Abstract
Tear hyperosmolarity is a key event in dry eye. In this work, we analyzed whether hyperosmolar challenge induces ATP release on the ocular surface. Moreover, as extracellular ATP can activate P2X7 receptor, the changes in P2X7 protein levels and its involvement in pathological process triggered by hypertonic treatment were also examined. High-performance liquid chromatography analysis revealed that ATP levels significantly increased in human corneal and conjunctival epithelial cells exposed to hyperosmotic challenge as well as in dry eye patients as compared to control subjects. A significant reduction in cell viability was detected after hyperosmolar treatment, indicating that the rise in ATP release was mainly due to cell lysis/death. Additionally, vesicular nucleotide transporter was identified in both cell lines and their protein expression was upregulated in hypertonic media. P2X7 receptor truncated form together with the full-length form was identified in both cell lines, and experiments using specific antagonist and agonist for P2X7 indicated that this receptor did not mediate cell death induced by hyperosmolar stress. In conclusion, hyperosmotic stress induces ATP release. Extracellular ATP can activate P2X7 receptor leading to cytotoxicity in many cells/tissues; however, this does not occur in human corneal and conjunctival epithelial cells. In these cells, the presence of P2X7 receptor truncated form together with the full-length form hinders a P2X7 apoptotic behavior on the ocular surface.
Keywords: ATP, P2X7, VNUT, Cornea, Conjunctiva, Dry eye
Introduction
Osmotic stress, triggered by increased extracellular osmolarity, is a highly relevant challenge to normal cell function in a variety of tissues, including the ocular surface epithelium. Tear film hyperosmolarity occurs in dry eye disorders, and this elevated tear osmolarity is considered as one of central events in dry eye pathophysiology, triggering apoptosis of corneal and conjunctival cells and inducing inflammatory pathways that leads to further cell death [1]. Considering the key involvement of hyperosmolarity in dry eye pathophysiology, several in vitro dry eye models induced by hyperosmotic challenge have been used to study dry eye [2–4].
ATP is the central energy carrier in cells; under homeostatic conditions, extracellular ATP levels are low and are acutely regulated by ectonucleotidases [5]. However, elevated extracellular concentrations of ATP can be detected when this nucleotide is released under stress conditions such as hypoxic, inflammatory, mechanical, and osmotic stress [6–11] via conductive and vesicular pathways as well as from cells subjected to damage resulting in cell death. Conductive mechanisms implicate diffusion through volume-regulated ion channels or pore-forming connexins/pannexins [12]. Exocytotic mechanisms involve ATP storage and Ca2+-regulated exocytotic release and SLC17A9 protein has been identified as a vesicular nucleotide transporter (VNUT) that plays a key role in ATP exocytosis [13]. VNUT expression has been detected in different types of cells [13–19]; however, its presence in corneal and conjunctival epithelial cells remains elusive.
After its release, extracellular ATP can act through various membrane receptors belonging to purinergic receptor family. One member of this receptor family is the P2X7 receptor, a trimeric ATP-gated cation channel of homomeric subunits [20]. Depending on the cell type and the presence of alternative P2X7 splice isoforms, P2X7 activation can induce cell death or also promote cell proliferation via a number of different intracellular pathways [21].
On the ocular surface, a role of the P2X7 receptor in the apoptotic and cytolytic effect induced by different ocular stresses (such as preservative exposure) in conjunctival and corneal cells has been suggested [22, 23]. On the other hand, in the cornea, P2X7 has been shown to facilitate corneal wound healing and epithelial migration [24] and regulate corneal stroma organization by modulating collagen and proteoglycan expression [25, 26].
In this study, we have investigated the release of ATP from human corneal and conjunctival epithelial cells subjected to hyperosmotic challenge and the levels of ATP in tears of control subjects and dry eye patients, as well as the mechanism involved in ATP release. Additionally, we have analyzed whether P2X7 receptor activation by released ATP could be involved in cell death process induced by hyperosmolarity as well as the protein levels of this receptor under these hyperosmotic conditions.
Materials and methods
Subjects
This study was performed according to the Declaration of Helsinki. An informed consent was obtained from all participants and they were free to give up the session at any time. The McMonnies test [27] was made to identify possible symptoms of ocular dryness.
Two groups of subjects were studied: the first group comprised 37 normal subjects who had no dry eye symptoms and a Schirmer I test result ≥10 mm. The second group included 46 symptomatic subjects and was divided into two groups: symptomatic subjects with normal tear secretion (n = 34) showing a Schirmer I test result ≥10 mm and symptomatic subjects with low tear secretion (n = 12), whose values of tear secretion were ≤5 mm.
Tear collection
Tear secretion was assessed in all groups with the Schirmer I test. The Schirmer strip was placed on the temporal tarsal conjunctiva of the lower lid for 5 min with the closed eyes. Secretion was measured without any type of stimulation.
Cell culture and treatments
Telomerase-immortalized human corneal limbal epithelial (HCLE) cells and human conjunctival epithelial cells (HCjE) were previously established [28] and kindly supplied by Dr. Ilene Gipson. Keratinocyte serum-free medium (Invitrogen, Carlsbad, CA) supplemented with 0.2 ng/ml epidermal growth factor, 0.4 mM calcium chloride, 25 μg/ml bovine pituitary extract, and antibiotics was used to cell growth. After reaching the confluence and in order to induce stratification and differentiation, this culture medium was substituted by DMEM/F12 medium supplemented with 10 ng/ml epidermal growth factor and 10% calf serum and for 7 days [28].
Stratified cells growing on 24-well plates were incubated with serum-free medium containing 0, 90, or 120 mM sodium chloride (NaCl) (corresponding to osmolarities of 312, 500, and 550 mOsm, respectively) for 24 h. Osmolarity values were selected based on previous data that indicated that osmolarity in areas of breakup of the precorneal tear layer can reach up to 560 mOsm [29].
For P2X7 inhibition assays, the antagonist A438079 (3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine) (10 μM) was incubated together the hypertonic treatment with NaCl. In the experiments with the selective P2X7 agonist BzATP 2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate, the compound was added at 300 μM for 24 h.
ATP levels measurement for HPLC analysis
After the corresponding treatments, the conditioned media of the stratified cells were collected and centrifuged, and the supernatants were stored at −20 °C until its use. Supernants were then heated in a 98 °C bath for 2 min and transferred to ice 10 min, to precipitate the proteins. To pellet the proteins, the tubes were centrifuged at 22,000×g for 10 min at 4 °C. For tear samples, Schirmer strips were located in tubes containing 500 μl of ultrapure water, and vortexed for 5 min. The strips were rinsed, and the liquid in the tubes was heated in a 100 °C bath for 20 min to precipitate proteins and centrifuged for 30 min to pellet the proteins.
ATP concentrations were measured by HPLC. A C18 reverse-phase column, 250 mm length, and 4.6 mm diameter (Hyperchrome, Scharlab, Madrid, Spain) and a mobile phase composed of 10 mM KH2PO4, 2 mM tetrabutylammonium, and 20% acetonitrile, pH 7.5, were used. The flow rate was 2 ml/min and ATP was detected at 260 nm wavelength.
MTT assay
HCLE and HCjE stratified cultures were grown on 24-well plates and exposed to the different treatments for 24 h. After exposure, MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay was used to evaluate cellular viability as previously reported [30]. The mean absorbance values of non-treated cells were considered as 100%, and results were expressed as a percentage of cell viability compared to non-treated (control) cells. Experiments were conducted in triplicate.
Western blot analysis
RIPA buffer (150 mM NaCl, 25 mM Tris HCL pH 7.6, 0.1% SDS, 1% sodium deoxycholate, 1% Nonidet P-40) supplemented with Protease Inhibitor Cocktail Kit (Thermo Scientific, Rockford, IL, USA) was used to total cellular protein extraction. PIERCE BCA Protein Assay kit (Thermo Scientific) was used to determine protein concentration and samples were separated by electrophoresis SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-dry fat milk and then incubated with antibodies against P2X7 (1:200, Alomone Labs, Jerusalem, Israel) or VNUT (1:500, Milipore, CA, USA). Binding of GAPDH antibody (1:000, Santa Cruz Biotechnology, CA, USA) worked as a loading control. After incubation with secondary antibodies (Jackson Immunoresearch, PA, USA), signals were detected with an ECL detection reagent (Amersham, Buckinghamshire, UK). Densitometric analysis was done using Kodak Molecular Imaging software (Kodak, Rochester, NY, USA). Data shown are representative of three independent experiments.
Statistical analysis
Statistical comparisons of treated and non-treated control cells were performed using the one-way ANOVA analysis followed by the Dunnet test using InStat3 software (GraphPad Software, La Jolla, CA, USA). Data of control subjects and dry eye patients were analyzed with Student’s t test. Differences were considered significant when p values <0.05.
Results
ATP release
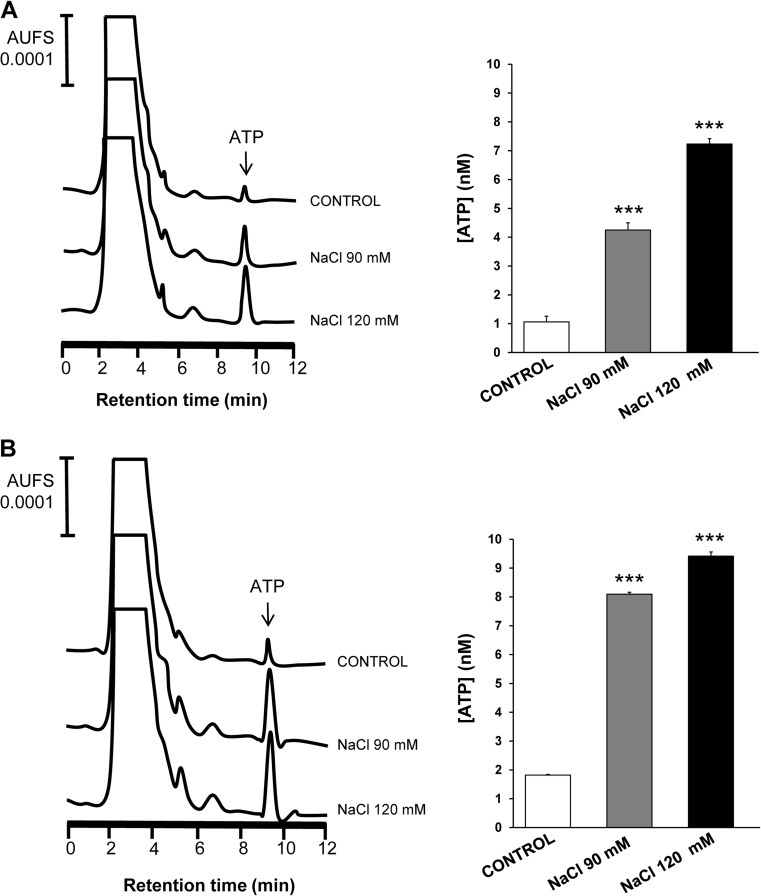
The samples of supernatants of stratified culture cells were collected after hyperosmotic challenge and the ATP levels analyzed by HPLC (Fig. 1). An increase in ATP concentration after hypertonic treatment as compared to untreated cells was detected in both cell lines. In particular, human corneal epithelial cells presented ATP levels of 1.06 ± 0.23 nM (Fig. 1a). After hypertonic treatment, the ATP levels were significantly increased to 4.25 ± 0.25 nM (p < 0.001) for treatment with 90 mM NaCl and 7.23 ± 0.19 (p < 0.001) nM for treatment with 120 mM NaCl. In human conjunctival epithelial cells (Fig. 1b), the ATP levels significantly increased (p < 0.001) from 1.82 ± 0.02 nM in control media to 8.09 ± 0.07 nM in 90 mM NaCl-added media and 9.42 ± 0.15 nM in 120 mM NaCl-added media.
Fig. 1.
Effect of hyperosmotic stress on ATP release by stratified human corneal and conjunctival epithelial cells. Supernants of stratified human corneal epithelial cells (a) or conjunctival cells (b) treated with 90 and 120 mM NaCl-added media as well as the corresponding control cells without treatment were collected and the concentrations of ATP were measured by HPLC analysis. HPLC elution profiles representative of ATP release for both types of cells are displayed in left panels, and quantification of the chromatograms is shown in right panels. Data are expressed as the mean ± standard deviation (n = 3). *** p < 0.001 vs control
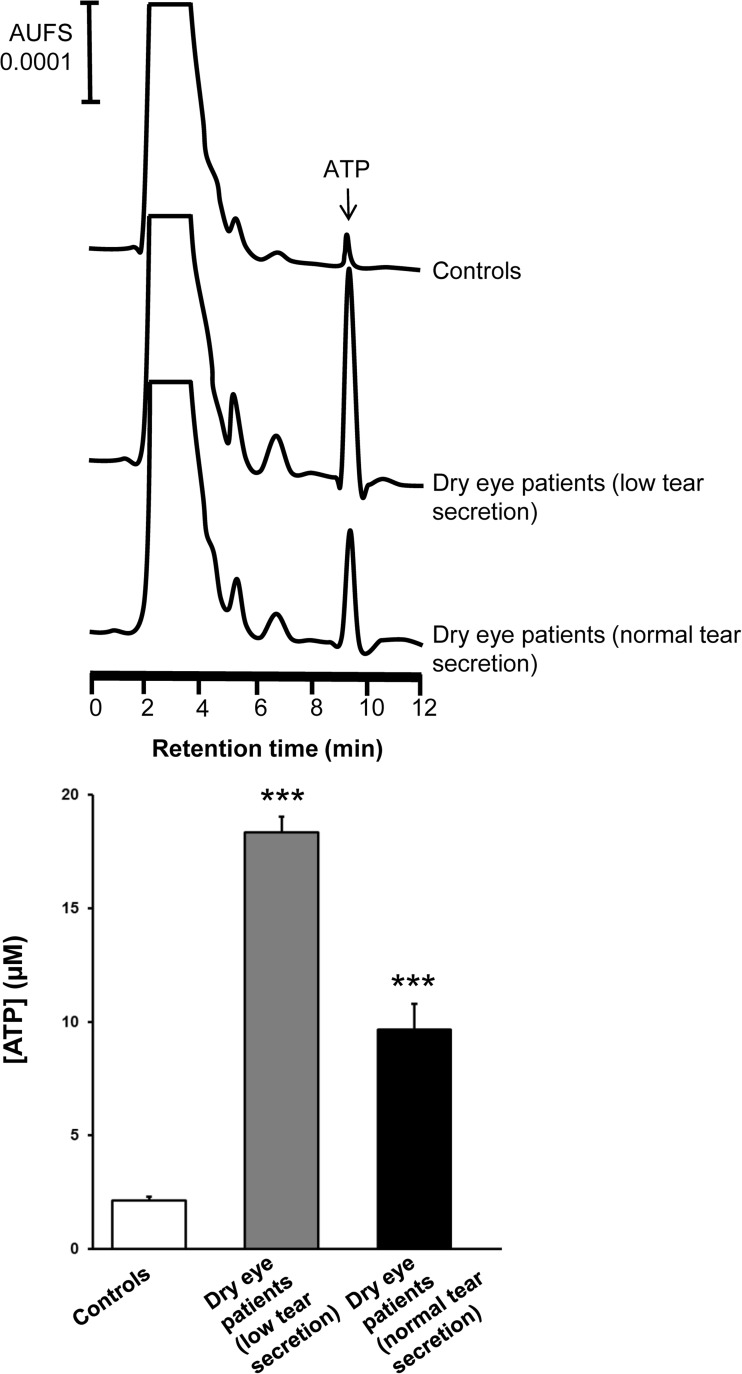
In addition, the concentration of ATP in tears from dry eye patients with normal and low secretion was also examined (Fig. 2). A 8.65- and a 4.56-fold increase in ATP levels for dry eye patients with low and normal tear secretion, respectively, was detected as compared to control subjects.
Fig. 2.
Concentration of ATP in tears of normal and dry eye subjects. Individuals with no symptoms and normal tear secretion (control subjects) were compared with symptomatic patients with low or normal tear secretion. Representative HPLC profiles of ATP (upper panel) and quantification of ATP levels (lower panel) are shown. Data from both eyes were pooled and are expressed as the mean ± standard deviation. *** p < 0.001 vs control
Mechanisms of ATP release
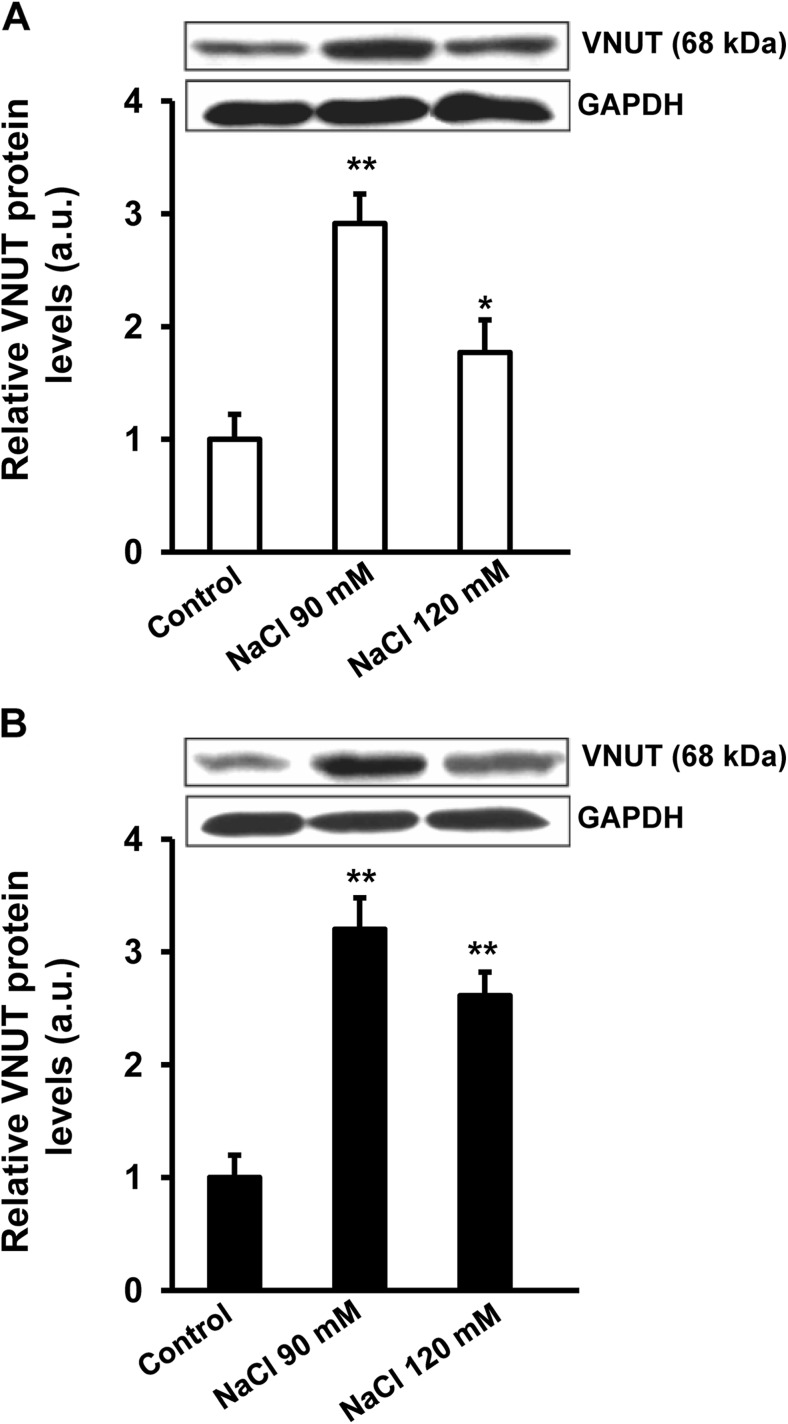
The presence of VNUT was evaluated by western blot analysis (Fig. 3). The protein expression of VNUT was identified under control conditions in both cell lines and hyperosmotic treatment induced a significant increase in VNUT protein levels. Human corneal (Fig. 3a) and conjunctival (Fig. 3b) epithelial cells treated with 90 mM NaCl showed a 2.9- and a 3.2-fold increase, respectively, in VNUT protein levels over the control (p < 0.01). When cells were exposed to 120 mM NaCl, the increase was around double for both types of cells.
Fig. 3.
VNUT expression in control cells and cells exposed to hypertonic treatment. Stratified human corneal epithelial cells (a) and stratified human conjunctival epithelial cells (b) were exposed to 90 and 120 mM NaCl-added media for 24 h. Cell lysates were successively immunoblotted with anti-VNUT and anti-GAPDH receptor to verify equal loading. The histograms represent the levels VNUT. Data (mean ± standard deviation; n = 3) are represented in arbitrary units (a.u.) and normalized to the intensity of the band corresponding to control cells. * p < 0.05, ** p < 0.01 vs control
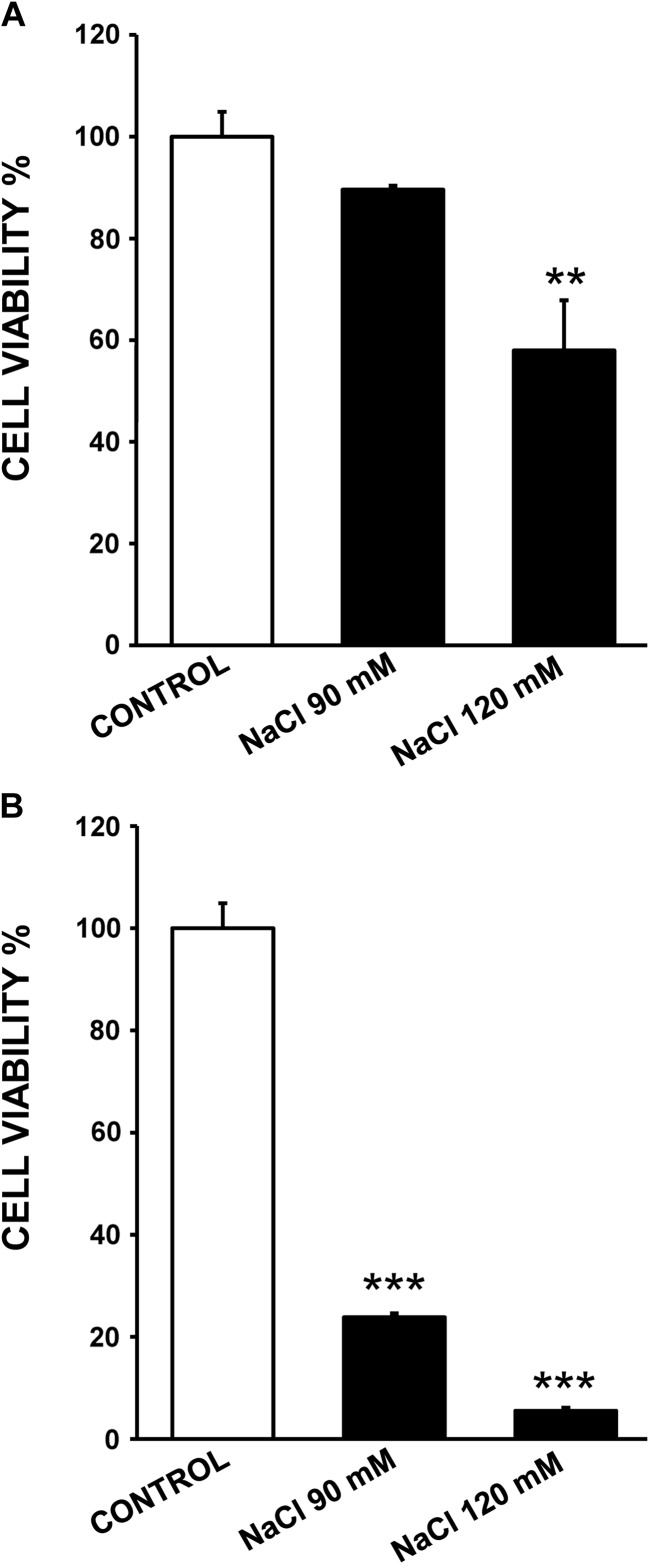
On the other hand, hyperosmolarity could induce physical cell damage or cell death leading to ATP release. As shown in Fig. 4a, in human corneal epithelial cells, exposure to 90 mM NaCl-added media only decreased cell viability to 89% of the control value whereas treatment with 120 mM NaCl-added media induced a significant reduction of cell viability to 58% of the control value (p < 0.01), indicating that cytotoxic effects were concentration-dependent. Human conjunctival cells seem to be more sensitive than human corneal cells to hyperosmolarity since a higher decrease in cell viability was detected. Thus, cell viability values were significantly reduced to 24% (p < 0.001) for 90 mM NaCl treatment and 120 mM NaCl treatment displayed a significantly more deleterious effect on cell viability, decreasing to 5.5% (Fig. 4b).
Fig. 4.
Effects of hyperosmotic exposure on cell viability. Stratified human corneal epithelial cells (a) and stratified human conjunctival epithelial cells (b) were exposed to 90 and 120 mM NaCl-added media for 24 h. Cell viability was determined with MTT assay and data were normalized to the control value (100%) and expressed as the mean ± standard deviation (n = 3). ** p < 0.01 vs control, *** p < 0.001 vs control
Study of P2X7 influence on cell viability
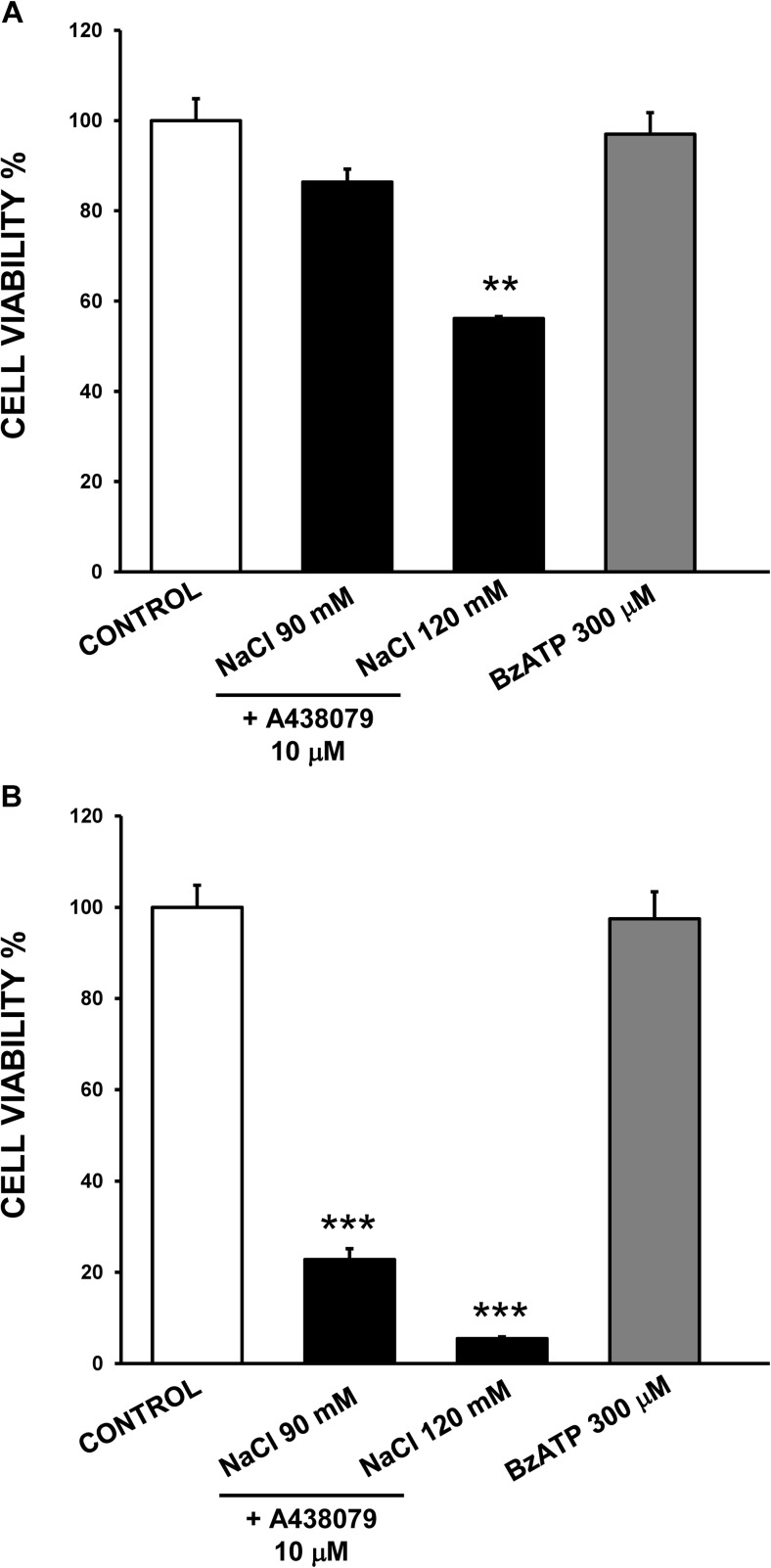
ATP released from damaged or dead cells could act in a paracrine manner, influencing surrounding cells. In particular, stimulation of P2X7 by extracellular/released ATP could contribute to cell death detected after exposure to hyperosmolarity conditions. To analyze this point, the highly selective P2X7 antagonist A438079 was used (Fig. 5). In human corneal epithelial cells, treatment with the antagonist did not abrogate the cytotoxic effect triggered by hyperosmotic challenge (Fig. 5a). Thus, cell viability levels were around 86% for exposure to 90 mM NaCl-added media in the presence of the antagonist and 56% when 120 mM NaCl and the antagonist were added, values similar to that found in the absence of A438079 antagonist (Fig. 4a).
Fig. 5.
Studies with the P2X7 receptor antagonist A438079 and the agonist BzATP on the effects of hyperosmotic exposure on cell viability. Stratified human corneal epithelial cells (a) and stratified human conjunctival epithelial cells (b) were incubated with 90 and 120 mM NaCl-added media for 24 h in the presence of the P2X7 receptor antagonist A438079 or treated with the P2X7 agonist BzATP. Cell viability was determined with MTT assay and data were normalized to the control value (100%) and expressed as the mean ± standard deviation (n = 3). ** p < 0.01 vs control, *** p < 0.001 vs control
Likewise, in human conjunctival epithelial cells, the antagonist did not reverse the cell death induced by 90 mM NaCl and 120 mM NaCl treatment (Fig. 5b). Moreover, treatment with the selective P2X7 agonist BzATP for 24 h did not modify cell viability in human corneal (Fig. 5a) or conjunctival epithelial cells (Fig. 5b), indicating that P2X7 on the ocular surface did not work as a cell death receptor.
Changes in P2X7 receptor levels with the hyperosmotic treatment
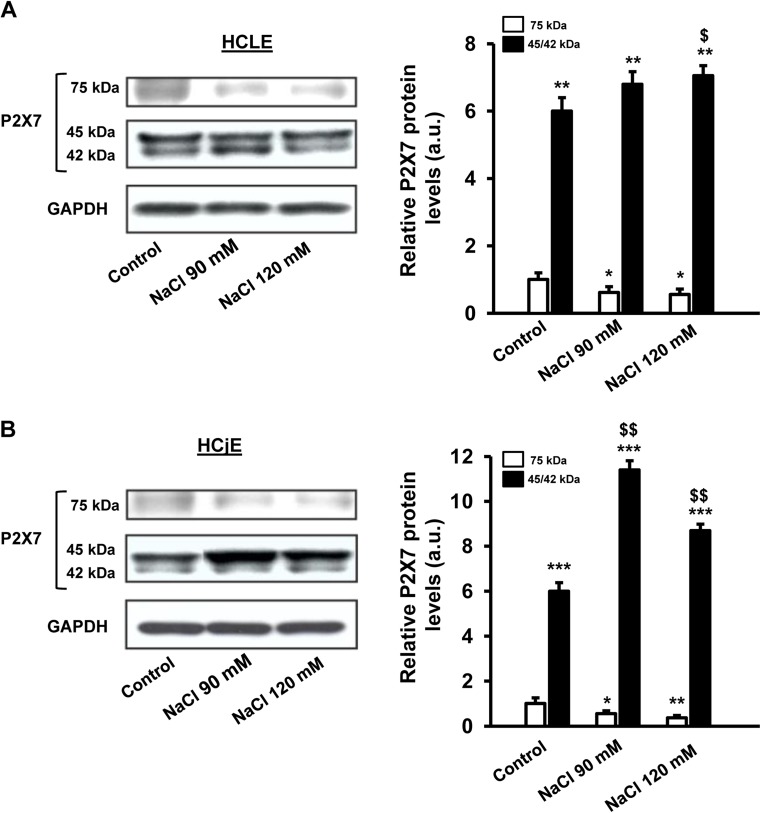
Western blot assays (Fig. 6) showed that both stratified human corneal and conjunctival epithelial cells expressed a full-length P2X7 receptor form of 75 kDa (Fig. 6a, b). Moreover, a truncated form (45–42 kDa), previously identified as the P2X7j variant [31, 32], was also detected in addition to a 75 kDa form, in both cell lines. Densitometric analysis revealed significant higher levels of truncated form (45–42 kDa) as compared to 75-kDa form in both cell lines (Fig. 6a; p < 0.01 and Fig. 6b; p < 0.001).
Fig. 6.
Effects of hyperosmotic exposure on P2X7 protein receptor levels. Stratified human corneal epithelial cells (a) and stratified human conjunctival epithelial cells (b) were treated with 90 and 120 mM NaCl-added media for 24 h. Cell lysates were successively immunoblotted with anti-P2X7 and anti-GAPDH receptor to confirm identical loading. The histograms indicate the levels of full-length and truncated form of P2X7 receptor. Data (mean ± standard deviation; n = 3) are given in arbitrary units (a.u.) and normalized to the intensity of the band corresponding to the full-length form (75 kDa band) in control cells. * p < 0.05, ** p < 0.01 and *** p < 0.001 vs control (75 kDa band). $ p < 0.05, $$ p < 0.01 vs control (45–42 kDa band)
Moreover, there were noteworthy changes in the protein levels of both forms after hyperosmotic treatment with NaCl (Fig. 6a, b). Thus, in human corneal epithelial cells, the 75-kDa form significantly decreased around 38% after 90 mM NaCl treatment as compared to control and 44% of reduction was determined when human corneal epithelial cells were incubated with 120 mM NaCl-added media (Fig. 6a) (p < 0.05). A similar behavior was observed in human conjunctival epithelial cells and a marked decrease of 63.5% (p < 0.001) was found for the 75-kDa form protein levels after 120 mM NaCl treatment as compared to control (Fig. 6b). In contrast, there was a significant increased trend in the protein expression of P2X7j variant after hypertonic challenge in both cell lines (Fig. 6a; p < 0.05 and Fig.6b; p < 0.01).
Discussion
Hyperosmolarity is considered as a key event in dry eye pathophysiology. In in vitro experiments, hyperosmotic exposure led to increased ATP release. Consistent with the in vitro results, dry eye patients exhibited higher levels of ATP as compared to control subjects.
In good agreement with previous reports [4], the hypertonic treatment caused a significant reduction in cell viability in both cell lines. Considering the effect that hyperosmotic stress induced on cell viability, the increased ATP concentration found in in vitro assays as well as in tears of dry eye patients could be originated by cell rupture.
Moreover, VNUT was for the first time detected in control human corneal and conjunctival epithelial cells and their protein levels were increased with the hypertonic treatment, suggesting that vesicle transport could be also involved in ATP release on the ocular surface, particularly in the cases in which does not exist a marked cell death such as basal conditions as well as in human corneal epithelial cells treated with 90 mM NaCl.
On the other hand, it has been proposed that mechanical shear stress on the corneal epithelium also promotes ATP release [11]. Corneal and conjunctival epithelia are subjected to shear force produced by the upper eyelid during blinking, and higher levels of ATP have been detected concomitantly with an increase in blinking frequency [33]. Taking into account that augmented blinking frequency is a typical sign of eye dryness, which occurs to compensate tear instability [34], ATP released as a consequence of increased blinking could also contribute to the higher levels of this nucleotide detected in dry eye patients.
The released ATP acting in a paracrine way could then stimulate purinergic receptors such as P2X7 receptor, which has been considered by some authors as a cell death receptor on the ocular surface [22, 23].
Although the levels of extracellular ATP detected in in vitro assays using our experimental method were lower than the concentration usually needed to activate P2X7, the activation of P2X7 by nanomolar levels of ATP in epithelial cells has been reported [35]. In addition, it has been previously suggested that under these experimental conditions, the ATP released from cells is diluted in the culture medium and it can be rapidly metabolized by ectonucleotidases; however, the concentration of ATP on cell surface might be high enough to induce P2X7 receptor activation [36].
To evaluate whether P2X7 receptor activation by ATP could also participate in the process of cell death induced by hyperosmotic stress, the P2X7 antagonist A438079 was used. This P2X7 antagonist did not reverse the drastic decrease of cell viability caused by the hyperosmotic treatment. Furthermore, stimulation with the P2X7 receptor agonist, BzATP, at a concentration enough to activate P2X7 receptor, did not trigger significant changes in cell viability. These results suggest that P2X7 receptor does not seem to be involved in cell death processes on the ocular surface and show the functional diversity that this receptor presents in the eye.
One explanation to justify this functional diversity is the presence of different receptor isoforms in different ocular structures. In fact, we detected through western blot not only the full-length form of 75 kDa but also a truncated variant of the receptor (45–42 kDa). The presence of this variant has been previously suggested in corneal epithelium [32], but this is the first time that it has been identified in conjunctival epithelium. This truncated variant seems to be the P2X7j form, which lacks the intracellular carboxyl terminus, the second transmembrane domain, and the distal third of the extracellular loop of the full-length P2X7 receptor [31]. Functional P2X7 receptor functions as a homotrimers [20]. It has been demonstrated that oligomerization of the P2X7j form with the full-length P2X7 form generate non-functional complexes that did not exhibit canonical properties of P2X7 receptor such as pore formation and apoptosis induction [31, 37].
Thus, in the corneal and conjunctival epithelium where the levels of truncated variant are higher than the full-length form, levels could predominate heterotrimeric complex formation with non-canonical activity. Moreover, cells exposed to hypertonic treatment displayed a significant decreased expression of full-length P2X7 receptor as compared to control whereas truncated variant was upregulated. Therefore, in cells expressing both P2X7 receptor forms, higher expression of P2X7j form and/or reduced expression of the full-length P2X7 receptor form would favor hetero-oligomerization of P2X7j with the full-length P2X7 resulting in inactive complexes, inability to induce apoptosis, and protecting cells from cell death.
In summary, hyperosmotic challenge induced ATP release in in vitro assays and higher levels of ATP were also detected in dry eye patients as compared to normal subjects. Cell death induced by hypertonic treatment seems to be the main mechanism responsible for ATP release, although vesicular release mediated by VNUT could also contribute to this process. P2X7 receptor activation by the released ATP does not participate in the decrease in cell viability triggered by hyperosmotic shock. This finding highlights the functional variety of this receptor in the eye and point to the development of alternative studies to know exactly the biological processes that can trigger the activation of the purinergic receptor P2X7 by released ATP. On the other hand, released ATP could activate not only the P2X7 receptor but also other purinergic receptors located on the corneal-conjunctival epithelium; therefore, future studies analyzing whether the activation of these other receptors could have influence on the pathological processes associated to dry eye could be interesting.
Acknowledgments
We thank Dr. Ilene Gipson, Schepens Eye Research Institute, Massachusetts Eye and Ear, Harvard Medical School, Boston, MA, USA, for providing the HCLE cells. We are grateful to Leyre Nieto Roldan for her technical assistance. This work was supported by the Spanish Ministry of Economy (SAF2013-44416-R, SAF2016-77084R) and the Institute Carlos III (RETICS RD12/0034/0003, RD16/0008/0017).
Compliance with ethical standards
Conflict of interest
Ana Guzman-Aranguez declares that she has no conflict of interest.
María J. Pérez de Lara declares that she has no conflict of interest.
Jesús Pintor declares that he has no conflict of interest.
Ethical approval
This study was performed according to the Declaration of Helsinki. An informed consent was obtained from all participants and they were free to give up the session at any time.
References
- 1.Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G, Labetoulle M, Rolando M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf. 2013;11:246–258. doi: 10.1016/j.jtos.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Hu DN, Pan Z, Lu CW, Xue CY, Aass I. Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. 2010;90:437–443. doi: 10.1016/j.exer.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Corrales RM, Luo L, Chang EY, Pflugfelder SC. Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea. 2008;27:574–579. doi: 10.1097/ICO.0b013e318165b19e. [DOI] [PubMed] [Google Scholar]
- 4.Luo L, Li DQ, Pflugfelder SC. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea. 2007;26:452–460. doi: 10.1097/ICO.0b013e318030d259. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldred JA, Sanderson J, Wormstone M, Reddan JR, Duncan G. Stress-induced ATP release from and growth modulation of human lens and retinal pigment epithelial cells. Biochem Soc Trans. 2003;31:1213–1215. doi: 10.1042/bst0311213. [DOI] [PubMed] [Google Scholar]
- 7.Li A, Banerjee J, Leung CT, Peterson-Yantorno K, Stamer WD, Civan MM. Mechanisms of ATP release, the enabling step in purinergic dynamics. Cell Physiol Biochem. 2011;28:1135–1144. doi: 10.1159/000335865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol. 2010;299:C1308–C1317. doi: 10.1152/ajpcell.00333.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luna C, Li G, Qiu J, Challa P, Epstein DL, Gonzalez P. Extracellular release of ATP mediated by cyclic mechanical stress leads to mobilization of AA in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2009;50:5805–5810. doi: 10.1167/iovs.09-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahidullah M, Mandal A, Beimgraben C, Delamere NA. Hyposmotic stress causes ATP release and stimulates Na, K-ATPase activity in porcine lens. J Cell Physiol. 2012;227:1428–1437. doi: 10.1002/jcp.22858. [DOI] [PubMed] [Google Scholar]
- 11.Srinivas SP, Mutharasan R, Fleiszig S. Shear-induced ATP release by cultured rabbit corneal epithelial cells. Adv Exp Med Biol. 2002;506:677–685. doi: 10.1007/978-1-4615-0717-8_95. [DOI] [PubMed] [Google Scholar]
- 12.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geisler JC, Corbin KL, Li Q, Feranchak AP, Nunemaker CS, Li C. Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology. 2013;154:675–684. doi: 10.1210/en.2012-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–5. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 16.Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sesma JI, Kreda SM, Okada SF, van Heusden C, Moussa L, Jones LC, O'Neal WK, Togawa N, Hiasa M, Moriyama Y, Lazarowski ER. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol. 2013;304:C976–C984. doi: 10.1152/ajpcell.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S. Autocrine regulation of TGF-beta1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. J Cell Sci. 2012;125:5051–5060. doi: 10.1242/jcs.104976. [DOI] [PubMed] [Google Scholar]
- 19.Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicke A. Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun. 2008;377:803–808. doi: 10.1016/j.bbrc.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Di Virgilio F, Ferrari D, Adinolfi E. P2X(7): a growth-promoting receptor-implications for cancer. Purinergic Signal. 2009;5:251–256. doi: 10.1007/s11302-009-9145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutot M, Liang H, Pauloin T, Brignole-Baudouin F, Baudouin C, Warnet JM, Rat P. Effects of toxic cellular stresses and divalent cations on the human P2X7 cell death receptor. Mol Vis. 2008;14:889–897. [PMC free article] [PubMed] [Google Scholar]
- 23.Dutot M, Warnet JM, Baudouin C, Rat P. Cytotoxicity of contact lens multipurpose solutions: role of oxidative stress, mitochondrial activity and P2X7 cell death receptor activation. Eur J Pharm Sci. 2008;33:138–145. doi: 10.1016/j.ejps.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Minns MS, Teicher G, Rich CB, Trinkaus-Randall V. Purinoreceptor P2X7 regulation of Ca(2+) mobilization and cytoskeletal rearrangement is required for corneal reepithelialization after injury. Am J Pathol. 2016;186:285–296. doi: 10.1016/j.ajpath.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mankus C, Chi C, Rich C, Ren R, Trinkaus-Randall V. The P2X(7) receptor regulates proteoglycan expression in the corneal stroma. Mol Vis. 2012;18:128–138. [PMC free article] [PubMed] [Google Scholar]
- 26.Mayo C, Ren R, Rich C, Stepp MA, Trinkaus-Randall V. Regulation by P2X7: epithelial migration and stromal organization in the cornea. Invest Ophthalmol Vis Sci. 2008;49:4384–4391. doi: 10.1167/iovs.08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMonnies C, Ho A, Wakefield D. Optimum dry eye classification using questionnaire responses. Adv Exp Med Biol. 1998;438:835–838. doi: 10.1007/978-1-4615-5359-5_117. [DOI] [PubMed] [Google Scholar]
- 28.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 29.Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;152(900–909):e901. doi: 10.1016/j.ajo.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzman-Aranguez A, Calvo P, Ropero I, Pintor J. In vitro effects of preserved and unpreserved anti-allergic drugs on human corneal epithelial cells. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2014;30:790–798. doi: 10.1089/jop.2014.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281:17228–17237. doi: 10.1074/jbc.M602999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mankus C, Rich C, Minns M, Trinkaus-Randall V. Corneal epithelium expresses a variant of P2X(7) receptor in health and disease. PLoS One. 2011;6:e28541. doi: 10.1371/journal.pone.0028541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guzman-Aranguez A, Santano C, Martin-Gil A, Fonseca B, Pintor J. Nucleotides in the eye: focus on functional aspects and therapeutic perspectives. J Pharmacol Exp Ther. 2013;345:331–341. doi: 10.1124/jpet.112.202473. [DOI] [PubMed] [Google Scholar]
- 34.Tsubota K, Hata S, Okusawa Y, Egami F, Ohtsuki T, Nakamori K. Quantitative videographic analysis of blinking in normal subjects and patients with dry eye. Arch Ophthalmol. 1996;114:715–720. doi: 10.1001/archopht.1996.01100130707012. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol. 2004;287:C1349–C1358. doi: 10.1152/ajpcell.00256.2004. [DOI] [PubMed] [Google Scholar]
- 36.Takai E, Tsukimoto M, Harada H, Kojima S. Autocrine signaling via release of ATP and activation of P2X7 receptor influences motile activity of human lung cancer cells. Purinergic Signal. 2014;10:487–497. doi: 10.1007/s11302-014-9411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng YH, Li X, Zeng R, Gorodeski GI. Endogenously expressed truncated P2X7 receptor lacking the C-terminus is preferentially upregulated in epithelial cancer cells and fails to mediate ligand-induced pore formation and apoptosis. Nucleosides Nucleotides Nucleic Acids. 2006;25:1271–1276. doi: 10.1080/15257770600890921. [DOI] [PubMed] [Google Scholar]