Abstract
Previously, we localized ADP-activated P2Y12 receptor (R) in rodent kidney and showed that its blockade by clopidogrel bisulfate (CLPD) attenuates lithium (Li)-induced nephrogenic diabetes insipidus (NDI). Here, we evaluated the effect of prasugrel (PRSG) administration on Li-induced NDI in mice. Both CLPD and PRSG belong to the thienopyridine class of ADP receptor antagonists. Groups of age-matched adult male B6D2 mice (N = 5/group) were fed either regular rodent chow (CNT), or with added LiCl (40 mmol/kg chow) or PRSG in drinking water (10 mg/kg bw/day) or a combination of LiCl and PRSG for 14 days and then euthanized. Water intake and urine output were determined and blood and kidney tissues were collected and analyzed. PRSG administration completely suppressed Li-induced polydipsia and polyuria and significantly prevented Li-induced decreases in AQP2 protein abundance in renal cortex and medulla. However, PRSG either alone or in combination with Li did not have a significant effect on the protein abundances of NKCC2 or NCC in the cortex and/or medulla. Immunofluorescence microscopy revealed that PRSG administration prevented Li-induced alterations in cellular disposition of AQP2 protein in medullary collecting ducts. Serum Li, Na, and osmolality were not affected by the administration of PRSG. Similar to CLPD, PRSG administration had no effect on Li-induced increase in urinary Na excretion. However, unlike CLPD, PRSG did not augment Li-induced increase in urinary arginine vasopressin (AVP) excretion. Taken together, these data suggest that the pharmacological inhibition of P2Y12-R by the thienopyridine group of drugs may potentially offer therapeutic benefits in Li-induced NDI.
Keywords: Purinergic receptors, Extracellular nucleotides, Arginine vasopressin, Diabetes insipidus, Nephrogenic, Polyuria
Introduction
It is now well-established that extracellular nucleotides (ATP/ADP/UTP), acting through P2 purinergic receptors, play a significant role in fine-tuning salt and water reabsorption along the nephron and collecting duct. Studies also revealed that most of the effects of extracellular nucleotides on renal tubular transport of salt and water are mediated by UTP/ATP-activated P2Y2 receptor, which is expressed widely in the kidney [1–4]. It has been shown that P2Y2 receptor exerts its effect on salt and water reabsorption by its ability to oppose the actions of aldosterone and arginine vasopressin (AVP) on the medullary collecting duct [reviewed in 5–9].
Recently, we discovered that ADP-activated P2Y12 receptor is also expressed in the rodent kidney, and it may play a potential role in water handling by the kidney by opposing the action of AVP on the collecting duct [10]. Thus, it appears that the autocrine and/or paracrine actions of extracellular nucleotides through P2Y receptors constitute tonic inhibitory effect on water and sodium reabsorption mediated by the AVP and aldosterone. Based on this deduction, we posited that the same actions of extracellular nucleotides mediated by P2Y receptors may also contribute to the development of collecting duct resistance to AVP often seen in acquired nephrogenic diabetes insipidus (NDI), such as the one induced by chronic lithium administration for the treatment of bipolar disorder [11–13]. Accordingly, we demonstrated that genetic deletion of P2Y2 receptor confers significant resistance for the development of lithium-induced NDI [14, 15]. However, currently, there are no FDA-approved selective antagonists of the P2Y2 receptor, thus limiting our in vivo studies using pharmacological approach on this receptor. In contrast, the availability of FDA-approved and selective P2Y12 receptor antagonists allowed us to demonstrate that its pharmacological blockade significantly ameliorates lithium-induced NDI in rodents [10, 16]. These drugs are used to inhibit platelet ADP receptor and thus prevent acute episodes of cardiovascular or cerebrovascular events, such as heart attack and stroke, respectively.
In our studies we blocked the P2Y12 receptor in rodents by the administration of clopidogrel bisulfate (CLPD) [10, 16]. CLPD has been in the clinical use for about 19 years as an anticlotting drug by virtue of its ability to block the platelet P2Y12 receptor (ADP receptor) irreversibly. CLPD belongs to the thienopyridine group of P2Y12 receptor antagonists. It is a prodrug that needs to be activated in the liver by cytochrome P450 enzymes generating its active metabolite (Act-Met) [17]. The active metabolite, which constitutes about 15% of the ingested drug, irreversibly binds to the P2Y12 receptor by forming disulfide bridges and thus prevents its activation by ADP [18].
Prasugrel (PRSG), another FDA-approved drug, also belongs to the same thienopyridine group of P2Y12 receptor antagonists. Similar to CLPD, it is a prodrug activated in the liver and its active metabolite irreversibly binds to P2Y12 receptor. However, there are significant differences between CLPD and PRSG with respect to their activation, pharmacokinetics, and pharmacodynamics [19, 20]. For example, CLPD is oxidatively activated by CYP2C19, CYP3A4, and CYP3A5 cytochrome P450 enzymes, whereas PRSG is activated by ester hydrolysis followed by CYP2C19 oxidation. Furthermore, activation of CLPD shows large interindividual differences, drug interactions, delayed start of effect, and very long-lasting effect [21, 22]. In contrast, PRSG administration has more efficient activation and less resistance, faster onset of effect, more potent mechanism of action, and fewer drug interactions [23]. Similarly, there are differences in the clinical efficiency of CLPD and PRSG as antiplatelet drugs [24, 25], which have been attributed to their differences in activation and/or metabolism [26]. In view of these differences, in this communication, we evaluated whether PRSG has similar effect on lithium-induced NDI in mice as we demonstrated previously using CLPD [16]. Lithium-induced NDI is characterized by polydipsia, polyuria, and impaired concentrating ability of the kidney, associated with marked decrease in the protein abundance of AVP-regulated collecting duct water channel, aquaporin-2 (AQP2). In addition, in lithium-induced NDI, the urinary excretion of sodium and AVP are high. Hence, we investigated the effect of PRSG on these lithium-induced characteristics.
Methods
Experimental animals
The animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Veterans Affairs Salt Lake City Health Care System, Salt Lake City, Utah. Specific pathogen-free B6D2 mice bred in-house were used in the study. All mice were subcutaneously implanted with microchip transponders for efficient tracking of the mice and the samples derived from them before and after euthanasia (Locus Technology, Inc., Manchester, MD). This also ensured blinding of samples during analysis. Unless otherwise stated, mice were housed in groups in conventional plastic cages with bedding and regulated light-dark cycles and had free access to food and drinking water. NDI was induced by the administration of LiCl by the methods established in our laboratory [10, 14–16]. Age-matched adult male mice were randomly divided into four groups (five mice/group). Group 1 (CNT; control mice) was fed regular rodent chow, group 2 received prasugrel (PRSG; 10 mg/kg bw/day) in drinking water, group 3 was fed lithium chloride-added diet (40 mmol LiCl/kg chow; MP Biomedicals, Solon, OH), and group 4 received a combination of lithium in food and PRSG in drinking water. PRSG was administered by mixing finely powdered tablets (Eli Lilly and Company, Indianapolis, IN) in drinking water. Based on the water consumption of the animals on the previous day, the concentration of PRSG in the drinking water was adjusted daily. The dose of PRSG used here, when adjusted to the K m factor (ratio of body surface area to body weight of the species (mouse vs. man) as per Reagan-Shaw et al. [27]) was approximately 5-fold higher than the human effective dose (HED). Thus, it is comparable to the dose of clopidogrel bisulfate we used previously in mouse model of lithium-induced NDI [16]. Twenty-four-hour urine samples were collected prior to the start of the experiment (day 0), and then on days 7 and 8 and 13 and 14 by placing the mice in plastic metabolic cages (1 mouse/cage), with free access to food and water. All mice were euthanized on day 14 after collection of urine samples. Blood and kidneys were collected at the time of euthanasia. Serum was separated after blood clotting and retraction of the clot. Cortical and medullary regions of the kidneys were dissected out, flash frozen, and then processed for laboratory assays. One half of one kidney from each mouse was fixed in 10% buffered formalin and then embedded in paraffin blocks.
Urine and serum analysis
Clear supernatants of urine obtained by centrifugation were used for the determination of osmolality by vapor pressure method (Wescor, Logan, UT). The concentrations of sodium and lithium in the serum, and urinary sodium, were measured with an EasyLyte (Medica, Bedford, MA) analyzer. Urinary excretion of AVP was determined by an ELISA kit (Enzo Life Sciences, Farmingdale, NY) as described previously [16].
Western blotting for proteins
Semiquantitative immunoblotting approach was used to determine the protein abundances of aquaporin-2 (AQP2) water channel of the collecting duct, the bumetanide-sensitive Na, K, 2Cl cotransporter-1 (BSC1 or NKCC2) of the thick ascending limb and the NaCl cotransporter (NCC) of the distal convoluted tubule in the kidney, as described previously [10, 16]. Briefly, cortical and medullary tissues were homogenized in a buffer containing protease inhibitors. Protein concentrations of the homogenates were determined, and samples were solubilized in Laemmli sample buffer. Equal amounts of protein in the samples were applied on 7.5 or 12% polyacrylamide precast gels (Life Technologies, Grand Island, NY or Bior-Rad, Hercules, CA) and subjected to electrophoresis. The size-fractionated proteins in the gels were electrotransferred to nitrocellulose membranes. After blocking with fat-free milk, the membranes were probed with our own rabbit peptide-derived polyclonal antibody against aquaporin-2 (AQP2), NKCC2, or NCC as previously described [15, 16]. Our polyclonal antibodies were derived from the same peptide sequences identified and utilized by Dr. Mark Knepper to produce antibodies. They were further evaluated by us for their specificity in our laboratories. For loading accuracy, parallel-run blots were probed with rabbit β-actin monoclonal antibody (Sigma-Aldrich, St. Louis, MO or Biolegend, San Diego, CA). Band densities of channel/transporter proteins were determined and were normalized to the densities of the respective β-actin bands. Mean band densities in each group were expressed as percent of the mean values in the control group. To avoid individual bias, Western blots were prepared by technical staff that had no knowledge of the project outcome. Digitization and of blots and densitometry were done by two of the authors (YZ and CME).
Morphological examination and confocal immunofluorescence
Paraffin-embedded kidneys were sectioned at 5-μm thickness, deparaffinized and rehydrated. Sections were stained with hematoxylin-eosin and examined under light microscope for morphological profiles. Sections were at first randomly examined in blind fashion, to gain overall assessment, and photographs were taken during a second round of examination. For confocal immunofluorescence microscopy, kidney sections (5-μm thickness) were shipped to the laboratory of JP-P at the University of Southern California. These sections were processed by methods described previously [10, 16, 28]. Briefly, deparaffinized and rehydrated sections were heated for 2 × 10 min in a microwave with medium heat in PBS and allowed to cool for 40 min to retrieve antigen. Sections were then permeabilized for 10 min with 0.1% Triton X-100 in PBS. To block non-specific binding, goat serum (1:20 dilution, Jackson Immunoresearch Laboratories, Inc., West Grove, PA) in PBS was applied to sections for 1 h. Sections were then probed with AQP2 goat polyclonal antibody (sc-9882 from Santa Cruz, CA; 1:100 dilution) or our P2Y12 receptor rabbit polyclonal antibody [10, 16], overnight followed by incubation with secondary Alexa fluor 594-conjugated goat anti-rabbit antibody (Invitrogen) or Alexa fluor 488-conjugated donkey anti-goat antibody, respectively, for 1 h. AQP2 and P2Y12 immunofluorescence experiments were performed by one of the authors (AR-B), and the slides were evaluated and imaged independently and blindly by another (JP-P). The peptide-derived P2Y12 receptor antibody used here was designed, generated, and thoroughly characterized by us and published [10]. Using an array of approaches, such as shRNA knockdown, overexpression, and immunodetection in native cells known to express P2Y12 receptor, such as platelets and microglia, we have established the specificity of our P2Y12 receptor antibody [10].
Statistical analysis
Quantitative data are expressed as mean ± SE. Data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey-Kramer Multiple Comparisons Test or Bonferroni Test. P values <0.05 were considered significant. GraphPad Instat software was used for statistical analysis (GraphPad Software, Inc., La Jolla, CA).
Results
Effect of PRSG on lithium-induced polydipsia and polyuria
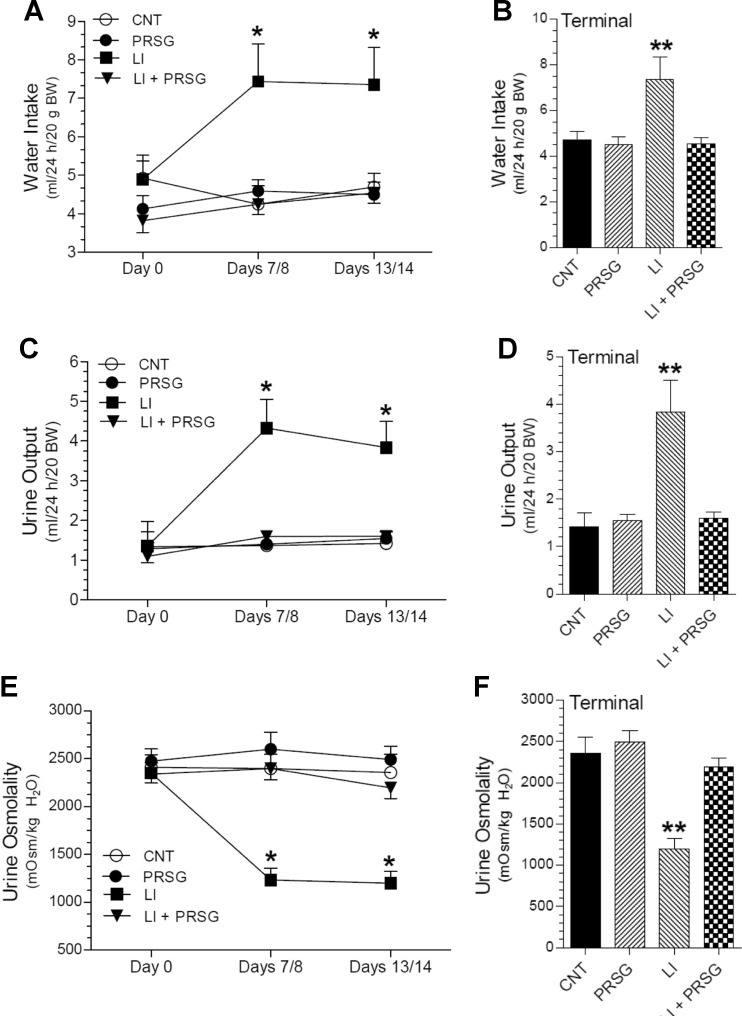
Lithium-induced NDI manifests as polydipsia and polyuria. Figure 1 shows the effect of PRSG on lithium-induced polydipsia and polyuria. The latter was assessed by urine output and urine osmolality. As expected, administration of lithium caused marked increase in water intake (Fig. 1a, b) and urine output (Fig. 1c, d) associated with a marked decrease in urine osmolality (Fig. 1e, f). Lithium-induced polydipsia and polyuria reached maximal values by day 7/8, and after that, they remained unchanged until the time of euthanasia on day 14. In contrast, in the group that received lithium in combination with PRSG, both polydipsia and polyuria were markedly suppressed from the beginning of the experimental period (P < 0.05 or better). In fact, during the experimental period, the water consumption, urine output, and urine osmolality in this group were comparable to the ones seen in the control (CNT) group. Administration of PRSG without lithium had no significant effect on basal water consumption, urine output, or urine osmolality.
Fig. 1.
Effect of prasugrel on lithium-induced polydipsia and polyuria. Groups of mice were fed regular diet (CNT) or lithium-added diet (LI) or prasugrel in drinking water (PRSG) or a combination of Li in the diet and PRSG in drinking water (LI + PRSG) for 14 days and euthanized. Twenty-four-hour water intake and urine output were determined, prior to the experimental period (day 0), on days 7 and 8, and prior to euthanasia (days 13 and 14; terminal). Mean values for days 7 and 8 and 13 and 14 were used in computing the data. a, b show water intake during the three time points or terminally, respectively. c, d show urine output during the three time points or terminally, respectively. e, f show urine osmolality during the three time points or terminally, respectively. Values shown are mean ± SE (N = 5 mice/per group). *significantly different from other groups at the same time point by ANOVA (P < 0.05 or better). **significantly different from all other groups by Tukey-Kramer multiple comparison test followed by ANOVA (P < 0.05 of better)
Effect of PRSG on lithium-induced decrease in AQP2 protein abundance
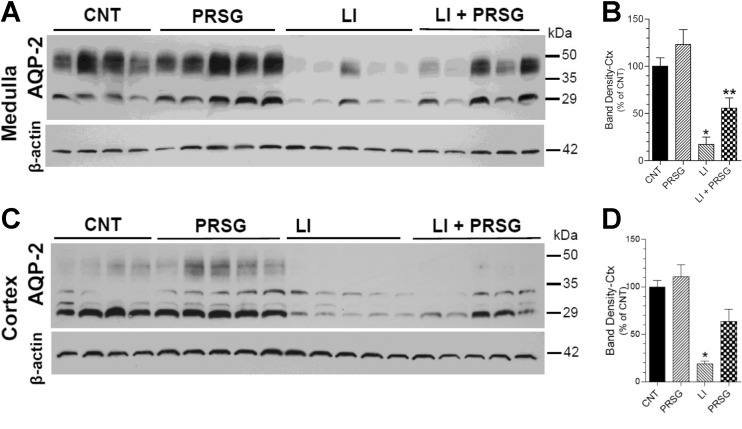
Lithium-induced polyuria is attributed to a decrease in the protein abundance of the AVP-regulated collecting duct water channel AQP2. In order to assess whether the observed suppressing effect of PRSG on lithium-induced polyuria is associated with changes in the protein abundance of AQP2, we performed semiquantitative immunoblotting using kidney cortical and medullary tissue homogenates. As shown in Fig. 2a, b, lithium treatment resulted in a marked decrease in the AQP2 protein abundance in the medulla (P < 0.01). Administration of PRSG significantly restored AQP2 protein abundance to higher levels as compared to the mice treated with lithium alone (P < 0.03). Although PRSG restored the AQP2 protein abundance to 3.2-fold higher than the mean value in lithium-treated mice, it corresponds to 56 ± 11% of the mean value seen in untreated control mice. Administration of PRSG alone tended to increase the AQP2 protein abundance, but the increase was not statistically significant. A similar pattern of changes in AQP2 protein abundance was seen in the cortical tissue homogenates (Fig. 2c, d). Lithium treatment caused marked decrease in AQP2 protein in the cortex (P < 0.001), which was significantly restored by the administration of PRSG (P < 0.05). The restored value was 64 ± 13% of the mean value in the control group. Thus, administration of PRSG significantly, but not completely, countered lithium-induced decreases in AQP2 protein abundance in the renal cortex and medulla.
Fig. 2.
Effect of prasugrel on lithium-induced decreases in AQP2 protein abundances in the kidney. Groups of mice were fed regular diet (CNT) or lithium-added diet (LI) or prasugrel in drinking water (PRSG) or a combination of Li in the diet and PRSG in drinking water (LI + PRSG) for 14 days and euthanized. Whole tissue homogenates of renal medulla or cortex were processed for semiquantitative immunoblotting for abundances of AQP2 water channel and β-actin proteins. a shows immunoblot profiles for AQP2 protein in the medulla, where each lane represents sample for one mouse. AQP2 protein band densities were normalized by corresponding β-actin band densities and are shown in b (mean ± SE). *significantly different from the CNT and PRSG groups by Tukey-Kramer multiple comparison test following ANOVA (P < 0.01); **significantly different from the LI group by Bonferroni Test following ANOVA (P < 0.05). c shows immunoblot profiles for AQP2 protein in the cortex, where each lane represents sample from one mouse. AQP2 protein band densities were normalized by corresponding β-actin band densities and are shown in d (mean ± SE) for the corresponding immunoblots of medulla and cortex. *significantly different from all other groups by Tukey-Kramer multiple comparison test following ANOVA (P < 0.05 or better)
Effect of PRSG on sodium cotransporter protein abundances
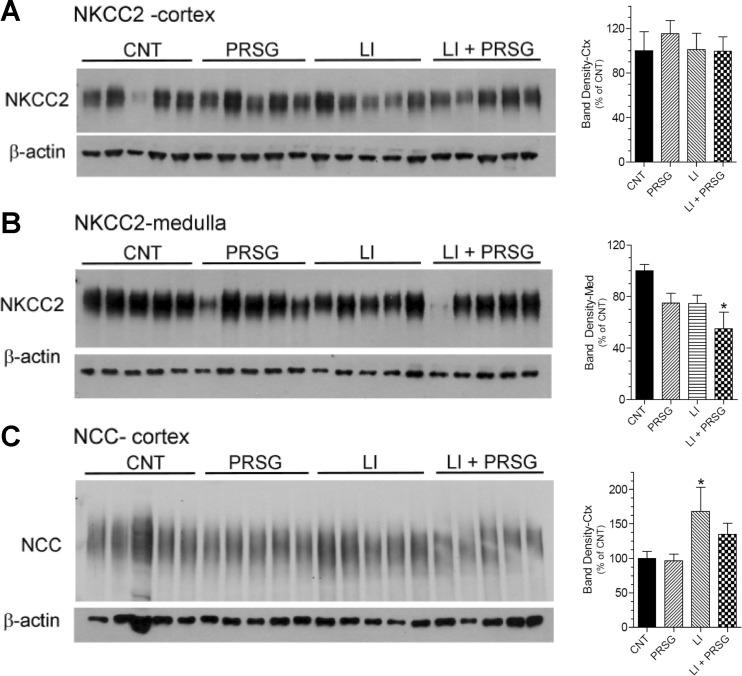
We also assessed the effect of prasugrel on the two major sodium-coupled cotransporters of the renal tubule that play a significant role in urinary concentration mechanism, i.e., NKCC2 of the thick ascending limb, and NCC of the distal convoluted tubule. As shown in Fig. 3a, we found no effect of lithium or PRSG on cortical NKCC2 protein abundance (two-way ANOVA P values for cortical NKCC2 were as follows: lithium 0.62, PRSG 0.64, interaction 0.56). Medullary NKCC2 protein (Fig. 3b) abundance was significantly reduced in the Li + PRSG group relative to the control group (P < 0.05). In fact, both PRSG and lithium significantly decreased medullary NKCC2 protein abundance by two-way ANOVA (P values, lithium 0.017, PRSG 0.018, interaction 0.73). On the other hand, NCC was increased by Li alone and effect that was countered by PRSG (Fig. 3c; P values, lithium 0.02, PRSG 0.38, interaction 0.47).
Fig. 3.
Effect of prasugrel alone or in combination with lithium on the protein abundances of NKCC2 and NCC in the renal cortex and/or medulla. Groups of mice were fed regular diet (CNT) or lithium-added diet (LI) or prasugrel in drinking water (PRSG) or a combination of Li in the diet and PRSG in drinking water (LI + PRSG) for 14 days and euthanized. Whole-tissue homogenates of renal medulla or cortex were processed for semiquantitative immunoblotting for abundances of sodium transporters, NKCC2 or NCC, and β-actin. a, b show immunoblot profiles for NKCC2 protein bands in the medulla and cortex, respectively, where each lane represents sample from one mouse. Bar graphs on the right side of each panel show corresponding densitometric values (mean ± SE) for the protein bands. *significantly different from the CNT group by Tukey-Kramer multiple comparison test following by ANOVA (P < 0.05). c immunoblot profile for NCC protein bands in the cortex, where each lane represents sample from one mouse. Bar graph on the right side of shows corresponding densitometric values (mean ± SE) for the immunoblot. *significantly different from the CNT and PRSG groups by Tukey-Kramer multiple comparison test following ANOVA (P < 0.03)
Confocal immunofluorescence imaging of AQP2 and P2Y12 receptor proteins in the medulla
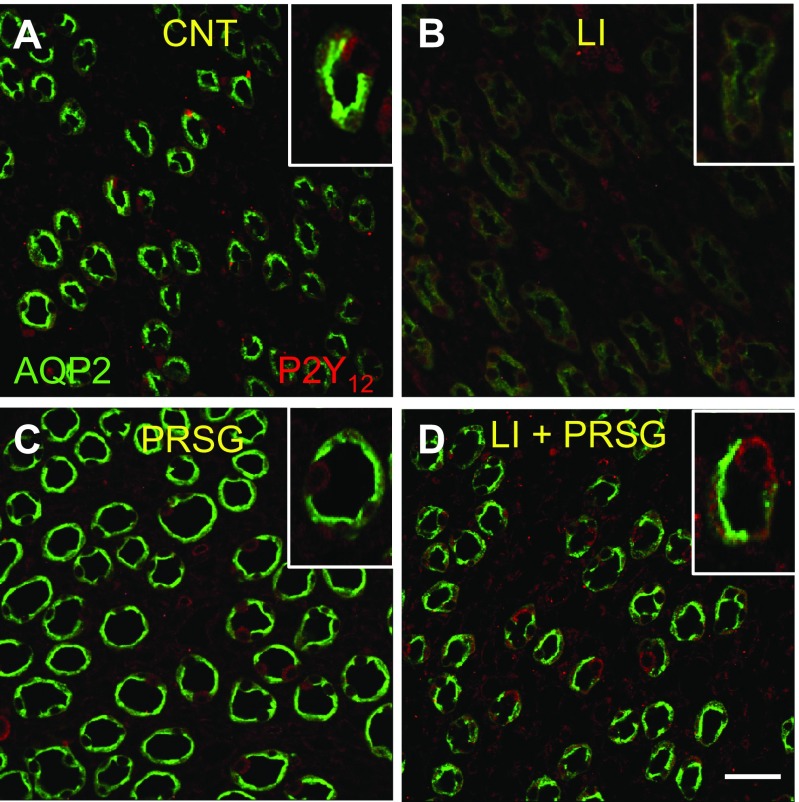
Confocal immunofluorescence imaging was used to visualize the cellular expression and disposition of AQP2 and P2Y12 receptor proteins in the inner medullary collecting ducts of mice treated with PRSG and/or lithium in comparison with control mice. Immunofluorescence labeling was even throughout the sections and equal among the different kidney samples belonging to the same experimental groups. Representative images of the inner medulla that contain the same density and orientation of collecting ducts were presented here. As shown in Fig. 4, in control mice, AQP2 protein (green) could be seen in abundance and both on apical domain and throughout the body of the cells in medullary collecting ducts (Fig. 4a). Lithium treatment caused a marked decrease in the cellular levels of APQ2 in the medullary collecting ducts (Fig. 4b). Administration of PRSG to the Li-treated mice restored the AQP2 protein abundance to the intensity seen in the control group, with comparable cellular distribution (Fig. 4d). Interestingly, administration of PRSG alone appeared to modestly increase AQP2 protein abundance in the cell, with more intense labeling at the apical domain (Fig. 4c). No detectable alterations in the labeling pattern of P2Y12 receptor protein (red) could be seen among the four groups.
Fig. 4.
Immunofluorescence (IF) imaging of cellular expression and disposition of APQ2 (green) and P2Y12 receptor (red) proteins in the inner medullary collecting ducts of mice treated with PRSG and/or lithium in comparison with control mice. Representative low magnification profiles show IF labeling in mice treated with no drug (a), LI (b), prasugrel (PRSG) (c), or a combination of LI and PRSG (d). Insets show corresponding higher magnification profiles. Fluorescence imaging settings were the same in all groups. Bar is 20 μm
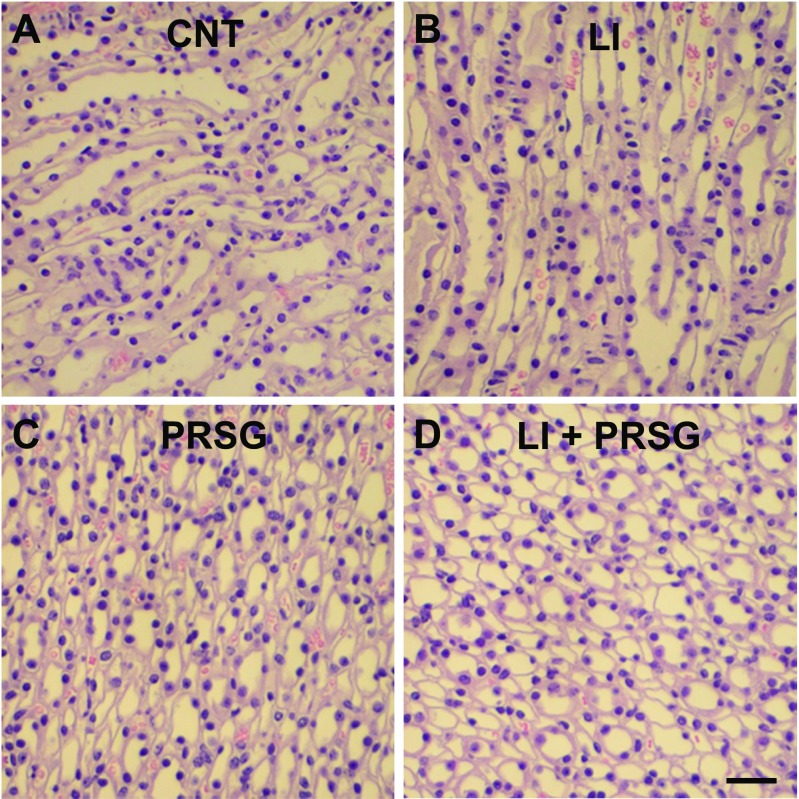
Effect of PRSG with/without lithium on medullary collecting duct morphology
Lithium-induced NDI is known to predominantly affect the medullary collecting ducts. Hence, to assess whether administration of PRSG with or without lithium had observably altered on the morphology of medullary collecting ducts, we examined paraffin sections of the kidneys stained with hematoxylin-eosin under a light microscope. As shown in Fig. 5, administration of PRSG alone or in combination with lithium did not cause detectable alterations in the morphology of the medullary collecting ducts, which looked similar to the ones seen in control group. Under our experimental conditions, administration of lithium alone did not cause detectable alterations in the morphology of medullary collecting ducts. Furthermore, examination of kidney sections stained with periodic acid–Schiff (PAS) reagent revealed that the integrity of tubular and glomerular cell membranes was not affected (not shown here).
Fig. 5.
Morphological appearance of medullary collecting ducts in mice treated with PRSG and/or lithium. Formalin-fixed and paraffin-embedded kidney samples were sectioned and stained with hematoxylin-eosin. Representative profiles of medullary collecting duct in a control mouse (a) or a mouse fed lithium (b) or treated with prasugral (c) or a combination of lithium and prasugrel (d). Bar is 20 μm
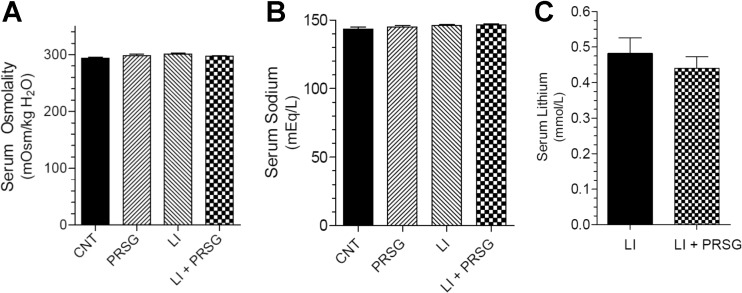
Effect of PRSG and/or lithium on serum parameters
The administration of PRSG with/without lithium did not have any effect on serum osmolality (Fig. 6a) or serum sodium levels (Fig. 6b), which are comparable to the respective mean values seen in the control group. Furthermore, administration of PRSG did not affect the mean serum lithium levels (Fig. 6c).
Fig. 6.
Effect of prasugrel and/or lithium on blood parameters. a Terminal serum osmolalities in all the groups. b Terminal serum sodium levels in all the groups. c Terminal serum lithium levels in lithium-treated groups. Values shown are mean ± se (N = 5 mice/group)
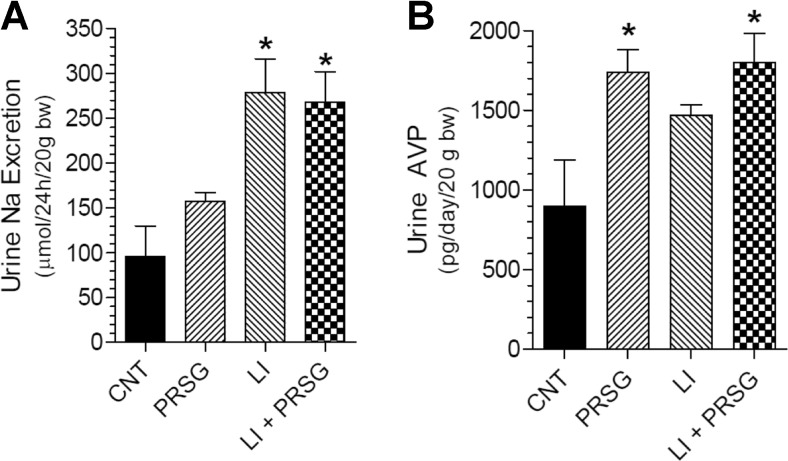
Effect of PRSG and/or lithium on urinary excretion of sodium and AVP
Lithium-induced NDI is known to result in increased urinary sodium and AVP excretion. As expected and shown in Fig. 7a, lithium feeding resulted in significant 2.9-fold increase in urinary excretion of sodium (P < 0.01). Administration of PRSG did not affect lithium-induced urinary excretion of sodium, which remained significantly high (P < 0.01). Interestingly, administration of PRSG alone resulted in a 60% increase in mean urinary excretion of Na as compared to the control group, although the difference was not significant due to large variation in the control group. Similarly and as expected, feeding lithium resulted in a 1.6-fold increase in urinary excretion of AVP, although the increase was not significant due to large variation in the control group. Administration of PRSG modestly increased the mean value in the lithium-treated group; but the increase was not significant. PRSG administration alone caused a significant 1.9-fold increase in mean urinary AVP level as compared to the control group (P < 0.05).
Fig. 7.
Effect of prasugrel and/or lithium on urinary parameters. a Urinary sodium excretion. *significantly different from CNT group by Tukey-Kramer multiple comparison test following ANOVA (P < 0.01). b Urinary AVP excretion. *significantly different from CNT group by Tukey-Kramer multiple comparison test following ANOVA (P < 0.05). All data are mean ± se, N = 5 mice/group
Discussion
In this communication, we demonstrated that administration of PRSG, a selective and irreversible antagonist of the P2Y12 receptor, ameliorated the key features of lithium-induced NDI, namely, polydipsia, polyuria, and decrease in AQP2 protein abundance in kidney cortex and medulla. Confocal immunofluorescence imaging revealed a reversal of lithium-induced alterations in cellular disposition of AQP2 protein by PRSG. However, PRSG administration has minimal impact on the protein abundances of sodium transporters, NKCC2 and NCC. These effects of PRSG were not due to a decrease in blood lithium levels. Furthermore, we showed that PRSG treatment did not have any effect on the lithium-induced increase in urinary sodium levels. Finally, we showed that PRSG alone caused a significant increase in urinary excretion of AVP, but that it did not significantly potentiate the effect of lithium on urinary AVP excretion.
Previously, using confocal immunofluorescence microscopy, we showed that P2Y12 receptor protein is expressed in several structures of mouse kidney, such as the proximal tubule brush border and blood vessels in the cortex, thick ascending limbs and collecting ducts in the medulla. However, the intensity of labeling is less in the medulla as compared to the cortex [16]. We also demonstrated that administration of CLPD significantly attenuated lithium-induced NDI and alterations in the kidney [10, 16]. This study extended our earlier observations to another P2Y12 receptor antagonist, PRSG, which is a distinct molecule as compared to CLPD, and also marketed as such. Overall, the effects of PRSG on lithium-induced NDI observed here are comparable to the effects of CLPD we reported previously in the mouse model of lithium-induced NDI [16]. However, we also observed a few differences between these two drugs with respect to lithium-induced NDI. Before elaborating those differences, it should be noted that the doses of CLPD and PRSG used by us in the mouse models are 5-fold higher than their respective human effective doses (HEDs), and thus are comparable between the two models. The effect of PRSG on lithium-induced polyuria (assessed by an increase in urine output and decrease in urine osmolality) and the decrease in AQP2 protein abundances in the renal cortex and medulla are comparable to those we previously reported for CLPD [16]. The cellular disposition of AQP2 in the medullary collecting ducts in mice treated with lithium in combination with CLPD or PRSG was also comparable.
The effects of PRSG on NCC and NKCC2 abundances were somewhat different than what we observed with CLPD. CLPD resulted in a 30–200% increase in NKCC2 and NCC band densities even in the absence of lithium. In the present study, PRSG led to a modest reduction in NKCC2 in the medulla, with little change in NCC or cortical NKCC2 band densities. We do not know the mechanism underlying these differences. It should be noted that only a fraction (∼15%) of the administered CLPD or PRSG is converted in the liver into the so-called active metabolite that irreversibly binds to the P2Y12 receptor. We do not know the biological effects of the other non-active metabolites, if any, especially on the kidney, through which they are excreted. Obviously, more studies are needed to delineate the effects of different metabolites of CLPD and PRSG.
With respect to blood and urine parameters, PRSG administration did not alter serum lithium levels. This is in contrast to the administration of CLPD, which caused a significant, but modest, increase in serum lithium levels [16]. Previously, we showed that administration of CLPD alone did not have any effect on urinary sodium excretion [16], whereas administration of PRSG alone tended to have numerically higher (1.6-fold) urinary sodium excretion. But, neither CLPD nor PRSG protected against lithium-induced increase in urinary sodium excretion.
Previously, we showed that administration of CLPD alone to rats increased urinary AVP excretion, a surrogate for circulating AVP levels. We also observed a similar CLPD-induced increase in the mouse model; however, the increase did not reach statistical significance [16] as it did in the rat model [10]. In contrast, in this study, we observed that administration of PRSG alone caused a significant 1.9-fold increase in urinary excretion of AVP, which may account for numerically higher values for urinary osmolality and AQP2 protein abundance in the medulla of the PRSG-treated group.
Thus, in spite of the differences in activation, bioavailability, pharmacokinetics, and pharmacodynamics, in our hands, CLPD and PRSG had comparable ameliorating effect on lithium-induced NDI in mice. This observation opens an avenue to develop new therapies for lithium-induced NDI in the clinic. Despite the advent of newer and safer drugs, lithium remains the main choice for the treatment of bipolar disorder by virtue of its ability to prevent suicidal tendencies [29–31]. To boost its therapeutic value, in recent years, lithium has emerged as a robust neuroprotective agent for the treatment of acute brain injury (e.g., stroke) and chronic neurodegenerative diseases (e.g., Alzheimer’s) [32–35]. In view of these potential current and future clinical applications, lithium is expected to stay on the market for a long time.
In the clinical setting, treatment of bipolar patients with lithium lasts for several years or decades. But, the major limitation in the chronic lithium therapy is development of NDI. NDI is a debilitating condition resulting in social inconvenience to morbidity. Elderly patients with NDI have an elevated risk of dehydration, hypernatremia, alterations in consciousness, and hemodynamic instability from hypovolemia [36, 37]. Currently used therapies for the treatment of NDI are often associated with adverse effects. For example, the use of amiloride, which blocks the entry of lithium to collecting duct cells by interacting with epithelial sodium channel (ENaC) enhances lithium-induced natriuresis [38, 39]. Thiazides, which reduce glomerular filtration rate (GFR) by activating the tubuloglomerular feedback (TGF) and thus decrease polyuria, also reduce renal excretion of lithium potentially causing lithium intoxication [40]. Finally, the long-term use of non-steroidal anti-inflammatory drugs (NSAID; e.g., indomethacin) or cyclooxygenase (COX) inhibitors is associated with adverse outcomes, including lithium intoxication [41–43], although they are effective in controlling the lithium-induced polyuria. Hence, there is an unmet need to find safer and efficacious drugs for the treatment of lithium-induced NDI.
In this context, our work shows that pharmacological blockade of the P2Y12 receptor by CLPD or PRSG, which is proven safer, has the potential for the treatment of lithium-induced NDI. More importantly, this approach shifts the current focus of research and therapies for lithium-induced NDI from the ones that counter anti-AVP effects of lithium to the ones that enhance the sensitivity of the kidney to AVP action, i.e., relieves the AVP-resistant state in lithium-induced NDI by eliminating the tonic inhibition of AVP action by the P2Y12 receptor. Thus, in addition to safety, there is novelty in this approach.
Acknowledgements
This work was supported by a grant from the US Department of Veterans Affairs Merit Review Program (to B. K. Kishore), the resources and facilities at the VA SLC Health Care System, Salt Lake City, Utah, and Marriott Cardiovascular Fellowship (to C. M. Ecelbarger). A. Brandes has been supported by Undergraduate Research Opportunities Program (UROP) of the University of Utah. Additional funding sources include National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-64324 (to J. Peti-Peterdi), and an Established Investigator Award from the American Heart Association (to C. M. Ecelbarger). The authors thank Kristina M. Heiney and Hwal Lee for the technical assistance.
Compliance with ethical standards
Conflict of interest
Yue Zhang declares that s/he has no conflict of interest.
János Peti-Peterdi declares that s/he has no conflict of interest.
Anna U. Brandes declares that s/he has no conflict of interest.
Anne Riquier-Brison declares that s/he has no conflict of interest.
Noel G. Carlson declares that s/he has no conflict of interest.
Christa E. Müller declares that s/he has no conflict of interest.
Carolyn M. Ecelbarger declares that s/he has no conflict of interest.
Bellamkonda K. Kishore declares that s/he has no conflict of interest.
Parts of this work were presented at the Experiment mal Biology 2016 meeting organized by FASEB, April 2016 in San Diego, CA, and appeared as a printed abstract in the proceedings of that meeting [44].
Ethical approval
The animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Veterans Affairs Salt Lake City Health Care System, Salt Lake City, Utah.
References
- 1.Rieg T, Bundey RA, Chen Y, Deschens G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007;21:3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol. 2008;295:F1715–F1724. doi: 10.1152/ajprenal.90311.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wildman SS, Marks J, Turner CM, Yew-booth L, Peppiat-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol. 2008;19:731–742. doi: 10.1681/ASN.2007040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol. 2010;21:1903–1911. doi: 10.1681/ASN.2010040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol. 2008;294:F10–F27. doi: 10.1152/ajprenal.00432.2007. [DOI] [PubMed] [Google Scholar]
- 6.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y2 receptors and water transport in the kidney. Puriner Signal. 2009;5:491–499. doi: 10.1007/s11302-009-9151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prætorius H, Leipziger J. Intrarenal purinergic signaling in the control or renal tubular transport. Annu Rev Physiol. 2010;72:377–393. doi: 10.1146/annurev-physiol-021909-135825. [DOI] [PubMed] [Google Scholar]
- 8.Vallon V, Rieg T. Regulation of renal transport mechanisms. Am J Physiol Renal Physiol. 2011;301:F463–F475. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leipziger J. Luminal nucleotides are tonic inhibitors of renal tubular transport. Curr Opin Nephrol Hyperten. 2011;20:518–522. doi: 10.1097/MNH.0b013e3283487393. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Peti-Peterdi J, Müller CE, Carlson NG, Baqi Y, Strasburg DL, Heiney KM, Villanueva K, Kohan DE, Kishore BK. P2Y12 receptor localizes in the renal collecting duct and its blockade augments arginine vasopressin action and alleviates nephrogenic diabetes insipidus. J Am Soc Nephrol. 2015;26:2978–2987. doi: 10.1681/ASN.2014010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Nelson RD, Carlson NG, Kamerath CD, Kohan DE, Kishore BK. Potential role of purinerigic signaling in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2009;296:F1194–F1201. doi: 10.1152/ajprenal.90774.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5:270–276. doi: 10.1038/nrneph.2009.43. [DOI] [PubMed] [Google Scholar]
- 13.Kishore BK, Ecelbarger CM. Lithium: a versatile tool for understanding renal physiology. Am J Physiol Renal Physiol. 2013;304:F1139–F1149. doi: 10.1152/ajprenal.00718.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Pop IL, Carlson NG, Kishore BK. Genetic deletion of the P2Y2 receptor offers significant resistance to development of lithium-induced polyuria accompanied by alterations in PGE2 signaling. Am J Physiol Renal Physiol. 2012;302:F70–F77. doi: 10.1152/ajprenal.00444.2011. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Li L, Kohan DE, Ecelbarger CM, Kishore BK. Attenuation of lithium-induced natriuresis and kaliuresis in P2Y2 receptor knockout mice. Am J Physiol Renal Physiol. 2013;305:F407–F416. doi: 10.1152/ajprenal.00464.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Peti-Peterdi J, Heiney KM, Riquier-Brison A, Carlson NG, Müller CE, Ecelbarger CM, Kishore BK. Clopidogrel attenuates lithium-induced alterations in renal water and sodium channels/transporters in mice. Puriner Signal. 2015;11:507–518. doi: 10.1007/s11302-015-9469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics. 2010;20:463–465. doi: 10.1097/FPC.0b013e3283385420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savi P, Zachayus J-L, Delesque-Touchard N, Labouret C, Herve C, Uzbiaga M-F, Pereillo J-M, Culouscou J-M, Bono F, Ferrara P, Herbert J-M. The active metabolite of clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci U S A. 2006;103:11069–11074. doi: 10.1073/pnas.0510446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallentin L. P2Y12 inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J. 2009;30:1964–1977. doi: 10.1093/eurheartj/ehp296. [DOI] [PubMed] [Google Scholar]
- 20.Giorgi MA, Cohen Arazi H, Gonzalez CD, Di Girolamo G. Beyond efficacy: pharmacokinetic differences between clopidogrel, prasugrel and ticagrel. Expert Opin Pharmacother. 2011;12:1285–1295. doi: 10.1517/14656566.2011.550573. [DOI] [PubMed] [Google Scholar]
- 21.Savi P, Pereillo JM, Uzabiaga ME, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- 22.Brophy JM, Babapulle MN, Costa V, Rinfret S. A Pharmacoepidemiology study of the interaction between atorvastatin and clopidogrel after percutaneous coronary intervention. Am Heart J. 2006;152:263–269. doi: 10.1016/j.ahj.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Berniochner I, Sibbing D. Thienopyridine and other ADP-receptor antagonists. Handb Exp Pharmacol. 2012;210:165–198. doi: 10.1007/978-3-642-29423-5_7. [DOI] [PubMed] [Google Scholar]
- 24.Wallentin L, Vaenhorst C, James S, Erlinge D, Braun Ö, Jakubowski JA, Sugidachi A, Winters KJ, Siebahn A. Prasugrel achieves greater and faster P2Y12 receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008;29:21–30. doi: 10.1093/eurheartj/ehm545. [DOI] [PubMed] [Google Scholar]
- 25.Norgard NB, Abu-Fadel M (2009) Comparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Vasc Health Risk Manag:873–882 [DOI] [PMC free article] [PubMed]
- 26.Hagihara K, Kazui M, Kurihara A, Yoshiike M, Honda K, Okazaki O, Farid NA, Ikeda T. A possible mechanism for the differences in efficiency and variability of active metabolite formation from thienopyridine antiplatelet agents, prasugrel and clopidogrel. Drug Met Disp. 2009;17:2145–2152. doi: 10.1124/dmd.109.028498. [DOI] [PubMed] [Google Scholar]
- 27.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 28.Hanner F, Lan L, Nguyen TX, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane channel pannexin 1. Am J Physiol Renal Physiol. 2012;303:F1454–F1459. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldenssarini RJ, Pompili M, Tondo L. Suicide in bipolar disorder: risks and management. CNS Spectr. 2006;11:465–471. doi: 10.1017/S1092852900014681. [DOI] [PubMed] [Google Scholar]
- 30.Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systemic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- 31.Geddes J, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381:1672–1682. doi: 10.1016/S0140-6736(13)60857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med. 2004;18:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- 33.Wade A, Yokoo H, Yanagita T, Kobayashi H. Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sc. 2005;99:307–321. doi: 10.1254/jphs.CRJ05009X. [DOI] [PubMed] [Google Scholar]
- 34.Florenza OV, de Paula VJ, Machado-Vieira R, Diniz BS, Gattaz WF. Does lithium prevent Alzheimer’s disease? Drugs Aging. 2012;29:335–342. doi: 10.2165/11599180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Chiu CT, Want Z, Hunsberger JG, Chuang DM. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev. 2013;65:105–142. doi: 10.1124/pr.111.005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luckey AE, Parsa CJ. Fluid and electrolytes in the aged. Arch Sug. 2003;138:1055–1060. doi: 10.1001/archsurg.138.10.1055. [DOI] [PubMed] [Google Scholar]
- 37.Rej S, Herrmann N, Shulman K. The effects of lithium on renal function in older adults—a systematic review. J Geriatr Psychiatry Neurol. 2012;25:51–61. doi: 10.1177/0891988712436690. [DOI] [PubMed] [Google Scholar]
- 38.Bedford JJ, Leader JP, Jing R, Walker LJ, Klein JD, Sands JM, Walker RJ. Amiloride restores renal medullary osmolytes in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2008;294:F812–F820. doi: 10.1152/ajprenal.00554.2007. [DOI] [PubMed] [Google Scholar]
- 39.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM. Amiloride blocks lithium entry through sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int. 2009;76:44–53. doi: 10.1038/ki.2009.91. [DOI] [PubMed] [Google Scholar]
- 40.Finley PR, Warner D, Peabody CA. Clinical relevance of drug interactions with lithium. Clin Pharmacokinet. 1995;29:172–191. doi: 10.2165/00003088-199529030-00004. [DOI] [PubMed] [Google Scholar]
- 41.Murray MD, Brater DC. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol. 1993;32:435–465. doi: 10.1146/annurev.pa.33.040193.002251. [DOI] [PubMed] [Google Scholar]
- 42.Phelan KM, Mosholder AD, Lu S. Lithium interaction with the cyclooxygenase-2 inhibitors reofecoxib and celecoxib and other nonsteroidal anti-inflammatory drugs. J Clin Psychiatry. 2003;64:1328–1334. doi: 10.4088/JCP.v64n1108. [DOI] [PubMed] [Google Scholar]
- 43.Conaghan PG. A turbulent decade of NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int. 2012;32:1491–1502. doi: 10.1007/s00296-011-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishore BK, Brandes AU, Carlson NG, Zhang Y. Prasugrel suppresses development of lithium-induced nephrogenic diabetes insipidus in mice (abstract) FASEB J. 2016;30:1220.5. doi: 10.1007/s11302-017-9555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]