Abstract
Type 2 diabetes mellitus (T2DM) accounts for more than 90% of all cases of diabetes mellitus (DM). Diabetic neuropathic pain (DNP) is a common complication of T2DM. Sinomenine is a natural bioactive component extracted from the Sinomenium acutum and has anti-inflammatory effects. The aim of our study was to investigate the effects of sinomenine on DNP mediated by the P2X3 receptor in dorsal root ganglia (DRG). The mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) in T2DM rats were lower than those of control rats. MWT and TWL in T2DM rats treated with sinomenine were higher compared with those in T2DM rats. The expression levels of the P2X3 protein and mRNA in T2DM rat DRG were higher compared with those of the control, while those in T2DM rats treated with sinomenine were significantly lower compared with those of the T2DM rats. Sinomenine significantly inhibited P2X3 agonist ATP-activated currents in HEK293 cells transfected with the P2X3 receptor. Sinomenine decreased the phosphorylation and activation of P38MAPK in T2DM DRG. Therefore, sinomenine treatment may suppress the up-regulated expression and activation of the P2X3 receptor and relieve the hyperalgesia potentiated by the activation of P38MAPK in T2DM rats.
Keywords: P2X3 receptor, Sinomenine, Diabetic neuropathic pain, Dorsal root ganglia
Introduction
Chronic neuropathic pain is a major public health problem, greatly impairs quality of life, and has a high economic impact on society [1]. The mechanism of chronic neuropathic pain is extremely complex, and it is very difficult to treat [2, 3]. Type 2 diabetes mellitus (T2DM) accounts for more than 90% of all cases of diabetes and is a complex heterogeneous disease [4–7]. Diabetic peripheral neuropathy (DPN) is a very common complication of T2DM. DPN is a frequent complication of T2DM and a major cause of morbidity and increased mortality [8, 9]. DNP is characterized by spontaneous pain, allodynia (pain to normally innocuous stimuli), and hyperalgesia (increased pain perception to noxious stimuli) [5, 6, 10–12]. Diabetic neuropathic pain treatment is difficult because no specific relief medication is available [6, 10, 12]. Although numerous studies have examined the mechanisms underlying hyperglycemia-induced nerve damage, the exact mechanism by which T2DM causes neuropathy has not been clearly elucidated.
Adenosine-5′-triphosphate (ATP), a non-selective agonist for each of the ionotropic P2X receptors, is involved in the transmission of painful sensory information from the periphery to the CNS [13–15]. The P2X3 receptor is a trimeric cation channel gated by extracellular ATP and most highly expressed in small diameter neurons of the DRG [13–15]. The P2X3 receptor is an important transducer of nociceptive stimuli and altered P2X3 receptor after nerve injury contributes to neuropathic pain hypersensitivity [16–19]. Expression levels of the P2X3 receptor in the DRG are increased in chronic constriction injury (CCI) rat models [13–17]. The P2X3 receptor is up-regulated during diabetic neuropathy and changes in the expression of P2X3, correlating with the development of hyperalgesia [20–23].
Sinomenine is a natural bioactive component extracted from the climbing plant Sinomenium acutum [24–28]. Sinomenine reduces joint swelling, erythrocyte sedimentation rate, production of antibodies, and secretion of cytokines in animal models of arthritis [24, 26]. It was reported that sinomenine attenuated neuropathic pain hypersensitivity [24–28]. However, its exact mechanism of action is not clearly understood. Sinomenine also alleviates high glucose-induced diabetic complications [29, 30]. Our studies showed that the effects of sinomenine on hyperalgesia correlate with modulating the expression of the P2X3 receptor in DRG in a T2DM rat model. Thus, the P2X3 receptor may be a target for the analgesic properties of sinomenine. The aim of this study was to investigate the effects of sinomenine on the P2X3 receptor in DRG-mediated DNP.
Materials and methods
Animals groups
Male Sprague–Dawley (SD) rats (180–230 g) were provided by the Center of Laboratory Animal Science of Nanchang University. The procedures were approved by the Animal Care and Use Committees of Nanchang University Medical Schools. The animals were housed in plastic boxes in groups of three at 21–25 °C. The IASP’s ethical guidelines for pain research in animals were followed. All animals were treated according to the ARVO Statement for the use of Animals in Ophthalmic and Vision Research in China. Type 2 diabetic model rats were given a high-fat diet (consisting of 22% fat, 48% carbohydrate, and 20% protein with a total calorific value of 44.3 kJ/kg) for 4 weeks and were subsequently injected intraperitoneally (i.p.) with a low dose of streptozotocin (STZ; 30 mg/kg) [31–33]. After 1 week of STZ injection, rats with a fasting blood glucose >7.8 mmol/L or non-fasting blood glucose >11.1 mmol/L were considered to be type 2 diabetic model rats. Control rats were fed regular chow (consisting of 5% fat, 53% carbohydrate, 23% protein, with a total calorific value of 25 kJ/kg) and were treated with vehicle citrate buffer (pH 4.4) in a volume of 0.25 mL/kg (i.p.) [31–33].
Rats were assigned in a random blind manner to one of three groups: the control group (control group), the diabetic model group (DM group), and DM rats treated with the sinomenine group (DM + sinomenine group); each group contained ten animals. The DM + sinomenine group consisted of diabetic animals treated with sinomenine (40 mg kg−1 day−1, i.p.) on the 1st through the 14th day after the DM rats were identified. Sinomenine (standard substance) was purchased from the National Institute for Food and Drug Control (Beijing, China), and the purity is 99%.
Thermal hyperalgesia
The latency to hind paw withdrawal from a thermal stimulus was determined by exposing the plantar surface of the hind paw to radiant heat using the Thermal Paw Stimulation System (BME-410C, Tianjin) [21, 22, 31]. Rats were placed in a transparent, square, bottomless acrylic box (22 × 12 × 22 cm) on a glass plate under which a light was located. After a 30-min habituation period, the plantar surface of the paw was exposed to a beam of radiant heat applied through the glass floor. Activation of the bulb simultaneously activated a timer, and both were immediately turned off by paw withdrawal or at the 25-s cutoff time. The hind paws were tested by a blinded observer in triplicate at 5-min intervals.
Mechanical hyperalgesia
The mechanical nociceptive threshold was evaluated by observing withdrawal responses to mechanical stimulation using von Frey filaments (Stoelting, Wood Dale, IL, USA) [21, 22, 31]. A linearly increasing pressure by a von Frey filament was applied, starting with 0.13 g and continuing until a withdrawal response occurred or the force reached 20.1 g (the cutoff value). The pressure was applied through a cone-shaped plastic tip with a diameter of 1 mm onto the dorsal surface of the hind paws. The tip was positioned between the third and fourth metatarsus, and the force was applied until the rat attempted to withdraw its paw (paw withdrawal threshold to pressure). The hind paws were tested alternately at 2-min intervals. The pain threshold was determined by a blinded observer as the mean of three consecutive stable values and expressed in grams.
Western blotting
Animals were anesthetized with penthiobarbital sodium, and the DRG were dissected. Approximately six to ten samples were harvested from each rat. The DRG were isolated immediately and rinsed in ice-cold PBS [21, 22]. After dilution with sample buffer (250 mmol/L Tris–Cl, 200 mmol/L dithiothreitol, 10% sodium dodecyl sulfate (SDS), 0.5% bromophenol blue, and 50% glycerol) and heating to 95 °C for 10 min, 20-μg samples of total protein (for P2X3 receptor analysis) were separated using 10% SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. After incubation with the primary antibody against the P2X3 receptor (CHEMICON International, Inc. USA), the membrane was incubated with peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA). Immunodetection was performed using the Pierce-enhanced chemiluminescence substrate (Thermo Scientific, Waltham, MA, USA). The β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a loading control. The following were the primary antibodies and dilutions used: rabbit polyclonal anti-P2X3 (1:1000; Chemicon International Company of America), monoclonal β-actin (1:10,000; Advanced Immunochemicals, Long Beach, CA), rabbit anti-p-P38MAPK antibody (1:1000 dilutions; Cell Signaling Technology, Beverly, MA, USA), and rabbit anti-P38MAPK antibody (1:1000; Cell Signaling Technology, Beverly, MA, USA). Band intensity was quantified using Image Pro-Plus software. The relative band intensity of target proteins was normalized against the intensity of respective β-actin bands as internal controls.
Real-time RT-PCR
Total RNA was isolated from DRG using the Trizol Total RNA Reagent. Complementary DNA (cDNA) synthesis was performed with 2 μg of total RNA using the RevertAid™ H Minus First Strand cDNA Synthesis Kit. The primers were designed with Primer Express 3.0 software (Applied Biosystems), and the sequences were as follows: P2X3, forward: 5′-ACAGAGTCATGGACGTGTCG-3′, and reverse: 5′-TGAGGTTAGGCAGGAGG TTT-3′; β-actin, forward: 5′-TAAAGACCTCTATGCCAACACAGT-3′, and reverse: 5′-CACGATGGAGGGGCCGGACTCATC-3′. quantitative PCR was performed using the SYBR® Green MasterMix in an ABI PRISM® 7500 Sequence Detection System (Applied Biosystems Inc., Foster City, CA). The quantification of gene expression was performed using the ΔΔCT calculation with CT as the threshold cycle. The relative levels of target genes, normalized to the sample with the lowest CT, were given as 2−ΔΔ CT [7, 22].
HEK293 cell culture and transfection
HEK 293 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% penicillin, and streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. Cells were transiently transfected with the human pcDNA3.0-EGFP-P2X3 plasmid using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions [34, 35]. When HEK293 cells were 70–80% confluent, cell culture media was replaced with Opti-MEM 2 h before transfection. The transfection media were prepared as follows: (a) 4 μg of plasmid DNA was diluted to a final volume of 250 μl with Opti-MEM; (b) 10 μl of Lipofectamine 2000 was diluted into 250 μl final volume of Opti-MEM; and (c) the Lipofectamine-containing solution was mixed with the plasmid-containing solutions and incubated at RT for 20 min. Subsequently, 500 μl of cDNA/Lipofectamine solution was added to each well. The cells were incubated for 6 h at 37 °C, 5% CO2. After incubation, the cells were washed in Opti-MEM containing 10% FBS and incubated for 24–48 h. The GFP fluorescence was assessed as a reporter for the efficiency of transfection. Whole cell patch clamp recordings were carried out 1–2 days after transfection.
Electrophysiological recordings
Electrophysiological recording was carried out using a patch/whole cell clamp amplifier (Axopatch 200B) [34, 35]. Single HEK293 cells that expressed green fluorescence implied P2X3 receptor expression. HEK293 cells expressing green fluorescence were subjected to electrophysiological recording [34, 35]. Microelectrodes were filled with internal solution composed of (mM) 145 K gluconate, 0.75 EGTA, 10 HEPES, 0.1 CaCl2, 2 MgATP, and 0.3 Na3GTP. The bath was continuously perfused with extracellular solution containing (mM) 126 NaCl, 2.5 KCl, 10 glucose, 1.2 MgCl2, 2.4 CaCl2, and 18 NaHCO3. The osmolarity of the extracellular and internal solutions was adjusted to 340 mOsm with sucrose, and the pH in the extracellular solution was adjusted to 7.4 with NaOH while that of the internal solution was adjusted to 7.3 with KOH for HEK293 cells. The resistance of recording electrodes was 2–6 MΩ. A small patch of membrane underneath the tip of the pipette was aspirated to form a seal (1–10 GΩ), and then more negative pressure was applied to rupture it. The holding potential (HP) was set at −70 mV. ATP (Sigma), sinomenine, and A317491 (Sigma) were dissolved in the external solution. Drugs were applied rapidly through a manifold comprising ten capillaries made of fused silica coated with polyimide with a 200-μm internal diameter. The distance from the tubule mouth to the cell examined was approximately 100 μm. Solutions were delivered by gravity flow from independent reservoirs. One barrel was used to apply drug-free external solution to enable rapid termination of drug application. Drugs were separately applied for 2 s at 4-min intervals, a time which was sufficient for responses to be reproducible. Data were low-pass filtered at 2 Hz, digitized at 5 kHz, and stored on a laboratory computer using a Digidata 1200 interface and pClamp10.0 software (Axon instruments). Concentration-response curves were performed by Sigmaplot12.0 software. Traces were acquired using pClamp software and plotted using Origin 8 (Microcal, Northampton, MA, USA).
Molecular docking
Molecular docking computations were performed using AutoDock 4.2 [36, 37]. Molecular docking is a computer simulation tool that attempts to predict the binding mode of a ligand in the active site of a protein. Molecular docking studies mimic the natural interaction of a ligand with the protein. The technique of docking is to position the ligand in different orientations and conformations within the binding site to calculate optimal binding geometries and energies. Therefore, after the docking procedure, the proper conformation of ligand in the active site of protein is obtained and used for calculation of molecular descriptors. For each ligand, a number of configurations called poses are generated and scored [37]. The score can be calculated as either a free energy of binding, which takes into account salvation and entropy, or the enthalpic term of the free energy of binding, or a qualitative shaped-based numerical measure. The final top-scoring poses, along with their scores and conformation energies, are written to a database where they are ready for further analysis.
Protein Data Bank entry 5SVK, which is a crystal structure of the open-state ATP-gated human P2X3 ion channel in the ATP bound [38], was used as a target protein [39]. Sinomenine and pubchem CID 5459308 were used as ligands. Both of them were prepared with AutoDockTools (ADT) [36] and Python scripts named prepare_ligand4.py and prepare_receptor4.py, which are associated with the AutoDock4.2 program. The binding pocket position in target protein was specified with the ADT molecular viewer. The parameters were kept at their default values. Finally, the output files were viewed using MGLtools [36] and PyMol (http://www.pymol.org/).
Statistical analysis
Statistical analyses of the data were performed on a computer (SPSS 11.5). All of the results were expressed as the mean ± SE. Statistical significance was determined using one factor analysis of variance (ANOVA) followed by the Fisher post hoc test for multiple comparisons. p < 0.05 was considered significant.
Results
Effects of sinomenine on mechanical or thermal hyperalgesia in T2DM rats
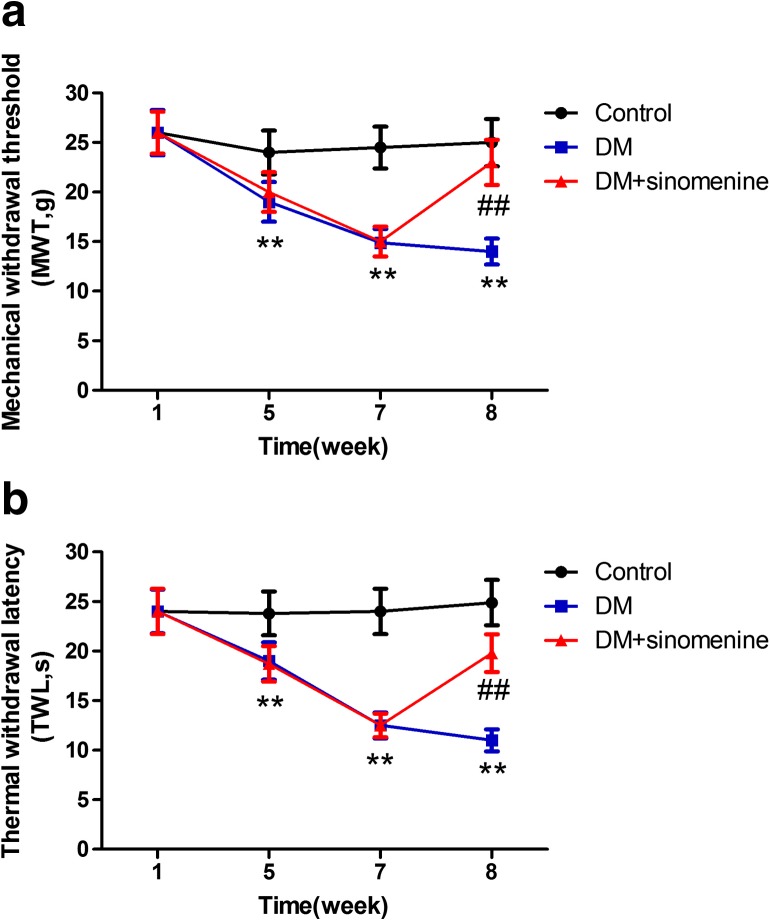
The mechanical withdrawal threshold (MWT) was measured. The MWT in the DM group was lower than the control group (p < 0.05). The MWT in T2DM rats treated with sinomenine was enhanced by about 50% compared with that of T2DM rats (p < 0.01) (Fig. 1a). These results showed that sinomenine inhibited pain behaviors by increasing the mechanical hyperalgesia threshold in DM rats.
Fig. 1.
Effects of sinomenine on the mechanical withdrawal threshold and thermal withdrawal latency in T2DM rats. a The mechanical withdrawal threshold (MWT) was measured. The MWT in T2DM rats treated with sinomenine was higher than that of the T2DM rats (p < 0.01). The upper limit of MWT detection was 26.0 g. Mean ± SEM, n = 10. **p < 0.01 compared with the control group; ## p < 0.01 compared with the DM group. b The thermal withdrawal latency (TWL) was measured. The TWL in DM rats treated with sinomenine was higher than that of the DM rats (p < 0.01). The upper limit of the thermal withdrawal latency detection was 30 s. Mean ± SEM. n = 10; **p < 0.01 compared with the control group; ## p < 0.01 compared with the DM group
The thermal withdrawal latency (TWL) in the T2DM group was lower compared with that in the control group (p < 0.05). The TWL in T2DM rats treated with sinomenine was increased by about 80% compared with that in the T2DM rats (p < 0.01) (Fig. 1b). These results revealed that sinomenine treatment also elevated the thermal hyperalgesia threshold in T2DM rats, suggesting that sinomenine treatment may relieve diabetic neuropathic pain.
Effects of sinomenine on the expression levels of DRG P2X3 mRNA, and protein in T2DM rats
The expression of DRG P2X3 messenger RNA (mRNA) was examined in each group using real-time RT-PCR. The expression of P2X3 mRNA in the DM group were higher compared with that in the control group (p < 0.01, n = 10 for each group). The expression values of P2X3 mRNA in the T2DM rats treated with sinomenine were decreased by about 28% compared with those of rats in the DM group (p < 0.01, n = 8 for each group) (Fig. 2a). These results indicated that sinomenine treatment may cut down the up-regulated expression of DRG P2X3 mRNA in T2DM rats.
Fig. 2.
Effects of sinomenine on the expression levels of DRG P2X3 mRNA and protein in T2DM rats. a The expression levels of DRG P2X3 mRNA were examined using real-time RT-PCR. The relative values of P2X3 mRNA expression in the T2DM group were higher compared with those in the control group (p < 0.01, n = 10 for each group). The relative values of P2X3 mRNA expression in T2DM rats treated with sinomenine were significantly decreased compared with the DM group (p < 0.01, n = 10 for each group). Mean ± SEM, n = 10. **p < 0.01 compared with the control group; ## p < 0.01 compared with the DM group. b The expression levels of P2X3 protein in DRG were examined using western blotting analyses. Image analysis revealed that the intensity values (integrated optical density, IOD) of P2X3 protein expression (normalized to each β-actin internal control) in the DM group were higher compared with those in the control group. The intensity values of P2X3 protein expression in T2DM rats treated with sinomenine were significantly lower compared with those in the DM group. Mean ± SEM, n = 10. ** p < 0.01 compared with the control group; ## p < 0.01 compared with the DM group
The expression of P2X3 protein in DRG was further studied by western blotting analyses. The expression of P2X3 protein (normalized against the β-actin internal control) in the DM group was higher compared with the control group (p < 0.01, n = 10 for each group). The expression of P2X3 protein in the T2DM rats treated with sinomenine was reduced by 35% compared with that in the DM group (p < 0.01, n = 8 for each group) (Fig. 2b). These results revealed that sinomenine treatment may decrease the expression of DRG P2X3 protein in T2DM rats.
Effects of sinomenine on the expression levels of DRG P38MAPK and p-P38MAPK in T2DM rats
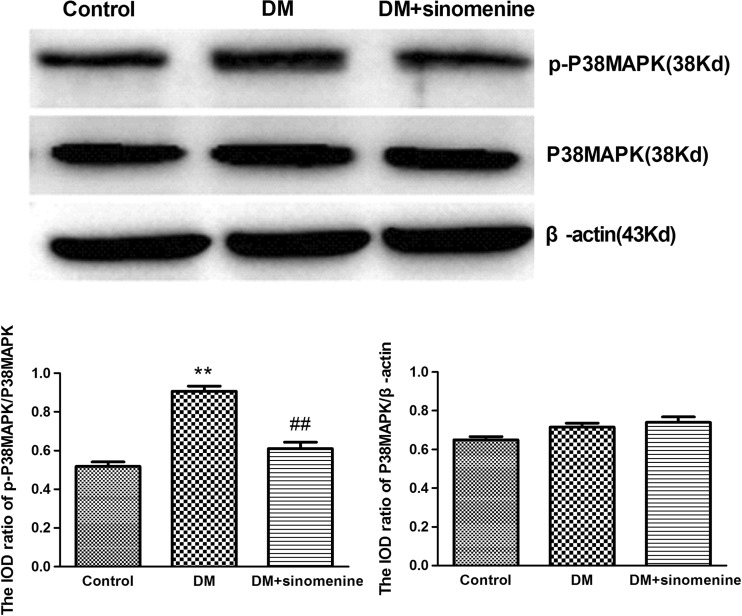
Phosphorylation and activation of P38MAPK are involved in inflammatory pain. The expression levels of P38MAPK and p-P38MAPK in DRG were analyzed using western blotting. The integrated optical density (IOD) ratio of P38MAPK to β-actin was not significantly different between the two groups (p > 0.05). However, the IOD ratio of p-P38MAPK to P38MAPK in the T2DM group was higher than that of the control group (n = 10). These results indicated phosphorylation of P38MAPK in DRG is correlated with hyperalgesia mediated by the P2X3 receptor in T2DM rats.
In addition, we examined whether the administration of sinomenine could affect the phosphorylation of P38MAPK in T2DM DRG. The IOD ratio of p-P38MAPK to P38MAPK in the T2DM rats treated with sinomenine was reduced by 5% compared with that in the T2DM group (p < 0.05, n = 10 for each group) (Fig. 3). Taken together, these results suggest that the effects of sinomenine treatment on hyperalgesia mediated by the P2X3 receptor may contribute to decreasing the phosphorylation and activation of P38MAPK in DRG in T2DM rats.
Fig. 3.
Effects of sinomenine on the expression levels of DRG P38MAPK and p-P38MAPK in T2DM rats. The expression levels of P38MAPK and p-P38MAPK in DRG were analyzed using western blotting analyses. The IOD ratio of P38MAPK to β-actin was not significantly different between the two groups (p > 0.05). The IOD ratio of p-P38MAPK to P38MAPK in the T2DM group was higher compared with that in the control group (n = 10). The IOD ratio of p-P38MAPK to P38MAPK in the T2DM rats treated with sinomenine was significantly lower compared with that in the T2DM group (p < 0.01, n = 10 for each group). Mean ± SEM, n = 10. **p < 0.01 compared with the control group; ## p < 0.01 compared with the DM group
Effects of sinomenine on the ATP-activated currents in HEK293 cells transfected with the pEGFP-hP2X3 plasmid
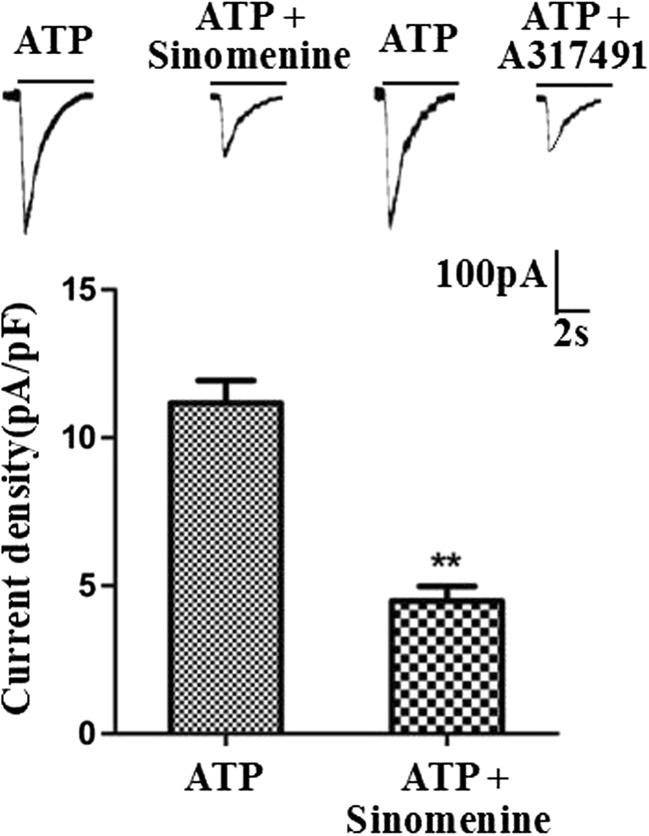
ATP-activated currents (IATP) in HEK293 cells transfected with the pEGFP-hP2X3 plasmid were recorded by whole cell patch clamp. Sinomenine (10 μM) significantly inhibited about 5% ATP-activated (100 μM) current (Fig. 4). We also observed that the specific selective P2X3 antagonist A317491 (10 μM) can inhibit the transient ATP current. These results revealed that sinomenine treatment could relieve pain behaviors in T2DM rats by inhibiting the P2X3 signaling in DRG.
Fig. 4.
Effect of sinomenine on ATP-activated current in HEK293 cells expressing the P2X3 receptor. Whole cell patch clamp recordings were performed on the HEK293 cells transfected with the P2X3 receptor. The results showed that sinomenine (10 μM) inhibited ATP-activated current in HEK293 cells. ATP-induced current can also be inhibited by the P2X3-selective antagonist, A317491 (10 μM). **p < 0.01 vs. IATP without sinomenine
Molecular docking of sinomenine on a hP2X3 receptor
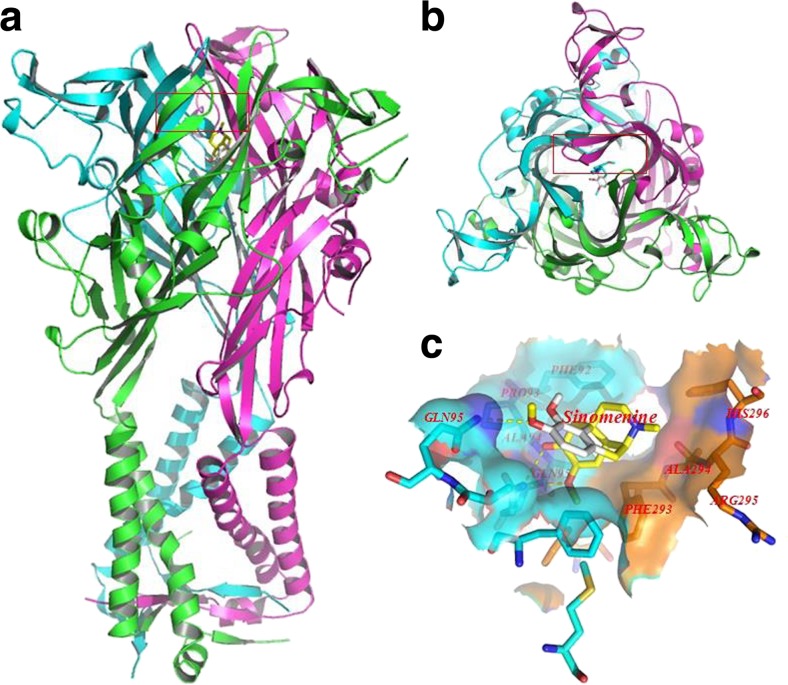
Molecular docking of sinomenine on a hP2X3 protein were generated by the AutoDock 4.2. Docking score of hP2X3 and sinomenine (kcal/mol) showed that sinomenine was enabled the perfect fit to interact with hP2X3 receptor (see Table 1). The perfect match enabled the sinomenine to interact with residues both deep in the ATP-binding pocket and in the outer sphere (Fig. 5).
Table 1.
MOE score of hP2X3 protein and sinomenine (kcal/mol)
| Mode/rank | Affinity (kcal/mol) | Dist from RMSDa lb | Best mode RMSE ub |
|---|---|---|---|
| 1 | −7.5 | 0.000 | 0.000 |
| 2 | −7.4 | 2.897 | 5.401 |
| 3 | −7.4 | 2.540 | 3.927 |
| 4 | −7.4 | 3.105 | 5.127 |
| 5 | −7.4 | 2.484 | 5.206 |
| 6 | −7.0 | 25.976 | 28.089 |
| 7 | −6.8 | 25.454 | 27.574 |
| 8 | −6.7 | 25.058 | 27.035 |
| 9 | −6.5 | 24.963 | 27.091 |
Explanation: The predicted binding affinity is in kilocalories per mole (energy)The docking energy in the 9 best sites are greater than -6kcal/mol, indicates a good docking effect between sinomenine and P2X3 receptor.
aRMSD values are calculated relative to the best mode and use only movable heavy atoms.RMSD matches each atom in one conformation with the closest atom of the same element type in the other conformation. Two variants of RMSD metrics are provided, RMSD lower bound (RMSD/lb) and RMSD upper bound (RMSD/ub), differing on how the atoms are matched in the distance calculation
Fig. 5.
Molecular docking of sinomenine on hP2X3 protein. Simulation modeling of sinomenine docking with hP2X3 protein was simulated by computer. a (forward map), b (top view) The best docking position between sinomenine and hP2X3. The docking position was in the outside of the cell membrane and the entrance of the channel. c The docking pocket; the blue surface and stick-like structure were the B chain of the hP2X3 protein and the orange surface and stick-like structure and the place covered by the blue surface were the C chain of the hP2X3 protein. The yellow dotted line was a hydrogen bond between the residues on the chains and sinomenine. The photo indicated that there was a strong binding energy between sinomenine and GLN95 in the B chain and ALA94 and GLN95 in the C chain. The results revealed that sinomenine could interact with the hP2X3 protein
Discussion
Up to 50% of people with diabetes are attacked by peripheral neuropathies [5, 6, 10–12]. DPN in T2DM patients constitutes a major risk factor for foot ulceration and amputation [6, 8–12]. Our results indicated that the threshold values of mechanical and thermal pain sensitivity in T2DM rat models were decreased compared with those in the control rats. Thermal and mechanical abnormal hyperalgesia in T2DM rats can be increased after lesion in diabetic peripheral neuropathy [21–23]. However, there are no specific relief medications for DNP, and current therapies are often associated with serious side effects [6, 10, 12, 40]. Our experiments showed that the MWT and TWL in T2DM rats treated with sinomenine were significantly enhanced compared with rats in the T2DM model group. Thus, we speculated that sinomenine treatment could reduce mechanical and thermal hyperalgesia in T2DM rats. What mechanism contributed to the inhibitory effects of sinomenine treatment on mechanical and thermal hyperalgesia in T2DM rats?
DM is often related to low-grade inflammation [6, 12]. ATP acts as an inflammatory mediator to participate in inflammatory-mediated pain [13–15, 17, 20, 41]. Damage to the peripheral nerve in neuropathic pain enhances the expression of the P2X3 receptor in the DRG neurons and is involved in pain transmission [13–18, 20]. Our results showed that the expression levels of P2X3 mRNA and protein in DM DRG are enhanced compared with those in the control. P2X3 receptor-mediated hyperalgesia is present in diabetic rat models [21–23]. Thus, the enhanced expression of the P2X3 receptor in DRG may be accompanied with mechanical and thermal hyperalgesia in T2DM rats. Our data also showed that up-regulated expression levels of the DRG P2X3 mRNA and protein in DM rats treated with sinomenine were significantly decreased compared with those in T2DM rats. Sinomenine treatment may decrease the up-regulated expression of the P2X3 receptor in T2DM DRG and relieve the mechanical and thermal hyperalgesia in T2DM rats.
P2X3 receptors are present mainly in small- to medium-sized neurons in the DRG. Selective distribution in small primary afferent neurons suggests a contributing role of the P2X3 receptor in nociception and pain [42]. Activation of the P2X3 receptor contributes to pain and hyperalgesia [43]. This study showed that the up-regulated expression of the P2X3 receptor was followed by an enhancement of mechanical and thermal hyperalgesia, resulting in the subsequent activation of the P2X3 receptor in the T2DM DRG. Cytokines liberated in the setting of peripheral inflammation contribute to pain. Thus, NONRATT021972 siRNA treatment could reduce the serum levels of TNF-α in T2DM rats, decrease the up-regulated expression of the P2X3 receptor in DRG, and relieve P2X3 receptor-mediated DNP in T2DM rats. This finding further confirmed that NONRATT021972 might be involved in the pathological process of T2DM.
To identify whether sinomenine could specifically inhibit signal transduction through the P2X3 receptor, a model of P2X3 receptor expression was established in HEK293 cells. HEK293 cells do not natively express any known P2X3 or other receptors; when unmodified HEK293 cells do not respond to P2X3 agonist stimulation. To validate this work, constructs expressing human P2X3 plasmid were transiently transfected into HEK293 cells as previously described [34, 35]. Our data showed that sinomenine significantly inhibited P2X3 agonist ATP-activated currents in the transfected HEK293 cells. The P2X3-specific antagonist, A317491, can inhibit the ATP-activated currents in the transfected HEK293 cells. The inhibition effect of sinomenine was similar to that of A317491, which suggested that a specific inhibitory effect of sinomenine on P2X3 may be a competitive blockade. These results confirmed that sinomenine inhibited mechanical and heat hyperalgesia mediated by the P2X3 receptor in T2DM rats. The electrophysiological results were consistent with down-regulation of P2X3 expression after sinomenine treatment, which relieved the mechanical and heat hyperalgesia in T2DM rats. As shown in Fig. 5, sinomenine could interact with the hP2X3. Interaction energies for the docked-complexes were calculated by AutoDock 4 and shown in Table 1. In Table 1, a higher value of negative interaction energy is an indicator of more efficient interaction between the hP2X3 and sinomenine. Sinomenine could bind to the hP2X3 to limit the combination of ATP, increasing the concentration of free ATP, leading to inhibition of the hP2X3 channel. Therefore, sinomenine may act as the inhibitor of P2X3 receptor.
P38 is activated in DRG nociceptor neurons by peripheral inflammation and participates in the generation and maintenance of inflammatory and neuropathic pain [44, 45]. Therefore, blockade of P38MAPK activation in DRG is likely able to decrease mechanical and heat hypersensitivity in inflammatory and neuropathic pain. The activation of the P2X3 receptor increases primary afferent nociceptor susceptibility to the action of inflammatory mediators [41]. The phosphorylated P38 levels are in response to inflammatory stimuli or nerve injury [44, 45]. ATP induced activation of P38 [41]. Activation of the P2X3 receptor may be related to the phosphorylation of P38MAPK in DRG, causing mechanical and heat hyperalgesia of the nerve injury. Our studies showed that the IOD ratio of p-P38MAPK to P38MAPK in the T2DM group was higher compared with that of the control group. P38MAPK phosphorylation in DRG may be involved in mechanical and heat hyperalgesia mediated by the P2X3 receptor in T2DM rats. After the administration of sinomenine, the IOD ratio of p-P38MAPK to P38MAPK in T2DM rats was significantly decreased compared with that in the T2DM rats that did not receive sinomenine. Sinomenine inhibited the phosphorylation of p38MAPK in the inflammatory condition [46]. Sinomenine treatment may decrease the phosphorylation and activation of DRG P38MAPK in T2DM rats and relieve mechanical and heat hyperalgesia mediated by the P2X3 receptor.
Conclusions
In summary, sinomenine treatment reduced the up-regulated expression and activation of the P2X3 receptor and decreased the phosphorylation and activation of P38MAPK in T2DM DRG, subsequently relieving thermal and mechanical hyperalgesia in T2DM rats. Thus, sinomenine treatment may alleviate DNP by inhibiting the abnormal excitatory transmission mediated by the P2X3 receptor in T2DM rats.
Acknowledgements
These studies were supported by grants (nos. 31560276, 81570735, 81171184, 31060139, and 81200853) from the National Natural Science Foundation of China, a grant (no. 20151BBG70250) from the Technology Pedestal and Society Development Project of Jiangxi Province, a grant (no. 20142BAB205028) from the Natural Science Foundation of Jiangxi Province, and grants (nos. GJJ13155 and GJJ14319) from the Educational Department of Jiangxi Province.
Compliance with ethical standards
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Shenqiang Rao and Shuangmei Liu are joint first authors
An erratum to this article is available at http://dx.doi.org/10.1007/s11302-017-9560-9.
References
- 1.Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med. 2009;10(5):918–929. doi: 10.1111/j.1526-4637.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- 2.Colvin LA, Dougherty PM. Peripheral neuropathic pain: signs, symptoms, mechanisms, and causes: are they linked? Br J Anaesth. 2015;114(3):361–363. doi: 10.1093/bja/aeu323. [DOI] [PubMed] [Google Scholar]
- 3.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 4.Ma RC, Chan JC. Type 2 diabetes in east Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes. 2015;6(3):432–444. doi: 10.4239/wjd.v6.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 8.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl 1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 9.Yoo M, Sharma N, Pasnoor M, Kluding PM. Painful diabetic peripheral neuropathy: presentations, mechanisms, and exercise therapy. J Diabetes Metab Suppl. 2013 doi: 10.4172/2155-6156.S10-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 11.Morales-Vidal S, Morgan C, Mccoyd M, Hornik A. Diabetic peripheral neuropathy and the management of diabetic peripheral neuropathic pain. Postgrad Med. 2012;124(4):145–153. doi: 10.3810/pgm.2012.07.2576. [DOI] [PubMed] [Google Scholar]
- 12.Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110(3):433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 15.Burnstock G. Purinergic receptors and pain. Curr Pharm Des. 2009;15(15):1717–1735. doi: 10.2174/138161209788186335. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Xu C, Liang S, Zhang A, Mu S, Wang Y, Wan F. Effect of tetramethylpyrazine on primary afferent transmission mediated by P2X3 receptor in neuropathic pain states. Brain Res Bull. 2008;77(1):27–32. doi: 10.1016/j.brainresbull.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Liang S, Xu C, Li G, Gao Y. P2X receptors and modulation of pain transmission: focus on effects of drugs and compounds used in traditional Chinese medicine. Neurochem Int. 2010;57(7):705–712. doi: 10.1016/j.neuint.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80(1–2):273–282. doi: 10.1016/S0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- 19.Zhang A, Gao Y, Zhong X, Xu C, Li G, Liu S, Lin J, Li X, Zhang Y, Liu H, Linag S. Effect of sodium ferulate on the hyperalgesia mediated by P2X3 receptor in the neuropathic pain rats. Brain Res. 2010;1313:215–221. doi: 10.1016/j.brainres.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 20.Burnstock G, Novak I. Purinergic signalling and diabetes. Purinergic Signal. 2013;9(3):307–324. doi: 10.1007/s11302-013-9359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng H, Zou L, Xie J, Hong W, Bing W, Zhu G, Lv Q, Xi Z, Liu S, Li G (2016) LncRNA NONRATT021972 siRNA decreases diabetic neuropathic pain mediated by the P2X 3 receptor in dorsal root ganglia. Mol Neurobiol :1–13 [DOI] [PubMed]
- 22.Wang S, Xu H, Zou L, Xie J, Wu H, Wu B, Yi Z, Lv Q, Zhang X, Ying M, Liu S, Li G, Gao Y, Xu C, Zhang C, Xue Y, Liang S. LncRNA uc.48+ is involved in diabetic neuropathic pain mediated by the P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2016;12(1):139–148. doi: 10.1007/s11302-015-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu GY, Li G, Liu N, Huang LY. Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Mol Pain. 2011;7:60. doi: 10.1186/1744-8069-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao T, Hao J, Wiesenfeld-Hallin Z, Wang DQ, XJ X. Analgesic effect of sinomenine in rodents after inflammation and nerve injury. Eur J Pharmacol. 2013;721(1–3):5–11. doi: 10.1016/j.ejphar.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 25.Gao T, Shi T, Wang DQ, Wiesenfeld Z. Repeated sinomenine administration alleviates chronic neuropathic pain-like behaviours in rodents without producing tolerance. Scandinavian Journal of Pain. 2014;5(4):249–255. doi: 10.1016/j.sjpain.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Lagerström MC. Sinomenine is a promising analgesic and anti-hyperalgesic for pain and hypersensitivity in rheumatoid arthritis. Scandinavian Journal of Pain. 2015;7:15–16. doi: 10.1016/j.sjpain.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Pertovaara A. Sinomenine against neuropathic pain hypersensitivity. Scandinavian Journal of Pain. 2014;5(4):248. doi: 10.1016/j.sjpain.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Q, Sun Y, Zhu J, Fang T, Zhang W, Li JX. Antinociceptive effects of sinomenine in a rat model of neuropathic pain. Sci Rep. 2014;4:7270. doi: 10.1038/srep07270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang AL, Li Z, Yuan M, Yu AC, Zhu X, Tso MO. Sinomenine inhibits activation of rat retinal microglia induced by advanced glycation end products. Int Immunopharmacol. 2007;7(12):1552–1558. doi: 10.1016/j.intimp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Yin Q, Xia Y, Wang G. Sinomenine alleviates high glucose-induced renal glomerular endothelial hyperpermeability by inhibiting the activation of RhoA/ROCK signaling pathway. Biochem Biophys Res Commun. 2016;477(4):881–886. doi: 10.1016/j.bbrc.2016.06.152. [DOI] [PubMed] [Google Scholar]
- 31.Islam MS. Animal models of diabetic neuropathy: progress since 1960s. J Diabetes Res. 2013;2013:149452. doi: 10.1155/2013/149452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Xu H, Zhu S, Xu W, Qin S, Liu S, Tu G, Peng H, Qiu S, Yu S, Zhu Q, Fan B, Zheng C, Li G, Liang S. Effects of neferine on CCL5 and CCR5 expression in SCG of type 2 diabetic rats. Brain Res Bull. 2013;90:79–87. doi: 10.1016/j.brainresbull.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125(3):451–472. [PubMed] [Google Scholar]
- 34.Liu S, Yu S, Xu C, Peng L, Xu H, Zhang C, Li G, Gao Y, Fan B, Zhu Q, Zheng C, Wu B, Song M, Wu Q, Liang S. Puerarin alleviates aggravated sympathoexcitatory response induced by myocardial ischemia via regulating P2X3 receptor in rat superior cervical ganglia. Neurochem Int. 2014;70:39–49. doi: 10.1016/j.neuint.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Zou L, Xie J, Xie W, Wen S, Xie Q, Gao Y, Li G, Zhang C, Xu C, Xu H, Wu B, Lv Q, Zhang X, Wang S, Xue Y, Liang S. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol Brain. 2016;9:44. doi: 10.1186/s13041-016-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansoor SE, Lu W, Oosterheert W, Shekhar M, Tajkhorshid E, Gouaux E. X-ray structures define human P2X3 receptor gating cycle and antagonist action. Nature. 2016;538(7623):66–71. doi: 10.1038/nature19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang R, Taly A, Lemoine D, Martz A, Cunrath O, Grutter T. Tightening of the ATP-binding sites induces the opening of P2X receptor channels. EMBO J. 2012;31(9):2134–2143. doi: 10.1038/emboj.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krimon S, Araldi D, Do PF, Tambeli CH, Oliveira-Fusaro MC, Parada CA. P2X3 receptors induced inflammatory nociception modulated by TRPA1, 5-HT3 and 5-HT1A receptors. Pharmacol Biochem Behav. 2013;112:49–55. doi: 10.1016/j.pbb.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95(2):229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2001;53(4):553–568. [PubMed] [Google Scholar]
- 44.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. P38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23(10):4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. P38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/S0896-6273(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 46.Oh YC, Kang OH, Kim SB, Mun SH, Park CB, Kim YG, Kim YI, Lee YS, Han SH, Keum JH, Shin DW, Ma JY, Kwon DY. Anti-inflammatory effect of sinomenine by inhibition of pro-inflammatory mediators in PMA plus A23187-stimulated HMC-1 cells. Eur Rev Med Pharmacol Sci. 2012;16(9):1184–1191. [PubMed] [Google Scholar]