Abstract
Objectives
Among American Indians, prior research has found associations between early life trauma and the development of post-traumatic stress disorder (PTSD) in adulthood. Given the physiological changes associated with PTSD, early life trauma could indirectly contribute to chronic disease risk. However, the impact of early life trauma on adult physical health in this population has not been previously investigated.
Methods
We evaluated associations among early life trauma, PTSD, and 13 physiological biomarkers that index cardiovascular, metabolic, neuroendocrine, anthropometric, and immune function in adulthood by conducting correlation and structural equation modeling path analyses (N = 197). Physiological systems were analyzed individually as well as in a composite measure of allostatic load.
Results
We found early life trauma was related to PTSD, which in turn was related to elevated allostatic load in adulthood. Among the various components of allostatic load, the neuroendocrine system was the only one significantly related to early life stress and subsequent PTSD development.
Conclusions
Changes in allostatic load might reflect adaptive adjustments that maximize short-term survival by enhancing stress reactivity, but at a cost to later health. Interventions should focus on improving access to resources for children who experience early life trauma in order to avoid PTSD and other harmful sequelae.
Keywords: allostatic load, American Indian, PTSD, trauma
Introduction
There is growing interest in the hypothesis that exposure to stress in early life increases the risk of chronic disease in adulthood (Alastalo et al, 2013; Goodwin and Stein, 2004; Hochberg et al, 2011; Miller et al, 2011; Turner et al, 2016). This process might reflect developmental tradeoffs that maximize short-term survival at a cost to health in later life (Gluckman and Hanson, 2004). For example, in stressful environments, a stronger physiological response to stress might enable people to respond more quickly to threatening situations (Del Giudice et al, 2013). However, such adjustments might compromise long-term health, since elevated stress reactivity could contribute to hypertension, neuronal degradation, or atherosclerosis (Sapolsky, 2004).
This early life developmental model may be particularly useful for explaining high rates of chronic disease among populations with elevated exposure to stress in early life, such as American Indians (Duran et al, 2004; Sinclair et al, 2011). Research in this population has found specifically high rates of trauma exposure, which can be considered a category of extreme stress exposures. However, extant research has not evaluated whether early life trauma exposure plays a significant role in the development of poor physical health among American Indian adults. Given other well-studied risk factors for chronic disease in this population, including poverty and substance abuse (Holm et al, 2010), it is unclear whether childhood trauma has an independent impact. Better understanding of this potential association could guide new prevention strategies to improve physical health for American Indian adults.
Mental illness is a pathway by which early life trauma can affect adult health (Enoch, 2011; Nikulina and Widom, 2013). A landmark study in mental health research among American Indians, the American Indian Service Utilization, Psychiatric Epidemiology, Risk and Protective Factors Project (AI-SUPERPFP), demonstrated an association between early life trauma and psychiatric illness, including post-traumatic stress disorder (PTSD) (Libby et al, 2005). As a mental disorder that can develop after a traumatic experience, PTSD is associated with a unique neuroendocrine and neurobiological profile (Zoladz and Diamond, 2013). The physiological alterations associated with PTSD development are likely proximately associated with the development of physical health disorders, such as musculoskeletal pain, cardiorespiratory symptoms, and poor gastrointestinal health (Pacella et al, 2013). In addition, PTSD has been associated with a doubled risk of developing coronary heart disease, even among PTSD-discordant twins (Vaccarino et al, 2013). PTSD is more prevalent in American Indians than in the general US population; AI-SUPERPFP reported a lifetime prevalence of PTSD as high as 22.5% among women in one Southwest tribe, compared with 9.1% among non-American Indian women in the National Comorbidity Survey (Beals et al, 2005). It is therefore possible that early life trauma in American Indians contributes to poor physical health through the development of PTSD and accompanying physiological changes. However, no prior research has evaluated the potential relationship between early life trauma, PTSD, and adult physical health in this population.
The concept of allostasis, or stability through change, can assist our understanding of this relationship (Danese and McEwen, 2012). Allostasis refers to the body's ability to maintain physiological functioning in the face of changing environmental conditions. While such responsiveness is generally adaptive, early life trauma and PTSD are thought to contribute to wear and tear on biological systems. Known as allostatic load, this cumulative deterioration has negative effects on individual physiology and health (Stewart, 2006). Allostatic load is assessed by analyzing a combination of anthropometric, neuroendocrine, immune, and metabolic biomarkers. These biomarkers can predict mortality risk, declines in cognitive and physical functioning, cardiovascular disease events, and diabetes (Mattei et al, 2010; Seeman et al, 2010), providing a useful measure for assessing chronic disease risk in a pre-clinical sample. Allostatic load is associated with factors such as older age, lower socioeconomic status, and being single (Juster et al, 2010; Rainisch and Upchurch, 2013). While PTSD has been associated with physiological changes consistent with allostatic load, to our knowledge only one study has specifically assessed the relationship between these variables. Conducted in an all-female sample, that study found a positive association between PTSD and allostatic load (Glover, 2006). In addition, some evidence associates early life stress with heightened allostatic load in adolescence (Evans and Schamberg, 2009) and adulthood (Carroll et al, 2013; Danese and McEwen, 2012; Glover et al, 2010). However, no extant research has empirically evaluated the relationship between early life trauma and allostatic load among middle-aged American Indian adults. This research is especially pertinent for American Indians and other populations with high rates of poor health behaviors that independently contribute to chronic disease.
The current study builds on the results of AI-SUPERPFP and its follow-up study, Healing Hearts, by investigating the potential contribution of early life trauma to heightened allostatic load in a subset of the original study sample. We sought to determine whether early life trauma has a direct link with allostatic load, or whether the relationship is mediated by PTSD. Specifically, we simultaneously tested if: 1) early life trauma was related to PTSD, 2) PTSD was related to allostatic load, and 3) if early life stress was directly related to allostatic load or if the relationship was fully explained by PTSD (i.e., full mediation). The results of this analysis promise to contribute to our understanding of whether and how early life trauma experience may influence physical health in adulthood among American Indians. These findings can inform interventions aimed at lowering rates of chronic disease in this population.
Materials and Methods
Data for our analysis were based on the study sample assembled by Healing Hearts, which enrolled a subset of the original AI-SUPERPFP sample. AI-SUPERPFP was conducted in 1997-1999 to evaluate the prevalence of mental health issues in a large sample of American Indians from the Northern Plains and the Southwest. Study methods were described in depth in a previous publication (Beals et al, 2003).
Healing Hearts, which was limited to a single Northern Plains site, compared participants with PTSD to non-PTSD controls who were matched for age, sex, and tribal affiliation. Approximately half of the Healing Hearts sample (90 of 197) had been diagnosed with lifetime PTSD. Exclusion criteria for Healing Hearts were overt cardiovascular disease, unstable angina, myocardial infarction in the past week, severe obstructive lung disease, decompensated heart failure, severe coronary disease, severe stroke, and other medical conditions that might compromise participant safety during travel to the study site in Aurora, Colorado. Oral steroids and medications such as theophylline or aminophylline were also criteria for exclusion. As a secondary data analysis of both AI-SUPERPFP and Healing Hearts study datasets, the present study received approval from the Colorado Multiple Institutional Review Board (#03-1028).
Study Measures
Early life trauma was assessed by an 11-item questionnaire during the AI-SUPERPFP study. It is analyzed in the present study as a continuous measure indexing reported exposure to various types of trauma before age 13. Exposures were categorized as direct trauma exposure, exposure to traumatic news, and witnessing violence (Table 3) (Whitesell et al, 2009). Each affirmative response to an exposure was scored as 1, and points were summed across items. Early life trauma subscales were analyzed both individually and in combination to create a total early life stress trauma, as analyzed in previous AI-SUPERPFP publications (Libby et al, 2005; Whitesell et al, 2009).
Table 3.
Summary of reported early life trauma exposures in study sample
| Trauma category | No. (%) |
|---|---|
| Experienced traumas | |
| In a disaster | 15 (8%) |
| Life threatening accident | 4 (2%) |
| Raped | 12 (6%) |
| Touched or forced to touch someone sexually | 15 (8%) |
| Physical abuse from parents | 29 (15%) |
| Physical abuse from other | 7 (4%) |
| Traumatic news | |
| Someone close in life threatening situation | 8 (4%) |
| Someone close sexually abused | 9 (5%) |
| Someone close committed suicide | 13 (7%) |
| Witnessed violence | |
| Witnessed someone being raped | 6 (3%) |
| Witnessed family violence | 66 (34%) |
| Participants with ≥ 1 exposure | 95 (48%) |
PTSD status was originally diagnosed through a computer-assisted structured interview in the AI SUPERPFP study by using criteria from the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV). This diagnosis was re-assessed prior to enrollment in Healing Hearts using the Structured Clinical Interview for DSM-IVR to evaluate current PTSD diagnosis. This meant that participants who were classified as having PTSD had both lifetime and current diagnoses, while those in the non-PTSD group had neither lifetime nor current diagnoses. To meet DSMIVR criteria, participants had to exhibit intrusion symptoms, avoidance, and enhanced arousal.
Biomarker measurements were conducted on samples collected and analyzed by the Healing Hearts study. Participants traveled to the University of Colorado Anschutz Medical School for a three-day visit. Anthropometric data, including height, weight, and waist circumference, were collected by physical examination. Blood pressure was collected in duplicate during the afternoon on two consecutive days, with the measures averaged across collection points. To measure blood lipids, blood was drawn at 8am following an overnight fast. Participants were instructed to sit quietly for 30 minutes before the blood draw. Lipids were analyzed by using a Hitachi 747-200 automated analyzer. Total cholesterol was measured by using cholesterol oxidase colorimetry. High density lipoprotein (HDL) cholesterol was measured colorimetrically by using polyethylene glycol-modified cholesterol esterase and cholesterol oxidase to convert HDL-cholesterol esters to 4-cholesternon and hydrogen peroxide. Glycosylated hemoglobin levels were determined in ethylenediaminetetraacetic acid-anticoagulated whole blood (3ml) by fluorescence microparticulate enzyme immunoassay with the Abbot Laboratories IMX automated immunoassay analyzer. Fibrinogen was analyzed by using a prothrombin time-based assay. C-reactive protein was measured by using the Stachrom Protein C kit from Diagnostica Stago. Dehydroepiandrosterone sulfate (DHEA-S) was assayed by radioimmunoassay with Diagnostic Systems Laboratories. Epinephrine and norepinephrine were analyzed by using a high-performance liquid chromatographic method involving electrochemical detection.
Allostatic load was assessed with 13 measures that represent the functioning of all physiological systems identified in a comprehensive review as important indices for this variable (Juster et al, 2010). The 13 measures represent the following systems: cardiovascular (systolic and diastolic blood pressure), immune (C-reactive protein, interleukin-6 (IL-6), and fibrinogen), metabolic (HDL, HDL to total cholesterol ratio, and glycosylated hemoglobin), neuroendocrine (norepinephrine, epinephrine, and DHEA-S), and anthropometric (body mass index and waist to hip ratio). Consistent with prior studies that utilize the allostatic load concept (Beckie, 2012), each component of allostatic load was divided into quartiles. For each biomarker, participants in the top quartile (or the bottom quartile in the case of DHEA-S and HDL-cholesterol) received a score of 1 (Juster et al, 2010). Scores were then summed across each system type (neuroendocrine, metabolic, immune, and anthropometric) to create a total score for allostatic load. The total allostatic load score and the score for each system type were analyzed as continuous variables.
Covariates included data on age, education, marital status, adult stressful events, and substance abuse, which were extracted from questionnaires administered by AI-SUPERPFP and analyzed in statistical models by the present study. Following the methodology of Libby et al. (2008), adult traumatic events were assessed by using the same 11 questions as the early life trauma measure, but were limited to traumas that occurred when participants were at least 18 years old (Whitesell et al, 2009). The adult trauma variable was analyzed as the total number of exposures across the three categories of trauma exposure (direct trauma exposure, traumatic news, and witnessing violence). Substance disorders for alcohol and other drugs were categorized by using criteria previously published for this sample (Libby et al, 2008; Mitchell et al, 2003). Participants were first asked if they had ever used one or more of 8 different types of drugs (excluding alcohol); those who answered affirmatively were asked about lifetime and past-year patterns of abuse. Drug use and dependence were assessed by using DSM-IV criteria and combined into a single drug disorder variable. For alcohol use, all participants who provided a year of first drink were asked, “In any 1-year period of your entire life, did you have at least 12 drinks of any kind of alcoholic beverage?” Those answering affirmatively were further screened to diagnose alcohol abuse and dependence, which were combined into a single alcohol disorder variable.
Current smoking status, wake and sleep time, and data on use of prescription medications for psychiatric and cardiovascular conditions were also considered covariates. These data were collected during the Healing Hearts study. For wake and sleep time, participants were asked for the average time they woke and went to sleep on weeknights. Since altered sleep patterns are known to affect many of the biomarkers of interest, an “altered sleep” category, which could reflect either shift work or other unusual sleep patterns, was created for participants who reported going to sleep after 3am. Participants who were taking medications for treatment of lipids, depression, diabetes, and hypertension were considered to be on medication. Lastly, biological sex was included as a covariate since there is evidence to suggest that males may be more sensitive to stressors experienced in early life than females (Stinson, 1985).
Data Analysis
In the present study all continuous variables were initially assessed for normality. Correlation analyses, linear regression, and structural equation modeling path analyses were used to evaluate our study question. Examining mediation in a path analysis does not require a significant relationship between early life trauma and allostatic load (MacKinnon & Fairchild, 2009; MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002), particularly among subgroups of participants (e.g., those reporting PTSD vs. those reporting no PTSD) for whom the mediated effect might be of the opposite sign (MacKinnon & Fairchild, 2009). Nonetheless, correlation analysis was first used to describe the bivariate relationships among early life trauma, PTSD, allostatic load, and sociodemographic and clinical variables. Path analyses were then used to determine if the link between stress in early life and allostatic load in adulthood was mediated by adult PTSD. One assumption of a single-mediator model is the absence of an interaction effect between the independent variable (early life trauma) and the mediating variable (PTSD) on the dependent variable (allostatic load) (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). This interaction was tested and found to be nonsignificant (unstandardized regression coefficient = −.11 (SE = .72), P = .88).
Path analyses were also used to analyze the three categories of early life trauma (direct trauma exposure, witnessed violence, and violent news) and allostatic load (cardiovascular, metabolic, neuroendocrine, inflammatory, and anthropometric markers). Saturated models were first run to examine mediation while controlling for all covariates. Reduced models were then run, including only covariate paths that were statistically significant in at least one model. Mplus was used for the path analyses using robust maximum likelihood estimation (Muthén and Muthén, 1998). Missing data were estimated using full information maximum likelihood estimation (90% covariance coverage). Unstandardized regression path coefficients are reported, because the primary mediating variable (PTSD) is dichotomous, while the dependent variables across the models are continuous.
Results
The sample was primarily female and middle-aged (Table 1). Participants who experienced early life trauma had higher rates of lifetime substance abuse disorders and PTSD, but the two study groups (PTSD vs. no PTSD) were otherwise similar across potential covariates. Tables 2 and 3 provide summaries of allostatic load scores and early life trauma exposures, respectively.
Table 1.
Summary statistics of study sample
| Total sample (N = 197) | No early life trauma (N =102) | Early life trauma (N = 95) | |
|---|---|---|---|
| Age, y | 44.1 (10.3) | 44.8 (10.1) | 43.4 (10.5) |
| Women, No. (%) | 143 (73%) | 71 (70%) | 72 (76%) |
| College graduates (%) | 19 (10%) | 9 (9%) | 10 (10%) |
| Married (%) | 63 (32%) | 34 (33%) | 29 (31%) |
| Substance abuse (%) | 82 (42%) | 27(27%) | 55 (58%)* |
| Smoker (%) | 147 (75%) | 76 (75%) | 71 (75%) |
| Altered sleep schedule (%) | 8 (4%) | 3 (3%) | 5 (5%) |
| Adult trauma exposure | 155 (79%) | 67 (66%) | 88 (93%)* |
| Current PTSD (%) | 90 (46%) | 33 (32%) | 57 (60%)* |
P < 0.001 in two-sided t-test for continuous variables, Pearson chi-square test for categorical variables comparing participants who did and did not experience early life trauma
Table 2.
Summary of allostatic load measures and cut-points
| Biomarker | Sample Mean (SD) | Cut-point |
|---|---|---|
| Cardiovascular | ||
| Systolic blood pressure, mm Hg | 131.5 (17.4) | >141 |
| Diastolic blood pressure, mm Hg | 79.4 (11.3) | >86.5 |
| Neuroendocrine | ||
| Epinephrine, pg/mL | 49.9 (86.9) | >34.0 |
| Norepinephrine, ug/L | 334.9 (205.7) | >32.2 |
| DHEA-S, ug/dL | 142.1 (94.9) | <73 |
| Inflammation | ||
| Il-6, pg/mL | 4.5 (8.5) | >4.4 |
| C Reactive Protein (in scale), ln(mg/L) | 6.5 (11.8) | >7.8 |
| Fibrinogen, mg/dL | 381.9 (89.4) | >430 |
| Metabolism | ||
| HDL, mg/DL | 47.1 (13.0) | <39 |
| HDL/TC ratio | 0.27 (0.07) | >0.31 |
| HbA1c, % | 6.3 (1.7) | >6.4 |
| Anthropometric | ||
| Waist to hip ratio | 0.95 (0.08) | >1.0 |
| Body mass index, kg/m2 | 32.0 (7.8) | >36.5 |
| Allostatic load | 3.3 (2.3) | ------- |
The bivariate relationships between early life trauma, PTSD, allostatic load, and sociodemographic and clinical variables are shown in Supplemental Table 1. Early life trauma was significantly correlated with adult trauma, PTSD, and substance abuse (all P < 0.001), but not with allostatic load (r = 0.02, p =0.81). Adult trauma and PTSD were significantly correlated with each other and with substance abuse (all P < 0.003). The dependent variable, allostatic load, was also significantly correlated with PTSD and medication use (both P < 0.003).
Path analysis assessed whether PTSD mediated the relationship between early life trauma and allostatic load (Figure 1). The path model for allostatic load demonstrated that early life trauma was related to PTSD (unstandardized regression coefficient = .32 (SE = .14), p = .02; OR = 1.38, 95%, CI = 1.04, 1.82), which in turn was related to allostatic load (unstandardized regression coefficient = .72 (SE = .36), p = .045). However, early life trauma was not directly related to allostatic load (unstandardized regression coefficient = .14 (SE = .10), p = .17). Therefore, PTSD fully mediated the relationship between these two variables. Analysis of the three categories of trauma exposure found that direct trauma exposure was significantly related to allostatic load (Figure 2), whereas witnessing violence and receiving traumatic news were not (Supplemental Figures 1-2).
Figure 1.
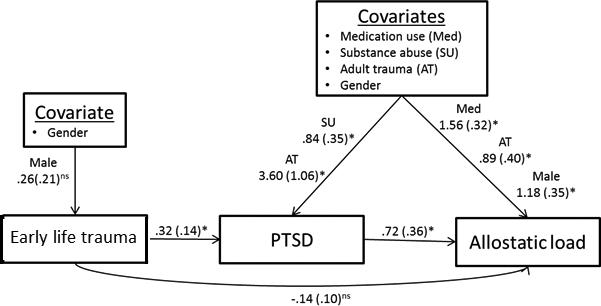
Early life trauma-Allostatic load final path model with reduced covariates. Covariates that were statistically significantly related to at least one primary variable were included in the model. For presentation purposes, mainly statistically significant covariate paths are reported. Unstandardized regression coefficients are reported.
Figure 2.
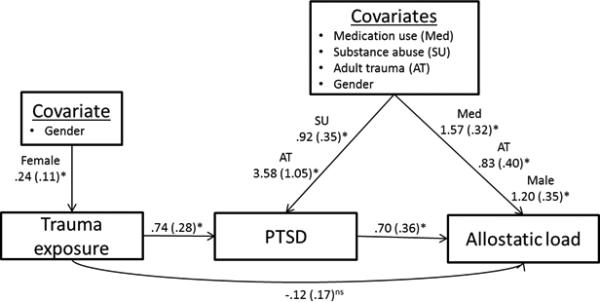
Trauma exposure-Allostatic load final path model with reduced covariates. For presentation purposes, mainly statistically significant covariate paths for the covariates are presented. Unstandardized regression coefficients are reported.
Finally, in analyses of the subscales of allostatic load, paths in mediation models were statistically significant only for the neuroendocrine summary score (Figure 3). Supplementary Figures 3-6 present the non-significant path models for early life trauma and the other allostatic load subscales (anthropometric, metabolic, inflammatory, and cardiovascular systems).
Figure 3.
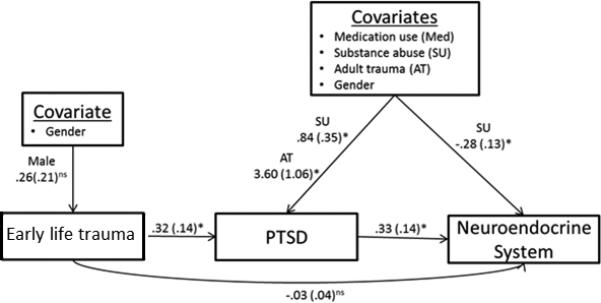
Early life trauma-Neuroendocrine System final path model with reduced covariates. Covariates that were statistically significantly related to at least one primary variable were included in the model. For presentation purposes, mainly statistically significant covariate paths are reported. Unstandardized regression coefficients are reported.
Discussion
Prior research in this study sample was pivotal in demonstrating the high prevalence of mental disorders, such as PTSD among American Indians, as well as the relationship between early life trauma and the development of these disorders (Beals et al, 2013; Libby et al, 2005). Here we build on those findings by establishing that individuals who experience early life trauma and subsequently develop PTSD have higher allostatic load, an index of physiological functioning, and that these effects are independent of other risk factors such as substance abuse, medication use, and education level.
Our measure of allostatic load reflects functioning in multiple physiological systems. However, our analysis of subcategories suggests that, at least by middle age, neuroendocrine markers associated with stress physiology are most strongly associated with early life trauma and PTSD. These findings are consistent with the hypothesis that stress physiology is very sensitive to trauma and other types of stress exposure in early life (Danese and McEwen, 2012; Del Giudice et al, 2013), particularly when such exposures occur in people who eventually develop PTSD. Our findings also accord with previous work that characterizes PTSD by alterations in neuroendocrine functioning (Zoladz and Diamond, 2013). Although we did not find associations between early life trauma and other subsystems of allostatic load, altered neuroendocrine activity could contribute to poor functioning in those subsystems over time. For example, lower levels of DHEA-S have been associated with higher risk of cardiovascular disease in postmenopausal women (Shufelt et al, 2010), and altered catecholamine levels can contribute to changes in immune function (Padgett and Glaser, 2003). It is therefore possible that the strength of the association between early life trauma, PTSD, and allostatic load, as measured in the other physiological systems, will increase as affected people grow older.
Early life stress, including that not specific to trauma, has been theorized to contribute to elevated allostatic load in adulthood (Danese and McEwen, 2012). Consistent with this hypothesis, one study of students in New York State found that adversity in childhood was associated with elevated allostatic load in adolescence (Evans et al, 2007). However, ours is the first study to empirically evaluate the relationship between early life stress and allostatic load in middle-aged American Indian adults. Notably, we controlled for adult exposure to stress, which prior research has shown to be greater in American Indians than in the general US population (Manson et al, 2005). Controlling for these exposures in analyses strengthens our finding that early life trauma had an independent impact on allostatic load among study participants. Nevertheless, given the robust correlation between early life trauma and adult trauma in our sample, it is likely that these exposures worked in concert to influence PTSD and increase allostatic load for most participants. Other literature suggests that chronic stress exposure in adults who also experienced chronic stress as children can have a significant effect on allostatic load and other health measures (Geronimus et al, 2006; Seeman et al, 2010). Importantly, a recent study found that the relationship between early life adversity and allostatic load was similarly not removed after adjusting for adult socioeconomic status and stress, while the relationship between early life adversity and doctor diagnosed health status and self-rated health were (Turner et al, 2016). This suggests that allostatic load may be particularly sensitive to early life trauma or other adverse experiences. However additional longitudinal research is needed to understand the independent or interactive role of exposures across the life course in shaping long-term physiological functioning.
Clinically, the results of this study suggest that screening for early life trauma and PTSD in American Indians might enable early identification and treatment of PTSD, potentially forestalling later morbidity. Several empirically supported treatments have been developed for PTSD in children and adults, and mental health systems increasingly focus on assessing and treating trauma in people of all ages (Foa et al, 2008; Pollio et al, 2014). Culturally acceptable efforts to prevent and treat PTSD are clearly needed for everyone exposed to early life stress.
A key strength of this study is our unique dataset. We used biomarker data collected for the Healing Hearts study to build on prior research with the same sample demonstrating a link between childhood stress and adult mental health. The high frequency of PTSD in this sample enabled us to evaluate whether PTSD mediated the relationship between early life stress and allostatic load. In addition, biomarker data were collected in a laboratory under controlled, standardized conditions, enhancing the reliability of markers sensitive to diurnal and activity-based variation.
Notably, there are many different ways to measure allostatic load (Juster et al, 2010). In addition to choosing different variables for inclusion, studies also sometimes consider the use of clinical cut points or sex-specific cut-points. Neither substituting clinical cut points that were below our cut off values (e.g. BMI, CRP, HDL, systolic and diastolic blood pressure) (Dobbelsteyn et al, 2001; Palaniappan et al, 2004; Ridker, 2003) nor generating sex-specific cut-points significantly changed our study results (data not shown). In addition, some studies choose to classify individuals who are on medication for specific disorders as having an elevated allostatic load score. Scoring individuals who were on medications for hypertension and dyslipidemia as having an elevated allostatic load score for systolic/diastolic blood pressure and HDL/HDL to total cholesterol ratio, respectively, also did not significantly change our results (data not shown).
While this analysis is the first to evaluate the relationship between early life trauma and adult allostatic load in American Indians, we note several limitations. First, early life trauma exposures were characterized only by retrospective data, which can be prone to bias. However, prior longitudinal research on child abuse has found that people tend to under-report rather than over-report abuse, a trend that would weaken rather than strengthen any proposed relationship between reported early life trauma and allostatic load (Keyes et al, 2011).
Second, the long-distance travel required for biomarker collection might have biased the Healing Hearts study sample, since the sickest participants in AI-SUPERPFP were the most likely to be excluded on the grounds of inability to travel. Again, this factor would likely weaken any statistical relationship between early life trauma and allostatic load.
Third, our study sample was primarily female. Nonetheless, menopausal status was not assessed by any measures, even though such status might have affected certain biomarkers under consideration. In addition, prior research suggests that men tend to be more sensitive than women to early environmental experiences (Stinson, 1985; Weinstock, 2007). It is therefore possible that our results err on the side of conservativism, since a sample with a better gender balance might have revealed even stronger associations among childhood trauma, PTSD, and allostatic load.
In conclusion, our findings indicate that early life trauma, when resulting in the development of PTSD, is an important contributor to elevated allostatic load among American Indian adults. This contribution is independent of the effects of substance abuse as well as of similar trauma exposures in adulthood. Our findings are consistent with a growing literature suggesting that early life should be considered a sensitive period during which the effects of environmental exposures on human biology and physiological functioning are amplified. This process might represent an evolved capacity that emerged to facilitate adaptation to varying environmental conditions, but became maladaptive in contemporary society, where psychosocial stress is persistently and disproportionately experienced by socially disadvantaged populations (Thayer and Kuzawa, 2011). Our results imply that interventions aimed at improving social environments and childhood development are more likely to optimize physiological functioning and well-being than interventions begun in adulthood (Shonkoff et al, 2009).
Supplementary Material
Acknowledgements
We thank the study participants and the participating tribes for their contribution to this research. Dr. Mark Laudenslagger, Dr. Raymond Harris, and two anonymous reviewers provided helpful feedback on earlier drafts of this manuscript. Dr. Ursula Running Bear provided helpful feedback on the dataset. This project was funded by NHLI R01 HL073824 and GCRC NIH grant M01 #RR00051. Thayer was supported by P20 MD006871 while working on this study.
Footnotes
Author contributions
ZT and CBL analyzed the data for the analysis. ZT, CBL, LN, and MM drafted the manuscript. DB and SM designed the original study, lead data collection, and provided critical comments on the manuscript.
We have no conflicts of interest to declare.
References
- Alastalo H, von Bonsdorff MB, Räikkönen K, Pesonen A-K, Osmond C, Barker DJP, Heinonen K, Kajantie E, Eriksson JG. Early Life Stress and Physical and Psychosocial Functioning in Late Adulthood. PloS one. 2013;8(7):e69011. doi: 10.1371/journal.pone.0069011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals J, Belcourt-Dittloff A, Garroutte E, Croy C, Jervis L, Whitesell N, Mitchell C, Manson S. Trauma and conditional risk of posttraumatic stress disorder in two American Indian reservation communities. Soc Psychiatry Psychiatr Epidemiol. 2013;48(6):895–905. doi: 10.1007/s00127-012-0615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals J, Manson SM, Mitchell CM, Spicer P, Team A-S. Cultural specificity and comparison in psychiatric epidemiology: walking the tightrope in American Indian research. Culture, Medicine and Psychiatry. 2003;27(3):259–289. doi: 10.1023/a:1025347130953. [DOI] [PubMed] [Google Scholar]
- Beals J, Novins DK, Whitesell NR, Spicer P, Mitchell CM, Manson SM. Prevalence of mental disorders and utilization of mental health services in two American Indian reservation populations: mental health disparities in a national context. American Journal of Psychiatry. 2005;162(9):1723–1732. doi: 10.1176/appi.ajp.162.9.1723. [DOI] [PubMed] [Google Scholar]
- Beckie TM. A Systematic Review of Allostatic Load, Health, and Health Disparities. Biological Research For Nursing. 2012;14(4):311–346. doi: 10.1177/1099800412455688. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proceedings of the National Academy of Sciences. 2013;110(42):17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. Making Sense of Stress: An Evolutionary—Developmental Framework. Adaptive and Maladaptive Aspects of Developmental Stress. Springer; 2013. pp. 23–43. [Google Scholar]
- Dobbelsteyn C, Joffres M, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. International Journal of Obesity & Related Metabolic Disorders. 2001;25(5) doi: 10.1038/sj.ijo.0801582. [DOI] [PubMed] [Google Scholar]
- Duran B, Malcoe LH, Sanders M, Waitzkin H, Skipper B, Yager J. Child maltreatment prevalence and mental disorders outcomes among American Indian women in primary care. Child Abuse & Neglect. 2004;28(2):131–145. doi: 10.1016/j.chiabu.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Enoch M-A. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Keane TM, Friedman MJ, Cohen JA. Guilford Press; 2008. Effective treatments for PTSD: practice guidelines from the International Society for Traumatic Stress Studies. [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96(5) doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DA. Allostatic load in women with and without PTSD symptoms. Annals of the New York Academy of Sciences. 2006;1071(1):442–447. doi: 10.1196/annals.1364.039. [DOI] [PubMed] [Google Scholar]
- Glover DA, Loeb TB, Carmona JV, Sciolla A, Zhang M, Myers HF, Wyatt GE. Childhood sexual abuse severity and disclosure predict posttraumatic stress symptoms and biomarkers in ethnic minority women. Journal of Trauma & Dissociation. 2010;11(2):152–173. doi: 10.1080/15299730903502920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the Past: Evolution, Development, and Patterns of Disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychological medicine. 2004;34(03):509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel J-C, Boileau P, Le Bouc Y, Deal C, Lillycrop K. Child health, developmental plasticity, and epigenetic programming. Endocrine Reviews. 2011;32(2):159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm JE, Vogeltanz-Holm N, Poltavski D, McDonald L. Assessing health status, behavioral risks, and health disparities in American Indians living on the northern plains of the US. Public Health Reports. 2010;125(1):68. doi: 10.1177/003335491012500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Keyes K, Hatzenbuehler M, Hasin D. Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors. Psychopharmacology. 2011;218(1):1–17. doi: 10.1007/s00213-011-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby AM, Orton HD, Beals J, Buchwald D, Manson SM. Childhood abuse and later parenting outcomes in two American Indian tribes. Child Abuse & Neglect. 2008;32(2):195–211. doi: 10.1016/j.chiabu.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby AM, Orton HD, Novins DK, Beals J, Manson SM. Childhood physical and sexual abuse and subsequent depressive and anxiety disorders for two American Indian tribes. Psychological Medicine. 2005;35(3):329–340. doi: 10.1017/s0033291704003599. [DOI] [PubMed] [Google Scholar]
- Manson SM, Beals J, Klein SA, Croy CD, team A-S. Social epidemiology of trauma among 2 American Indian reservation populations. American journal of public health. 2005;95(5):851. doi: 10.2105/AJPH.2004.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Social science & medicine. 2010;70(12):1988–1996. doi: 10.1016/j.socscimed.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological bulletin. 2011;137(6):959. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CM, Beals J, Novins DK, Spicer P. Drug use among two American Indian populations: prevalence of lifetime use and DSM-IV substance use disorders. Drug and Alcohol Dependence. 2003;69(1):29–41. doi: 10.1016/s0376-8716(02)00253-3. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. Statistical analyses with latent variables User's guide 3. 1998.
- Nikulina V, Widom C. Child neglect, race, childhood family and neighborhood poverty and adult physical health: does mental health mediate or moderate these relations? Comprehensive Psychiatry. 2013;54(1):e7–e8. [Google Scholar]
- Pacella ML, Hruska B, Delahanty DL. The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. Journal of anxiety disorders. 2013;27(1):33–46. doi: 10.1016/j.janxdis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Glaser R. How stress influences the immune response. Trends in immunology. 2003;24(8):444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Palaniappan L, Carnethon MR, Wang Y, Hanley AJG, Fortmann SP, Haffner SM, Wagenknecht L. Predictors of the Incident Metabolic Syndrome in Adults. The Insulin Resistance Atherosclerosis Study. 2004;27(3):788–793. doi: 10.2337/diacare.27.3.788. [DOI] [PubMed] [Google Scholar]
- Pollio E, Mclean M, Behl L, Deblinger E. Trauma-Focused Cognitive Behavioral Therapy. Treatment of Child Abuse: Common Ground for Mental Health, Medical, and Legal Practitioners. 2014;31 [Google Scholar]
- Rainisch BKW, Upchurch DM. Sociodemographic Correlates of Allostatic Load Among a National Sample of Adolescents: Findings From the National Health and Nutrition Examination Survey, 1999–2008. Journal of Adolescent Health. 2013;53(4):506–511. doi: 10.1016/j.jadohealth.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Social status and health in humans and other animals. Annual Review Of Anthropology. 2004;33(1):393–418. [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186(1):223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce W, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, Braunstein GD, Pepine CJ, Bittner V, Vido DA. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health—National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women's Ischemia Syndrome Evaluation (WISE). The Journal of Clinical Endocrinology & Metabolism. 2010;95(11):4985–4992. doi: 10.1210/jc.2010-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair KA, Bogart A, Buchwald D, Henderson JA. The Prevalence of Metabolic Syndrome and Associated Risk Factors in Northern Plains and Southwest American Indians. Diabetes Care. 2011;34(1):118–120. doi: 10.2337/dc10-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. The Detrimental Effects of Allostasis: Allostatic Load as a Measure of Cumulative Stress. Journal of Physiological Anthropology. 2006;25:133–145. doi: 10.2114/jpa2.25.133. [DOI] [PubMed] [Google Scholar]
- Stinson S. Sex differences in environmental sensitivity during growth and development. American Journal of Physical Anthropology. 1985;28(S6):123–147. [Google Scholar]
- Thayer ZM, Kuzawa CW. Biological memories of past environments: epigenetic pathways to health disparities. Epigenetics. 2011;6(7):798–803. doi: 10.4161/epi.6.7.16222. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Thomas CS, Brown TH. Childhood adversity and adult health: Evaluating intervening mechanisms. Social Science & Medicine. 2016;156:114–124. doi: 10.1016/j.socscimed.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, Bremner JD. Posttraumatic Stress Disorder and Incidence of Coronary Heart Disease: A Twin Study. Journal of the American College of Cardiology. 2013;62(11) doi: 10.1016/j.jacc.2013.04.085. 10.1016/j.jacc.2013.1004.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochemical Research. 2007;32(10):1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Whitesell NR, Beals J, Mitchell CM, Manson SM, Turner RJ. Childhood exposure to adversity and risk of substance-use disorder in two American Indian populations: the meditational role of early substance-use initiation. Journal of studies on alcohol and drugs. 2009;70(6):971. doi: 10.15288/jsad.2009.70.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Current status on behavioral and biological markers of PTSD: A search for clarity in a conflicting literature. Neuroscience & Biobehavioral Reviews. 2013;37(5):860–895. doi: 10.1016/j.neubiorev.2013.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


