Abstract
Leishmania amazonensis is the etiological agent of diffuse cutaneous leishmaniasis. The immunopathology of leishmaniasis caused by L. amazonensis infection is dependent on the pathogenic role of effector CD4+ T cells. Purinergic signalling has been implicated in resistance to infection by different intracellular parasites. In this study, we evaluated the role of the P2X7 receptor in modulating the immune response and susceptibility to infection by L. amazonensis. We found that P2X7-deficient mice are more susceptible to L. amazonensis infection than wild-type (WT) mice. P2X7 deletion resulted in increased lesion size and parasite load. Our histological analysis showed an increase in cell infiltration in infected footpads of P2X7-deficient mice. Analysis of the cytokine profile in footpad homogenates showed increased levels of IFN-γ and decreased TGF-β production in P2X7-deficient mice, suggesting an exaggerated pro-inflammatory response. In addition, we observed that CD4+ and CD8+ T cells from infected P2X7-deficient mice exhibit a higher proliferative capacity than infected WT mice. These data suggest that P2X7 receptor plays a key role in parasite control by regulating T effector cells and inflammation during L. amazonensis infection.
Keywords: ATP, P2X7, L. amazonensis, T cells, Macrophages
Introduction
Leishmaniasis is a parasitic disease transmitted by the bite of infected sandflies of the genus Phlebotomus in the Old World and the genus Lutzomyia in the New World. Eleven million people are infected by the Leishmania parasite worldwide, and 2 million new infections are reported annually [1]. Approximately 90 % of all Leishmania infections are cutaneous forms; they are characterized as multilocular, chronic or mucocutaneous and are caused by species such as L. braziliensis, L. guyanensis and L. amazonensis in Brazil, Peru and Bolivia [2]. During transmission, the sandflies inoculate the infective forms of Leishmania, named promastigotes, into the skin, and the dermal macrophages internalize them. Promastigotes become amastigotes inside the macrophages and multiply intracellularly until the cells are disrupted [1]. The high capacity of L. amazonensis to evade the microbicidal effects of macrophages appears to be the most important factor that allows this parasite to sustain the infection. Nitric oxide (NO) and reactive oxygen species (ROS) are microbicidal molecules that are required to promote the resolution of in vitro infection [3]. However, L. amazonensis has been described as resistant to ROS-induced death [4].
Extracellular nucleotides and their metabolites have been recognized as highly important signalling molecules that can mediate an array of physiological processes, including pain sensation and immune responses. These extracellular nucleotides trigger multiple downstream events by binding to cell surface receptors called P2 nucleotide receptors, which are divided into two major subfamilies: G-protein-coupled P2Y receptors that can bind ATP, ADP, UTP, UDP and UDP-glucose and ionotropic P2X receptors that are activated by ATP [5]. Most P2X and P2Y receptor subtypes are expressed in immune cells [6]. The stimulation of P2X receptors, in particular P2X7, is a key process in a variety of inflammatory conditions and responses to infectious agents, such as Mycobacterium tuberculosis, Chlamydia trachomatis and Toxoplasma gondii [7–11]. P2X7 receptor stimulation leads to the activation of multiple immune signalling pathways, including the MyD88/NFκB pathway and the phospholipase D pathway [12]. In addition, this receptor induces ROS production and apoptosis, mechanisms that have important anti-pathogenic roles [13]. ATP also rapidly induces autophagy in human and mouse macrophages, resulting in mycobacterial killing in a process dependent on Ca++ influx [14].
Previous studies from our group have already demonstrated that ATP induces resistance in macrophages infected by L. amazonensis through the stimulation of the P2X7 receptor [15] via a mechanism involving ROS and leukotriene B4 (LTB4) production, which is dependent on 5-lipoxygenase in the host cells [16]. In vivo infection by L. amazonensis leads to different immune responses compared to infection by other species that cause cutaneous leishmaniasis, such as L. major. The Th1/Th2 dichotomy is not observed in L. amazonensis infection because a mixed frequency of CD4+ T cells producing IFN-γ and IL-4 is found during the infection [17]. Interestingly, effector CD4+ T cells are associated with disease pathology [18], while regulatory T cells are associated with lesion resolution [19].
Considering that (i) P2X7 receptor has a role in host resistance against a wide variety of microbial pathogens, (ii) P2X7 activation regulates T cell activation and proliferation, and (iii) the immune response against L. amazonensis infection depends on the pathogenic role of effector T cells, in the present study, we investigated whether the P2X7 receptor modulates inflammatory responses during in vivo infection by L. amazonensis using P2X7-deficient mice.
Materials and methods
Parasites
Amastigotes of L. amazonensis (strain MHOM/BR/75/Josefa) were isolated from a mouse lesion and allowed to transform into axenic promastigotes by maintenance at 27 °C in 119 medium (M199) (Sigma) supplemented with 10 % heat-inactivated foetal bovine serum (FBS, Cultilab), 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 % hemin (Sigma).
Animals
Male C57BL/6 mice (WT) and P2X7 receptor knockout (KO) mice (originally from the Jackson Laboratory, USA) were bred in the Animal House of Transgenic Mice of Federal University of Rio de Janeiro. Mice aged 8–10 weeks were used for the experiments. The animals were maintained at 22 °C in a 12-h light/dark cycle. The Commission for Ethical Use of Research Animals (CEUA) of Federal University of Rio de Janeiro approved the animal experimentation protocols under number IBCCF154.
Macrophage infection
Peritoneal macrophages were isolated from WT and KO mice and plated (106 cells) in 24-well plate dishes containing glass slides for 1 h at 37 °C. Then, the cultures were washed with warm PBS to remove nonadherent cells and maintained in Gibco® Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % FBS (Cultilab), 100 U/ml penicillin, and 100 μg/ml streptomycin for 24 h at 37 °C. The cells were incubated with L. amazonensis promastigotes at a ratio of 1:5 for 4 h at 37 °C in 5 % CO2. The cellular monolayers were washed with PBS to remove free parasites and fixed with 4 % formaldehyde for 30 min before staining with May-Grumwald-Giemsa (Panótico Rápido, Laborclin). The same staining was performed after 24 h of infection in the cells maintained at 37 °C. The cells were photographed under an optical microscope, and the parasite loads were estimated by counting at least two hundred macrophages. The index of infection was calculated by this formula: (% of infected macrophages × parasites/infected macrophages) / 100.
In vivo infection
P2X7 KO and WT mice were infected subcutaneously with 1 × 106 L. amazonensis promastigotes in the right footpad. The lesion was measured every 7 days with a dial calliper (Mitutoyo) and expressed as the difference between the thickness of the infected and uninfected paws [20, 21]. At 47 days postinfection (DPI), the mice were euthanized, and their footpads and popliteal lymph nodes were removed for analysis. The infected paw was cut off, weighed and individually homogenized in 1 mL of supplemented M199 using a tissue grinder. The number of viable cells was determined by a haemocytometer using Trypan blue exclusion. Parasite load was then analysed by the limiting dilution assay [22]. Briefly, under sterile conditions, serial fourfold dilutions were prepared and distributed in 96-well microtiter plates in triplicate. After 14 days of incubation at 27 °C, the wells were examined with an inverted microscope at ×200 magnification for the presence or the absence of promastigotes. The final titre was the last dilution for which the well contained at least one parasite. The result is expressed as parasites/footpad.
Cytokine dosage
The macerated footpad was filtered using a 40-μm cell strainer (BD). The cellular suspension was centrifuged for 10 min at 750×g. IL-10, IL-4, IL-12p40, IFN-γ, IL-17 and TGF-β levels were determined in cell supernatants as described by de Matos Guedes et al. [23].
Flow cytometry analysis
T cell proliferation was evaluated with viable cells obtained from popliteal lymph nodes. The cells were stained with 5 μM CFSE (Invitrogen) and incubated with 1.25 μg/ml anti-CD3 (mAb 145-2C11) in supplemented DMEM for 48 h at 37 °C. Then, the cells were incubated with anti-CD4 (PerCP Cy5.5 mAb RM4–5) and anti-CD8 (PE mAb 53–6.7) as recommended by the manufacturer (eBioscience) for 30 min. The staining was evaluated by flow cytometry acquisition of 50,000 events in a FACSCanto. The results were analysed using the FlowJo software.
Histological analyses
Each tissue specimen was fixed in formalin, decalcified in Morse solution and embedded in paraffin. Histological sections of 5 μm in thickness were stained with haematoxylin and eosin. The histopathological changes between WT and P2X7 KO mouse lesions were observed using an optical microscope (Olympus®) at ×100 and ×400 magnification. The morphological parameters analysed in the epidermis were acanthosis, dyskeratosis, papillomatosis, exocytosis, hyperkeratosis in the dermis, plasma exudation and cellular exudation, inflammatory infiltrate production (polymorphonuclear (PMNs), eosinophils and mononuclear cells), giant cells and granuloma formation.
Statistical analysis
The groups were compared using Student’s t test. The statistical significance of differences between groups was evaluated using a one-way ANOVA coupled to Tukey’s post hoc test. All statistical analyses were performed using the statistical GraphPad Prism® software, version 4, and p values <0.05 were considered significant.
Results
P2X7 KO mice are more susceptible to L. amazonensis infection
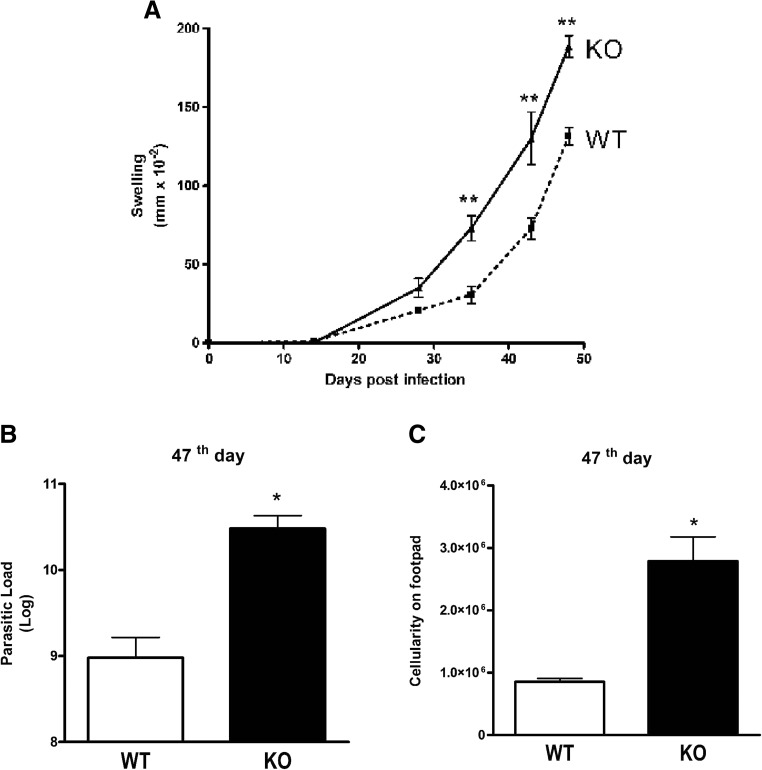
The in vitro treatment of macrophages with ATP has previously been shown to control L. amazonensis infection [15]. To determine if the ATP effect was due to stimulation of the P2X7 receptor, we infected P2X7 KO mice with L. amazonensis (Fig. 1). We observed that P2X7 KO mice were more susceptible to infection by L. amazonensis and had larger lesions in their footpad at 35 DPI (Fig. 1a). At day 47 after infection, we determined the parasite load in the paws. P2X7 KO mice had a higher parasite load (Fig. 1b) and a higher number of cells in the footpad compared to infected WT mice (Fig. 1c). These results suggest a more pronounced tissue inflammation in P2X7 KO mice.
Fig. 1.
Susceptibility to L. amazonensis infection in P2X7 KO and WT mice. P2X7 KO and WT mice were subcutaneously infected in the footpad, and the lesion development was monitored until the 47th day. a Lesions were significantly larger in P2X7 KO mice than in WT mice on day 36 (b, c). The parasitic load and total cells in P2X7 KO and WT mouse footpads. Bars represent the mean ± standard error of the mean of three independent experiments performed with three animals. *p < 0.05; **p < 0.01 using Student’s t test and one-way ANOVA coupled to Tukey’s post hoc test comparing infected P2X7 KO and WT mice at the same timepoint
P2X7 KO mice exhibit increased Th1 inflammation during L. amazonensis infection
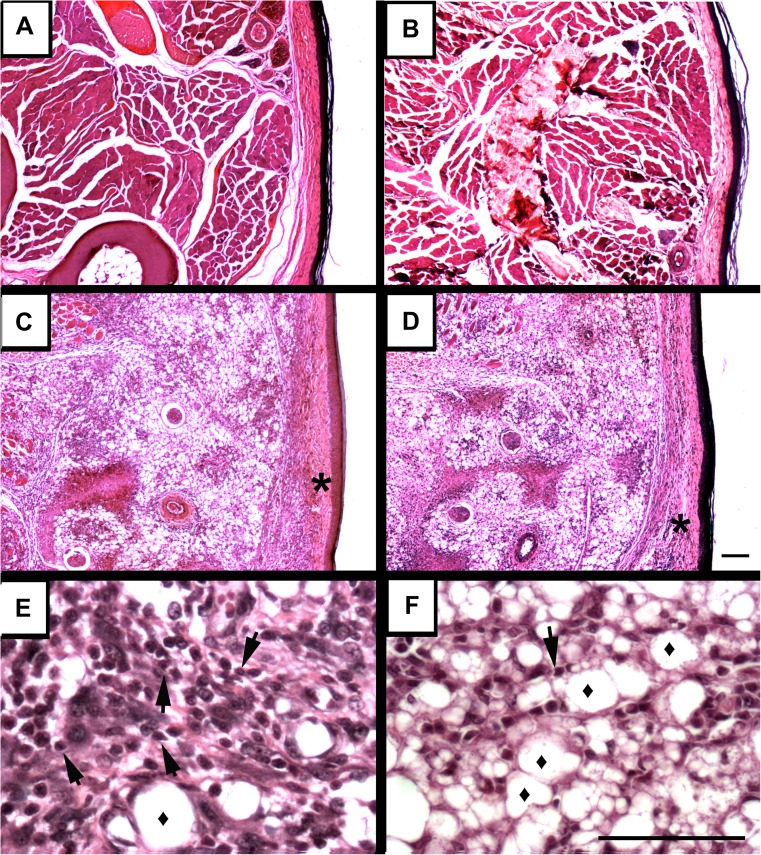
To further assess the accumulation of cells in the infected footpads of P2X7 KO mice (Fig. 1c), we performed tissue histology. In contrast to the uninfected footpads of WT (Fig. 2a) and P2X7 KO (Fig. 2b) mice, we observed that infection induced epidermal hyperplasia and cellular infiltration mainly by mononuclear cells in P2X7 KO footpads. Disruption of the dermal barrier (asterisks) was observed in both mouse genotypes but was more pronounced in P2X7 KO mice (Fig. 2c, d). In addition, we observed a more vacuolated mononuclear cell infiltrate (diamond), which is directly related to parasite load, in the P2X7 KO mice compared to WT mice (Fig. 2e, f).
Fig. 2.
Histological analysis of P2X7 KO and WT footpads infected by L. amazonensis. The footpads were removed 47 days after the infection. a WT-noninfected, b P2X7 KO-noninfected, c, e WT-infected and d, f P2X7 KO-infected footpad. Asterisk indicates the dermal barrier, diamond indicates the vacuolated macrophages, and arrowheads indicate infiltrating lymphocytes. Bars represent 60 μm. The figure is representative of results obtained from five animals
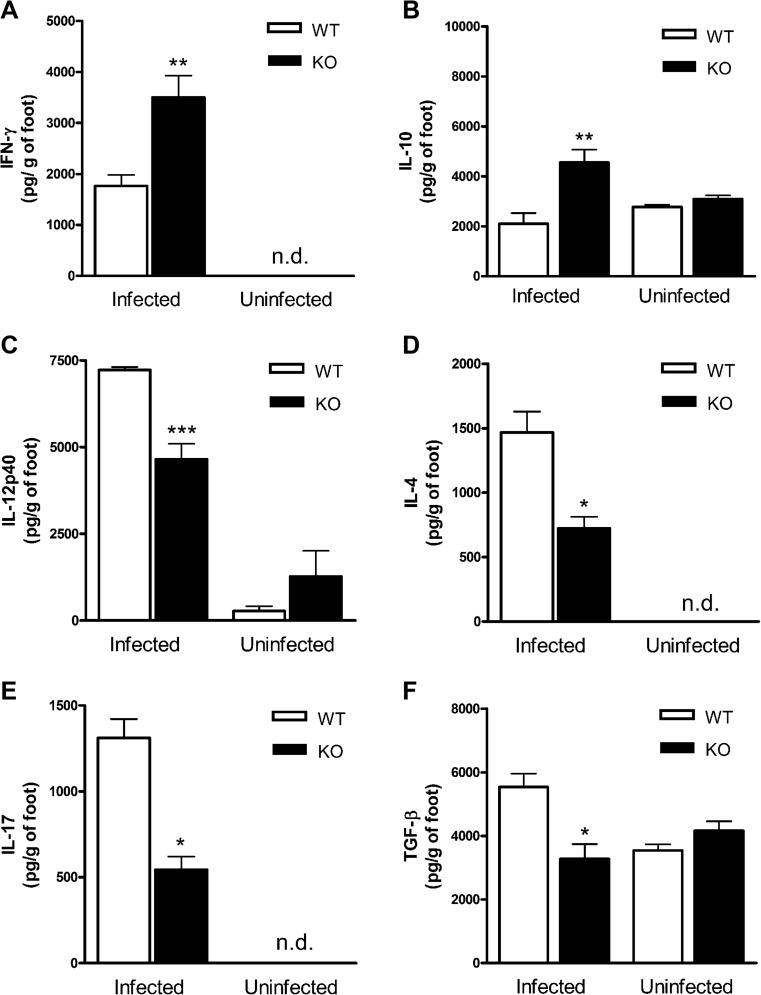
We quantified the cytokines in the infected tissues to determine which type of inflammatory response was associated with the increase in tissue damage and mononuclear cell infiltration. We observed higher levels of IFN-γ (Fig. 3a) and IL-10 (Fig. 3b) in P2X7 KO mice, with low IL-12p40 (Fig. 3c), IL-4 (Fig. 3d), IL-17 (Fig. 3e) and TGF-β (Fig. 3f) levels in P2X7 KO mice compared to infected WT mice. The reduced Th2 (IL-4), Th17 (IL-17) and Treg (TGF-β) cytokines and increased IFN-γ suggested that the inflammation was associated with the Th1 response.
Fig. 3.
Cytokine profile in infected and noninfected P2X7 KO and WT footpads. At 47 DPI, the footpad macerate was used to measure cytokines a IFN-γ, b IL-10, c IL-12p40, d IL-4, e IL-17 and f TGF-β by ELISA. Bars represent the mean ± standard error of representative group of experiments performed twice with five animals. *p < 0.05; ** p < 0.01; ***p < 0.001, using Student’s t test and one-way ANOVA coupled to Tukey’s post hoc test comparing infected P2X7 KO and WT mice
Importantly, we did not observe significant changes in IL-12p40, IL-10 and TGF-β levels footpad from WT in comparison with P2X7 KO noninfected mice. In addition, IFN-γ, IL-4 and IL-17 were not detected in the footpad from noninfected mice.
Excessive T cell proliferation is associated with P2X7 receptor deletion
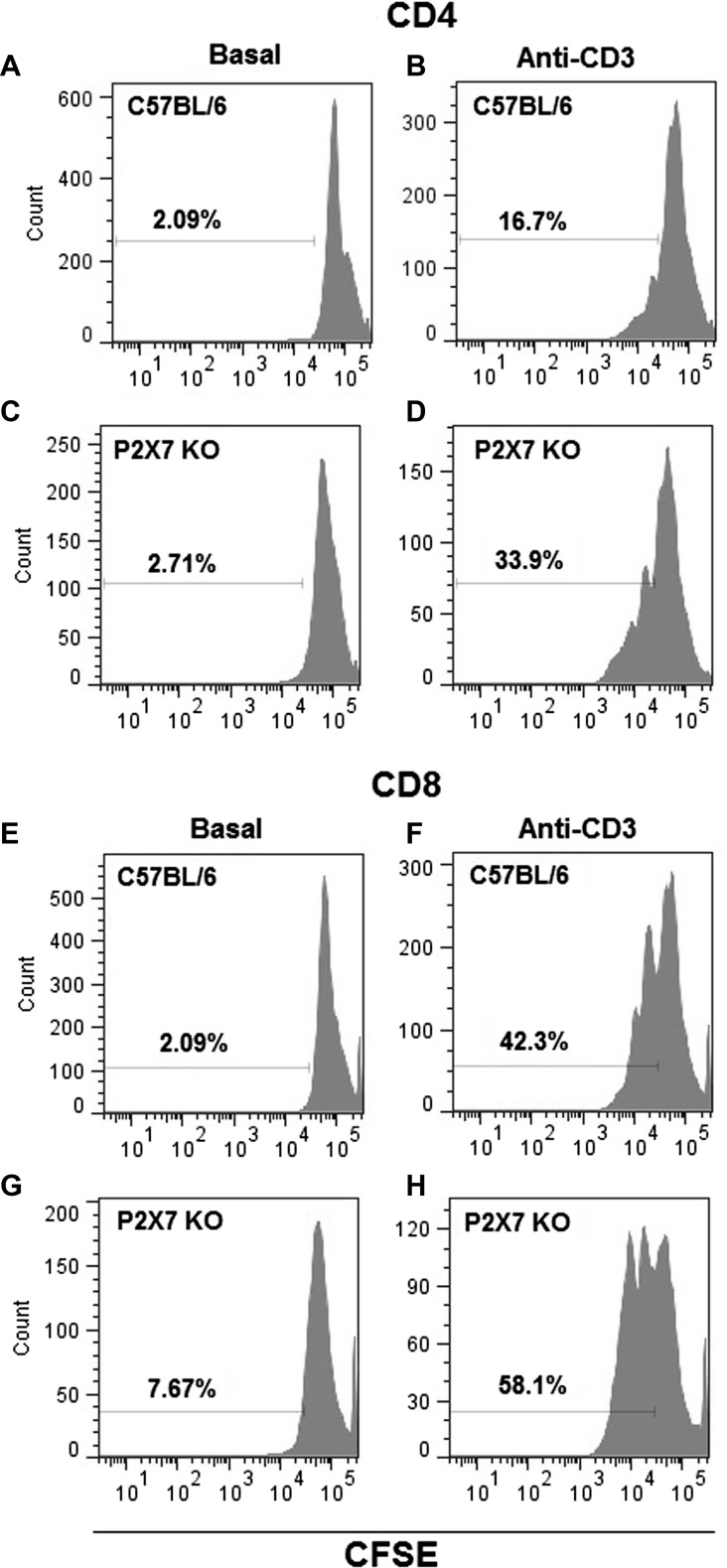
The increase in IFN-γ in the infected footpads of P2X7 KO mice suggests an increase in T cell number. We studied the anti-CD3-induced proliferation in T cells from popliteal lymph nodes of infected WT and P2X7 KO mice. We observed that the proliferation of CD4+ cells from infected P2X7 KO mice after stimulation with anti-CD3 was 2.5-fold higher (37 ± 2, mean ± SD) than the proliferation of CD4+ cells from infected WT mice (15 ± 2, mean ± SD) (Fig. 4b, d). No difference was observed between CD4+ cells from WT (2.03 ± 0.03, mean ± SD) and P2X7 KO mice (5 ± 1, mean ± SD) without anti-CD3 (Fig. 4a, c). The proliferation of CD4+ cells from uninfected P2X7 KO mice was only 1.16-fold higher than the proliferation of CD4+ cells from uninfected WT mice (data not shown). The CD8+ T cells from infected P2X7 KO mice also showed 1.94-fold higher proliferation (64 ± 3, mean ± SD) than the CD8+ cells from WT mice (33 ± 5, mean ± SD) (Fig. 4f, h). Additionally, the proliferation of CD8+ cells from uninfected P2X7 KO mice was only 1.24-fold higher than the proliferation of CD8+ cells from uninfected WT mice (data not shown). No difference was observed between CD8+ cells from WT (2.7 ± 0.4, mean ± SD) and P2X7 KO mice (6 ± 1, mean ± SD) without anti-CD3 (Fig. 4e, g).
Fig. 4.
Cell proliferation in popliteal lymph node cells from P2X7 KO and WT mice infected by L. amazonensis. The proliferation assay was performed at 47 DPI in CD4- and CD8-positive cells from popliteal lymph nodes of P2X7 KO and WT mice. The cells were incubated with anti-CD3 mAb to induce clonal expansion. The cell proliferation was evaluated by flow cytometry using CFSE. a, b Proliferation of CD4 cells from WT without or with anti-CD3 mAb. c, d Proliferation of CD4 cells from P2X7 KO without or with anti-CD3 mAb. e, f Proliferation of CD8 cells from WT without or with anti-CD3 mAb, and g, h proliferation of CD8 cells from P2X7 KO without or with anti-CD3 mAb. Histograms are representative of experiments performed once with five animals each
Discussion
Several lines of evidence indicate that purinergic receptors contribute to controlling and resolving parasite infections [13, 24, 25]. The role of the P2X7 receptor in a wide range of infectious diseases has been studied in vitro and in vivo using P2X7 KO mice [10, 26, 27]. The P2X7 receptor appears to be crucial during the immunological response against M. tuberculosis because P2X7 KO mice had a higher bacterial burden in the lungs than WT mice when infected with a low-virulence strain, H37Rv Mtb [7]. On the other hand, the P2X7 receptor contributed to tissue damage in infection caused by a highly virulent strain of M. tuberculosis [27]. In addition, P2X7 KO mice infected with Toxoplasma gondii lost significantly more weight than WT C57BL/6 J mice after infection. Interestingly, WT C57BL/6 J mice, in turn, also lost more weight than WT BALB/c mice [26] and had more gut damage in a model of ileitis [11]. This suggests that there is a relationship between the P2X7 receptor sensitivity and the ability of these mice to respond to and eliminate intracellular pathogens because WT C57BL/6 J mice have a proline to leucine polymorphism at amino acid 451 in the C-terminal tail of the P2X7 receptor, which reduces the P2X7 sensitivity to ATP [28]. In murine model of colitis, P2X7-deficient mice showed reduced tissue damage in the colon [29] and faster recovery from epithelial damage caused by an inducing agent [30]. Nevertheless, we observed that the absence of the P2X7 receptor during in vivo infection caused by L. amazonensis resulted in more severe injury in mice. The literature shows that the P2X7 receptor has an important role in controlling infection and inflammation, but its contribution to both of these processes depends on the inflammatory context and the pathogenic microorganism.
We previously reported that the P2X7 receptor plays a key role in the control of in vitro infection by L. amazonensis, using a selective P2X7 antagonist [15]. Recently, we showed that P2X7-mediated L. amazonensis elimination involves 5-lipoxygenase activation and LTB4 secretion in infected macrophages [16]. However, no studies have addressed the role of the P2X7 receptor during L. amazonensis in vivo infection. Here, we examined the contribution of the P2X7 receptor to the in vivo immune response against L. amazonensis using a murine model of leishmaniasis, which has been widely used to characterize the immunobiology of Leishmania infection. We observed a higher burden of L. amazonensis and an increased cellularity in footpads of P2X7 KO mice compared to infected WT mice. In addition, P2X7 KO mice developed lesions earlier compared to the control group, suggesting that P2X7-deficient mice are more susceptible to L. amazonensis infection. However, pathological severity is not always associated with parasite load in P2X7 KO mice, as observed in ileitis caused by Toxoplasma gondii. Instead, the inability of P2X7-deficient mice to limit production of NO can explain the damage in the infected gut in this model [11].
The P2X7 receptor is crucial for a productive immune response against pathogens because it not only modulates macrophage responses but also regulates T cell functions. Prolonged stimulation of P2X7 is essential to control T cell activation and proliferation by inducing apoptosis [31]. P2X7 receptor deletion exacerbated experimental autoimmune encephalomyelitis due to reduced apoptotic activity in T lymphocytes. In this model, T cells from P2X7-deficient mice proliferated more vigorously in response to antigen stimulation [32]. Furthermore, P2X7-deficient mice infected with L. monocytogenes exhibit higher frequencies of IFNγ+ CD8+ T cells [33]. In accordance with these reports, we also observed a higher proliferation of CD4+ and CD8+ effector T cells and increased IFN-γ levels in paws from infected P2X7 KO mice. This excessive T cell proliferation might contribute to the pathogenesis in leishmaniasis because CD4+ T cells have a pathogenic role [18], while regulatory T cells have a protective role in this disease [19].
The excessive Th1 response and the increased levels of IFN-γ observed in P2X7 KO mice in this study may be related to the failure in control L. amazonensis infection because macrophages pretreated with IFN-γ are more susceptible to Leishmania infection [34]. In addition, P2X7 receptor activation by ATP potentiated IFNγ-induced NO production in microglia [35]. Similarly, we observed a reduction in NO and ROS production in peritoneal macrophages from P2X7 KO mice compared to WT mice, suggesting an inefficient innate immune response in the inflammatory environment of P2X7 KO mice (unpublished data). Thus, the failure to respond by P2X7-deficient macrophages should not be ruled out.
Furthermore, following the increase in IFN-γ, we observed an increase in IL-10. This response could be related to the increase in parasite load. The production of IL-10 that is associated with disease is produced by T cells and not the macrophages in Leishmania infection [36, 37]. IL-10 has been shown to impair IFN-γ response in leishmaniasis [37, 38]. The production of IL-10 could be stimulated by negative feedback from a strong IFN-γ response by Th1 cells [39]. IL-10 production by Th1 cells has been shown to fail to control Toxoplasma and L. major infections [40, 41]. Thus, the increase of IL-10 levels might also be involved in the failure to control the parasite. Here, we showed lower levels of IL-12 in footpads of infected P2X7 KO mice compared to infected WT mice. The IL-12 observed in P2X7 KO mice can result from downregulation of IL-10 because IL-10 negatively regulates several cell types, including macrophages [42]. Finally, the differences observed in cytokine IL-4 may not be related to resistance or susceptibility to Leishmania amazonensis, as demonstrated previously in infected patients and C57BL/6 mice with infected footpads [21, 43]. Therefore, we propose that P2X7 receptor expression is important for proinflammatory cytokine IL-17 and IL-12 secretion and modulation of IL-10, which together leads to L. amazonensis infection control.
In summary, the P2X7 receptor deletion promotes an excessive inflammation that results in tissue damage and high parasite load. Our study describes for the first time a crucial role of the P2X7 receptor in the homeostatic regulation of T cell responses and function during Leishmania infection. Finally, our findings highlight the role of purinergic signalling in controlling leishmaniasis, supporting the idea that the P2X7 receptor may represent a suitable therapeutic target for the development of treatments against infectious inflammatory diseases.
Acknowledgements
This work was supported by funds from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Núcleos de Excelência (PRONEX), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), the Instituto Nacional de Ciência e Tecnologia para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica (INPeTAm/UFRJ), and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Abbreviations
- KO
Knockout
- DPI
Days postinfection
- ROS
Reactive oxygen species
- NO
Nitric oxide
- LTB4
Leukotriene B4
- FBS
Heat-inactivated foetal bovine serum
- CEUA
Commission for Ethical Use of Research Animals
- TCA
Trichloroacetic acid
- GFI-1
Growth factor-independent 1 transcription repressor
- M199
199 medium
- DMEM
Dulbecco’s modified Eagle’s medium
Compliance with ethical standards
Conflict of interest
Vanessa Ribeiro Figliuolo declares that she has no conflict of interest.
Suzana Passos Chaves declares that she has no conflict of interest.
Luiz Eduardo Baggio Savio declares that he has no conflict of interest.
Maria Luiza Prates Thorstenberg declares that she has no conflict of interest.
Érika Machado Salles declares that she has no conflict of interest.
Christina Maeda Takiya declares that she has no conflict of interest.
Maria Regina D’Império-Lima declares that she has no conflict of interest.
Herbert Leonel de Matos Guedes declares that he has no conflict of interest.
Bartira Rossi-Bergmann declares that she has no conflict of interest.
Robson Coutinho-Silva declares that he has no conflict of interest.
Ethical approval
All animals received care according to institutional guidelines, and all procedures were according to EU guidelines (2010/63) after approval by the Animal Care Committee of the Federal University of Rio de Janeiro.
Footnotes
Vanessa Ribeiro Figliuolo and Suzana Passos Chaves contributed equally
References
- 1.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, Den BM. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Showler AJ, Boggild AK. Cutaneous leishmaniasis in travellers: a focus on epidemiology and treatment in 2015. Curr Infect Dis Rep. 2015;17:489–0489. doi: 10.1007/s11908-015-0489-2. [DOI] [PubMed] [Google Scholar]
- 3.Mukbel RM, Patten C, Jr, Gibson K, Ghosh M, Petersen C, Jones DE. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. AmJTrop Med Hyg. 2007;76:669–675. [PubMed] [Google Scholar]
- 4.Henard CA, Carlsen ED, Hay C, Kima PE, Soong L. Leishmania amazonensis amastigotes highly express a tryparedoxin peroxidase isoform that increases parasite resistance to macrophage antimicrobial defenses and fosters parasite virulence. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos AA, Jr, Rodrigues-Junior V, Zanin RF, Borges TJ, Bonorino C, Coutinho-Silva R, Takyia CM, Santos DS, Campos MM, Morrone FB. Implication of purinergic P2X7 receptor in M. Tuberculosis infection and host interaction mechanisms: a mouse model study. Immunobiology. 2013;218:1104–1112. doi: 10.1016/j.imbio.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Kusner DJ, Adams J. ATP-induced killing of virulent mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol. 2000;164:379–388. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]
- 9.Correa G, Marques da SC, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R. Activation of the P2X(7) receptor triggers the elimination of toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Morandini AC, Savio LE, Coutinho-Silva R. The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed J. 2014;37:169–177. doi: 10.4103/2319-4170.127803. [DOI] [PubMed] [Google Scholar]
- 11.Miller CM, Zakrzewski AM, Robinson DP, Fuller SJ, Walker RA, Ikin RJ, Bao SJ, Grigg ME, Wiley JS, Smith NC. Lack of a functioning P2X7 receptor leads to increased susceptibility to Toxoplasmic ileitis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Xiao Y, Li Z. P2X7 receptor positively regulates MyD88-dependent NF-kappaB activation. Cytokine. 2011;55:229–236. doi: 10.1016/j.cyto.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Coutinho-Silva R, Ojcius DM. Role of extracellular nucleotides in the immune response against intracellular bacteria and protozoan parasites. Microbes Infect. 2012;14:1271–1277. doi: 10.1016/j.micinf.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biswas D, Qureshi OS, Lee WY, Croudace JE, Mura M, Lammas DA (2008) ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunol 9:35. doi:10.1186/1471-2172-9-35.:35-39 35-39 [DOI] [PMC free article] [PubMed]
- 15.Chaves SP, Torres-Santos EC, Marques C, Figliuolo VR, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B. Modulation of P2X(7) purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect. 2009;11:842–849. doi: 10.1016/j.micinf.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Chaves MM, Marques-da-Silva C, Monteiro AP, Canetti C, Coutinho-Silva R. Leukotriene B4 modulates P2X7 receptor-mediated Leishmania amazonensis elimination in murine macrophages. J Immunol. 2014;192:4765–4773. doi: 10.4049/jimmunol.1301058. [DOI] [PubMed] [Google Scholar]
- 17.Ji J, Sun J, Qi H, Soong L. Analysis of T helper cell responses during infection with Leishmania amazonensis. AmJTrop Med Hyg. 2002;66:338–345. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- 18.Soong L, Chang CH, Sun J, Longley BJ, Jr, Ruddle NH, Flavell RA, McMahon-Pratt D. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J Immunol. 1997;158:5374–5383. [PubMed] [Google Scholar]
- 19.Ji J, Masterson J, Sun J, Soong L. CD4 + CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J Immunol. 2005;174:7147–7153. doi: 10.4049/jimmunol.174.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonardoni MV, Russo M, Jancar S. Essential role of platelet-activating factor in control of Leishmania (Leishmania) amazonensis infection. Infect Immun. 2000;68:6355–6361. doi: 10.1128/IAI.68.11.6355-6361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felizardo TC, Gaspar-Elsas MI, Lima GM, Abrahamsohn IA. Lack of signaling by IL-4 or by IL-4/IL-13 has more attenuating effects on Leishmania amazonensis dorsal skin--than on footpad-infected mice. Exp Parasitol. 2012;130:48–57. doi: 10.1016/j.exppara.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 23.de Matos Guedes HL, da Silva Costa BL, Chaves SP, de Oliveira Gomes DC, Nosanchuk JD, De Simone SG, Rossi-Bergmann B. Intranasal vaccination with extracellular serine proteases of Leishmania amazonensis confers protective immunity to BALB/c mice against infection. Parasit Vectors. 2014;19(7):448. doi: 10.1186/1756-3305-7-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM, Wiley JS, Smith NC. The role of the P2X(7) receptor in infectious diseases. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coutinho-Silva R, Correa G, Sater AA, Ojcius DM. The P2X(7) receptor and intracellular pathogens: a continuing struggle. Purinergic Signal. 2009;5:197–204. doi: 10.1007/s11302-009-9130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller CM, Zakrzewski AM, Ikin RJ, Boulter NR, Katrib M, Lees MP, Fuller SJ, Wiley JS, Smith NC. Dysregulation of the inflammatory response to the parasite, toxoplasma gondii, in P2X7 receptor-deficient mice. Int J Parasitol. 2011;41:301–308. doi: 10.1016/j.ijpara.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Amaral EP, Ribeiro SC, Lanes VR, Almeida FM, de Andrade MR, Bomfim CC, Salles EM, Bortoluci KR, Coutinho-Silva R, Hirata MH, Alvarez JM, Lasunskaia EB, D’Imperio-Lima MR. Pulmonary infection with hypervirulent mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- 29.Neves AR, Castelo-Branco MT, Figliuolo VR, Bernardazzi C, Buongusto F, Yoshimoto A, Nanini HF, Coutinho CM, Carneiro AJ, Coutinho-Silva R, de Souza HS. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2014;20:444–457. doi: 10.1097/01.MIB.0000441201.10454.06. [DOI] [PubMed] [Google Scholar]
- 30.Hofman P, Cherfils-Vicini J, Bazin M, Ilie M, Juhel T, Hebuterne X, Gilson E, Schmid-Alliana A, Boyer O, Adriouch S, Vouret-Craviari V. Genetic and pharmacological inactivation of the purinergic P2RX7 receptor dampens inflammation but increases tumor incidence in a mouse model of colitis-associated cancer. Cancer Res. 2015;75:835–845. doi: 10.1158/0008-5472.CAN-14-1778. [DOI] [PubMed] [Google Scholar]
- 31.Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. P2X7 on mouse T cells: one channel, many functions. Front Immunol. 2015;19(6):204. doi: 10.3389/fimmu.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Brosnan CF. Exacerbation of experimental autoimmune encephalomyelitis in P2X7R−/− mice: evidence for loss of apoptotic activity in lymphocytes. J Immunol. 2006;176:3115–3126. doi: 10.4049/jimmunol.176.5.3115. [DOI] [PubMed] [Google Scholar]
- 33.Heiss K, Janner N, Mahnss B, Schumacher V, Koch-Nolte F, Haag F, Mittrucker HW. High sensitivity of intestinal CD8+ T cells to nucleotides indicates P2X7 as a regulator for intestinal T cell responses. J Immunol. 2008;181:3861–3869. doi: 10.4049/jimmunol.181.6.3861. [DOI] [PubMed] [Google Scholar]
- 34.Koutsoni O, Barhoumi M, Guizani I, Dotsika E. Leishmania eukaryotic initiation factor (LeIF) inhibits parasite growth in murine macrophages. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gendron FP, Chalimoniuk M, Strosznajder J, Shen S, Gonzalez FA, Weisman GA, Sun GY. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem. 2003;87:344–352. doi: 10.1046/j.1471-4159.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 36.Buxbaum LU. Interleukin-10 from T cells, but not macrophages and granulocytes, is required for chronic disease in Leishmania mexicana infection. Infect Immun. 2015;83:1366–1371. doi: 10.1128/IAI.02909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz T, Remer KA, Nahrendorf W, Masic A, Siewe L, Muller W, Roers A, Moll H. T cell-derived IL-10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell based vaccine. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de JA, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;19(204):239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;19(204):285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;19(204):273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci. 1993;685:713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- 43.Espir TT, Figueira LP, Naiff MF, da Costa AG, Ramalho-Ortigao M, Malheiro A, Franco AM (2014) The role of inflammatory, anti-inflammatory, and regulatory cytokines in patients infected with cutaneous leishmaniasis in Amazonas state, Brazil. J Immunol Res 2014. doi:10.1155/2014/481750 [DOI] [PMC free article] [PubMed]