolescents learn to respond during a partially reinforced Pavlovian appetitive cue (also reinforced one of every four presentations) faster than adults [126]. Subsequently, the differential encoding of the partial reinforcement schedule of the target may contribute to the adult impetus to learn about other cues in the environment that mediate this schedule (i.e. the feature) during negative occasion setting.
7. Conclusions and future directions
A central theme emerging from the research we have reviewed here and the related literature is that the development of basic associative learning processes may underlie age-differences in other forms of behavior [111]. An important note is that adolescents and adults may similarly encode the meaning of individual cues. The difference manifests in how these meanings are integrated, to provide a flexible picture in which the meaning of one cue can change in the presence of another stimulus. In agreement with this notion, adolescents often acquire information even if they do not utilize it to the same extent as adults in guiding appropriate behavior [19, 100, 112]. In keeping with this perspective, future research should continue to examine how the nature of individual cues, and perhaps more importantly the formation of contingencies between networks of cues, manifests across development.
The development of a heuristic facilitates the efficiency of response patterns by allowing an individual to rely on computations made previously in environments that are distinct but contain overlapping salient details. This can be very beneficial in everyday life when an individual experiences some degree of consistency in the surrounding environment. However, adolescents are much more likely to experience a broader range of environments as they strive to establish independence [71]. Thus, during this limited window it may in fact be beneficial for an individual to forgo the use of broad scale heuristics for guiding responding. By instead attending to individual stimuli, the adolescent can encode a wealth of information into a cue representation that may subsequently be of value when multiple cues are integrated during the formation of a response heuristic. The result is a tradeoff between enhanced behavioral flexibility in circumstances where behavior should be mediated by highly salient individual cues [e.g., 33, 113–117] and the potential for maladaptive behavior when responding biased by salient individual cues results in reckless or risky behavior [47]. Moreover, biases towards individual cues contribute to failures incorporating information based on contingencies between multiple cues in the environment, as we observe in negative occasion setting.
This is just one example of how behavior can differ depending on the drives of a particular life stage. Capitalizing on the behavioral tendencies of adolescents could contribute to interventions targeted at reducing the likelihood that such tendencies will manifest as maladaptive behaviors. Taking this into account, advances in understanding adolescent behavior will require consideration of how behavioral phenotypes evolve in more complex, dynamic environments that better reflect the natural world [118, 119]. Furthermore, emerging research suggests that adolescence does not merely reflect a time point on a linear developmental trajectory. Although this has become increasingly evident from research and the development of theoretical perspectives in recent years, research is still limited with regard to exactly what combination of factors make this period what it is.
Finally, contemporary research has made crucial contributions to elucidating the cognitive, behavioral and neurobiological changes that characterize adolescence. However, further study is necessary to establish the nuances that mediate the dynamic interactions between behavioral and neurobiological development. The changes that occur in the adolescent brain are believed facilitate more efficient transmission of information between brain regions and more selective and refined information processing through development [23, 120]. Relatedly, behavioral phenotypes prevalent during adolescence likely result from age-related differences in the coordination of brain systems that organize behavioral output. Thus, an important area for future research will be to consider the functional connectivity between prefrontal and subcortical regions as it relates to inhibitory control. As adolescence progresses, functional interconnectivity, especially between distributed networks, increases, providing a mechanism for top-down modulation of subcortical output that diminishes inappropriate responding [64, 121]. With the continued development of technologies like chemogenetics, future studies should employ viral mediated gene delivery during adolescence to see if targeted manipulations could somehow improve inhibitory control, for example by leveling out the contributions of prefrontal and subcortical regions that are imbalanced during this period.
Highlights.
We review factors contributing to the ontogeny of proactive inhibition
Difficulties using negative occasion setters are specific to adolescence
Adolescents differentially integrate the meanings and contingencies of multiple cues
Adolescent behavior may be biased towards individual rather than networks of cues
A functional balance between OFC and NAC is necessary for negative occasion setting
Acknowledgments
Research supported by NIH Grants F31MH107138 (HCM) and R01DA027688 (DJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 2.Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- 3.Somerville LH. The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science. 2013;22:121–127. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear LP. The Behavioral Neuroscience of Adolescence. New York: Norton; 2010. [Google Scholar]
- 5.Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- 6.Arnett JJ. Adolescent storm and stress, reconsidered. The American Psychologist. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- 7.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown MR, Benoit JR, Juhás M, Dametto E, Tse TT, MacKay M, Sen B, … Greenshaw AJ. fMRI investigation of response inhibition, emotion, impulsivity, and clinical high-risk behavior in adolescents. Frontiers in Systems Neuroscience. 2015;9:124. doi: 10.3389/fnsys.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CC, Chen WJ, Yang HJ, Hsiao CK, Tien AY. Performance on the Wisconsin Card Sorting Test among adolescents in Taiwan: Norms, factorial structure and relation to schizotypy. Journal of Clinical and Experimental Neurospychology. 2000;22:9–79. doi: 10.1076/1380-3395(200002)22:1;1-8;FT069. [DOI] [PubMed] [Google Scholar]
- 12.Anderson VA, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developmental Neuropsychology. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- 13.Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: Two behavioral features of adolescence in mice. Behavioral Neuroscience. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- 14.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 15.Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behavioral Neuroscience. 2011;125:93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- 16.Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: adolescent-limited and life-persistent patterns of impulsivity. Behavioral Neuroscience. 2011;125:194–201. doi: 10.1037/a0022919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behavioural Brain Research. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 18.Sun HS, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- 19.Meyer HC, Bucci DJ. The ontogeny of learned inhibition. Learning & Memory. 2014;21:143–152. doi: 10.1101/lm.033787.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer HC, Bucci DJ. Negative occasion setting in juvenile rats. Behavioural processes. 2016 doi: 10.1016/j.beproc.2016.05.003. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 22.Hare TA, Tottenham N, Galván A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galván A. The Teenage Brain Sensitivity to Rewards. Current Directions in Psychological Science. 2013;22:88–93. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Gilbert JE, Thomas KM. Inhibitory control during emotional distraction across adolescence and early adulthood. Child development. 2013;84:1954–1966. doi: 10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills KL, Dumontheil I, Speekenbrink M, Blakemore SJ. Multitasking during social interactions in adolescence and early adulthood. Royal Society Open Science. 2015;2:150117. doi: 10.1098/rsos.150117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson KA, Rudolph MD, … Casey BJ. When Is an Adolescent an Adult? Assessing Cognitive Control in Emotional and Nonemotional Contexts Psychological Science. 2016;27:549–562. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- 28.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry. 2011;69:e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Science. 2012;16:106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer HC, Bucci DJ. A learning perspective on proactive inhibitory control. Learning and Memory in press. [Google Scholar]
- 31.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 32.Logan GD. On the ability to inhibit thought and action: A user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- 33.Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response inhibition and behavioral flexibility between adolescent and adult rats. Behavioral Neuroscience. 2013;127:23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaffard M, Longcamp M, Velay JL, Anton JL, Roth M, Nazarian B, Boulinguez P. Proactive inhibitory control of movement assessed by event-related fMRI. NeuroImage. 2008;42:1196–1206. doi: 10.1016/j.neuroimage.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 35.Skinner BF. The behavior of organisms: an experimental analysis. New York: D. Appleton-Century; 1938. [Google Scholar]
- 36.Holland PC. Occasion setting in Pavlovian conditioning. Psychology of Learning and Motivation. 1992;28:69–125. [Google Scholar]
- 37.Bouton ME. Learning and Behavior: A Contemporary Synthesis. Sunderland, MA: Sinauer Associates; 2006. [Google Scholar]
- 38.Swartzentruber D. Modulatory mechanisms in Pavlovian conditioning. Animal Learning & Behavior. 1995;23:123–143. [Google Scholar]
- 39.Holland PC, Morell JR. The effects of intertrial and feature-target intervals on operant serial feature negative discrimination learning. Learning and Motivation. 1996;27:21–42. [Google Scholar]
- 40.Bouton ME, Nelson JB. Mechanisms of feature-positive and feature-negative discrimination learning in an appetitive conditioning paradigm. In: Schmajuk N, Holland PC, editors. Occasion setting: Associative learning and cognition in animals. Washington, D.C: American Psychological Association; 1998. pp. 69–112. [Google Scholar]
- 41.Bueno JL, Holland PC. Occasion setting in Pavlovian ambiguous target discriminations. Behavioural Processes. 2008;79:132–147. doi: 10.1016/j.beproc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 43.MacLeod JE, Bucci DJ. Contributions of the subregions of the medial prefrontal cortex to negative occasion setting. Behavioral Neuroscience. 2010;124:321–328. doi: 10.1037/a0019344. [DOI] [PubMed] [Google Scholar]
- 44.Meyer HC, Putney RB, Bucci DJ. Inhibitory learning is modulated by nicotinic acetylcholine receptors. Neuropharmacology. 2015;89:360–367. doi: 10.1016/j.neuropharm.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouton ME, Nelson JB. Context-specificity of target versus feature inhibition in a feature-negative discrimination. Journal of Experimental Psychology: Animal Behavior. 1994;20:51–65. [PubMed] [Google Scholar]
- 46.Ross RT, Holland PC. Conditioning of simultaneous and serial feature-positive discriminations. Animal Learning and Behavior. 1981;9:293–303. [Google Scholar]
- 47.van Duijvenvoorde AC, Peters S, Braams BR, Crone EA. What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neuroscience & Biobehavioral Reviews. 2016 doi: 10.1016/j.neubiorev.2016.06.037. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 48.Lang PJ, Simons RF, Balaban MT. Attention and orienting: Sensory and motivational processes. Hillsdale, NJ: Erlbaum; 1997. [Google Scholar]
- 49.Robinson A, Eggleston R, Bucci DJ. Physical exercise and catecholamine reuptake inhibitors affect orienting behavior and social interaction in a rat model of attention-deficit/hyperactivity disorder. Behavioral Neuroscience. 2012;126:762–771. doi: 10.1037/a0030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacLeod JE, Potter AS, Simoni MK, Bucci DJ. Nicotine administration enhances conditioned inhibition in rats. European journal of pharmacology. 2006;551:76–79. doi: 10.1016/j.ejphar.2006.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer HC, Chodakewitz MI, Bucci DJ. Nicotine administration enhances negative occasion setting in adolescent rats. Behavioural Brain Research. 2016;302:69–72. doi: 10.1016/j.bbr.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 53.Stuss DT. Biological and psychological development of executive functions. Brain Cognition. 1992;20:8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- 54.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, … Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 55.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 56.Romine CB, Reynolds CR. A model of the development of frontal lobe functioning: findings from a meta-analysis. Applied Neuropsychology. 2005;12:190–201. doi: 10.1207/s15324826an1204_2. [DOI] [PubMed] [Google Scholar]
- 57.Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology, Biochemistry and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- 59.Neill DB. Frontal-striatal control of behavioral inhibition in the rat. Brain Research. 1976;105:89–103. doi: 10.1016/0006-8993(76)90925-2. [DOI] [PubMed] [Google Scholar]
- 60.Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Annals of the New York Academy of Sciences. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- 61.Newman L, McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Developmental Psychobiology. 2011;53:391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juraska JM, Willing J. Pubertal onset as a critical transition for neural-development and cognition. Brain Research. 2016 doi: 10.1016/j.brainres.2016.04.012. Advance online publication. [DOI] [PMC free article] [PubMed]
- 63.Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casey BJ, Getz S, Galván A. The adolescent brain and risky decisions. Developmental Reviews. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Reviews. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 68.Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: Implications for the development of psychopathology. Cerebral Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- 69.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, … Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. The Journal of Neuroscience. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 72.Galván A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, … Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caballero A, Granberg R, Tseng KY. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience & Biobehavioral Reviews. 2016 doi: 10.1016/j.neubiorev.2016.05.013. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 78.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 79.Casey BJ, Galván A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinions in Neurobiology. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 80.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neuroscience and Biobehavioral Reviews. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 81.Rosenberg D, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 82.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacology Review. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer HC, Bucci DJ. Imbalanced activity in the orbitofrontal cortex and nucleus accumbens impairs behavioral inhibition. Current Biology. doi: 10.1016/j.cub.2016.08.034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ. Chemogenetic silencing of retrosplenial cortex neurons disrupts sensory preconditioning. The Journal of Neuroscience. 2014;34:10982–10988. doi: 10.1523/JNEUROSCI.1349-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, … Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature Neuroscience. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith KS, Bucci DJ, Luikart BW, Mahler SV. DREADDS: Use and application in behavioral neuroscience. Behavioral Neuroscience. 2016;130:137–155. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qu Y, Galván A, Fuligni AJ, Lieberman MD, Telzer EH. Longitudinal Changes in Prefrontal Cortex Activation Underlie Declines in Adolescent Risk Taking. The Journal of Neuroscience. 2015;35:11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 90.Balleine BW, Leung BK, Ostlund SB. The orbitofrontal cortex, predicted value, and choice. Annals of the New York Academy of Science. 2011;1239:43–50. doi: 10.1111/j.1749-6632.2011.06270.x. [DOI] [PubMed] [Google Scholar]
- 91.San-Galli A, Marchand AR, Decorte L, Di Scala G. Retrospective revaluation and its neural circuit in rats. Behavioural brain research. 2011;223:262–270. doi: 10.1016/j.bbr.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 92.Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annual Reviews in Psychology. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- 93.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 94.Tymula A, Belmaker LAR, Roy AK, Ruderman L, Manson K, Glimcher PW, Levy I. Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proceedings of the National Academy of Sciences. 2012;109:17135–17140. doi: 10.1073/pnas.1207144109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blankenstein NE, Crone EA, van den Bos W, van Duijvenvoorde ACK. Dealing with uncertainty: testing risk- and ambiguity-attitude across adolescence. Developmental Neuropsychology. 2016;41:77–92. doi: 10.1080/87565641.2016.1158265. [DOI] [PubMed] [Google Scholar]
- 96.Tottenham N, Phuong J, Flannery J, Gabard-Durnam L, Goff B. A negativity bias for ambiguous facial-expression valence during childhood: Converging evidence from behavior and facial corrugator muscle responses. Emotion. 2013;13:92–103. doi: 10.1037/a0029431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li R, Brannon EM, Huettel SA. Children do not exhibit ambiguity aversion despite intact familiarity bias. Frontiers in psychology. 2014;5:1519. doi: 10.3389/fpsyg.2014.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spear LP. Adolescent neurodevelopment. Journal of Adolescent Health. 2013;52:S7–13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proceedings of the National Academy of Sciences USA. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, … Lee FS. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences USA. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Galván A, McGlennen KM. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. Journal of cognitive neuroscience. 2013;25:284–296. doi: 10.1162/jocn_a_00326. [DOI] [PubMed] [Google Scholar]
- 103.Reyna VF. A new intuitionism: Meaning, memory, and development in fuzzy-trace theory. Judgment and Decision Making. 2012;7:332. [PMC free article] [PubMed] [Google Scholar]
- 104.Rivers SE, Reyna VF, Mills B. Risk taking under the influence: a fuzzy-trace theory of emotion in adolescence. Developmental Review. 2008;28:107–144. doi: 10.1016/j.dr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christakou A. Present simple and continuous: Emergence of self-regulation and contextual sophistication in adolescent decision-making. Neuropsychologia. 2014;65:302–312. doi: 10.1016/j.neuropsychologia.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 106.Hartley CA, Somerville LH. The neuroscience of adolescent decision-making. Current opinion in behavioral sciences. 2015;5:108–115. doi: 10.1016/j.cobeha.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palminteri S, Kilford EJ, Coricelli G, Blakemore SJ. The Computational Development of Reinforcement Learning during Adolescence. PLoS Computational Biology. 2016;12:e1004953. doi: 10.1371/journal.pcbi.1004953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tversky A, Kahneman D. Environmental Impact Assessment, Technology Assessment, and Risk Analysis. Springer; Berlin Heidelberg: 1985. The framing of decisions and the psychology of choice; pp. 107–129. [Google Scholar]
- 109.Pachur T, Hertwig R, Gigerenzer G, Brandstätter E. Testing process predictions of models of risky choice: a quantitative model comparison approach. Frontiers in Psychology. 2013;4:646. doi: 10.3389/fpsyg.2013.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reyna VF, Brainerd CJ. Fuzzy-trace theory: An interim synthesis. Learning and individual Differences. 1995;7:1–75. [Google Scholar]
- 111.Hunt PS, Burk JA, Barnet RC. Adolescent transitions in reflexive and non-reflexive behavior: Review of fear conditioning and impulse control in rodent models. Neuroscience & Biobehavioral Reviews. 2016 doi: 10.1016/j.neubiorev.2016.06.026. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stanton ME, Fox GD, Carter CS. Ontogeny of the conditioned eyeblink response in rats: acquisition or expression? Neuropharmacology. 1998;37:623–632. doi: 10.1016/s0028-3908(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 113.Steinberg L, Belsky J. An Evolutionary Perspective on Psychopathology in Adolescence. In: Cicchetti D, Toth S, editors. Adolescence: Opportunities and challenges. Rochester, NY: University of Rochester Press; 1996. pp. 93–124. [Google Scholar]
- 114.Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzi JD. Adolescent Development of Forebrain Stimulant Responsiveness: Insights from Animal Studies. Annals of the New York Academy of Sciences. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- 115.Qin Y, Carter CS, Silk EM, Stenger VA, Fissell K, Goode A, Anderson JR. The change of the brain activation patterns as children learn algebra equation solving. Proceedings of the National Academy of Sciences USA. 2004;101:5686–56891. doi: 10.1073/pnas.0401227101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson C, Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Developmental Cognitive Neuroscience. 2011;1:540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Teslovich T, Mulder M, Franklin NT, Ruberry EJ, Millner A, Somerville LH, Simen P, … Casey BJ. Adolescents let sufficient evidence accumulate before making a decision when large incentives are at stake. Developmental Science. 2014;17:59–70. doi: 10.1111/desc.12092. [DOI] [PubMed] [Google Scholar]
- 118.McNamara JM, Houston AI. Integrating function and mechanism. Trends in ecology & evolution. 2009;24:670–675. doi: 10.1016/j.tree.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 119.McNamara JM, Fawcett TW, Houston AI. An adaptive response to uncertainty generates positive and negative contrast effects. Science. 2013;340:1084–1086. doi: 10.1126/science.1230599. [DOI] [PubMed] [Google Scholar]
- 120.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stevens MC, Skudlarski P, Pearlson GD, Calhoun VD. Age-related cognitive gains are mediated by the effects of white matter development on brain network integration. NeuroImage. 2009;48:738–746. doi: 10.1016/j.neuroimage.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cox SM, Andrade A, Johnsrude IS. Learning to like: A role for human orbitofrontal cortex in conditioned reward. J Neurosci. 2005;25:2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Delamater AR. The role of the orbitofrontal cortex in sensory-specific encoding of associations in pavlovian and instrumental conditioning. Ann NY Acad Sci. 2007;1121:152–173. doi: 10.1196/annals.1401.030. [DOI] [PubMed] [Google Scholar]
- 124.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Ann N Y Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meyer HC, Bucci DJ. Age differences in appetitive Pavlovian conditioning and extinction in rats. Physiology & Behavior. 2016;167:354–362. doi: 10.1016/j.physbeh.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bradshaw SE, Agster KL, Waterhouse BD, McGaughy JA. Age-related changes in prefrontal norepinephrine transporter density: The basis for improved cognitive flexibility after low doses of atomoxetine in adolescent rats. Brain Research. 2016;1641B:245–257. doi: 10.1016/j.brainres.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]