Abstract
Kidney ischemia–reperfusion injury (IRI) is common during transplantation. IRI is characterised by inflammation and thrombosis and associated with acute and chronic graft dysfunction. P-selectin and its ligand PSGL-1 are cell adhesion molecules that control leukocyte-endothelial and leukocyte-platelet interactions under inflammatory conditions. CD39 is the dominant vascular nucleotidase that facilitates adenosine generation via extracellular ATP/ADP-phosphohydrolysis. Adenosine signalling is protective in renal IRI, but CD39 catalytic activity is lost with exposure to oxidant stress. We designed a P-selectin targeted CD39 molecule (rsol.CD39-PSGL-1) consisting of recombinant soluble CD39 that incorporates 20 residues of PSGL-1 that bind P-selectin. We hypothesised that rsol.CD39-PSGL-1 would maintain endothelial integrity by focusing the ectonucleotidase platelet-inhibitory activity and reducing leukocyte adhesion at the injury site. The rsol.CD39-PSGL-1 displayed ADPase activity and inhibited platelet aggregation ex vivo, as well as bound with high specificity to soluble P-selectin and platelet surface P-selectin. Importantly, mice injected with rsol.CD39-PSGL-1 and subjected to renal IRI showed significantly less kidney damage both biochemically and histologically, compared to those injected with solCD39. Furthermore, the equivalent dose of rsol.CD39-PSGL-1 had no effect on tail template bleeding times. Hence, targeting recombinant CD39 to the injured vessel wall via PSGL-1 binding resulted in substantial preservation of renal function and morphology after IRI without toxicity. These studies indicate potential translational importance to clinical transplantation and nephrology.
Keywords: CD39, NTPDase-1, PSGL-1, Purinergic signalling, Kidney ischemia–reperfusion injury
Introduction
Kidney ischemia–reperfusion injury (IRI) occurs when blood flow to the kidney is interrupted and re-established. It is an inherent feature of kidney transplantation and occurs during organ harvesting (ischemia) as well as engraftment (reperfusion). IRI leads to inflammation, kidney dysfunction, and increases graft immunogenicity and rejection. We and others have accumulated a wealth of evidence detailing the role of purinergic signalling in IRI, and strategies targeting pathways involved in purinergic signalling have shown promising results both in vitro and in vivo [1–5].
The endothelium presents a platelet-inhibitory surface due to the release of prostacyclin and nitric oxide, and by the cell surface expression of CD39 (NTPDase1). CD39 hydrolyses extracellular nucleotides ATP and ADP to AMP [6], which serves as a substrate for ecto-5′ nucleotidase, for further conversion to adenosine [7]. Thus CD39 transforms the platelet-aggregatory profile of ADP into platelet-inhibitory mediators at the crucial endothelial-platelet interface. CD39−/− mice exhibit a pro-thrombotic state [8] and CD39 expression is lost during endothelial injury or exposure to oxidant stress, while infusion of soluble NTPDases or adenovirus-mediated over expression of CD39 protects against xenograft rejection [9, 10]. Adenosine-mediated signalling is protective in kidney IRI. CD39 overexpression protects from renal IRI via an adenosine-dependent mechanism, and CD39−/− mice exhibit more severe renal histopathology and dysfunction than wild-type mice [1, 2]. In the kidney, adenosine controls renin release, renal vascular tone and the rate of glomerular filtration. Recombinant soluble CD39 (solCD39) produced by deletion of the two transmembrane domains that anchor CD39 to the cell surface, inhibits platelet reactivity and recruitment, and provides protection in a murine model of stroke [8, 11, 12]. However, solCD39 promotes systemic bleeding in a concentration-dependent manner and therefore targeting CD39 to the site of endothelial activation will be a valid therapeutic option for IRI. Indeed, we recently showed that targeting CD39 binding to activated platelets with the use of a novel anti-GPIIb/IIIa-CD39 construct inhibited thrombosis and maintained patency of carotid artery after ferric chloride-induced injury at doses that did not prolong tail-bleeding time [4].
P-selectin and P-selectin glycoprotein ligand 1 (PSGL-1) are vascular adhesion molecules with critical roles in thrombus formation and in leukocyte–endothelial and leukocyte–platelet interaction during inflammation. P-selectin, expressed on activated platelets and endothelial cells, binds PSGL-1 which is present on the leukocyte surface, thereby initiating the adhesion and rolling of leukocytes on the endothelial surface [13, 14]. Inhibition of the P-selectin-PSGL-1 interaction by means of antibodies or by recombinant soluble PSGL-1 has shown therapeutic benefit in deep vein thrombosis, arterial thrombosis, and for vascular remodelling after trauma [15]. PSGL-1 undergoes extensive posttranslational O-linked glycosylation, fucosylation and sialylation, and tyrosine sulfation, which is necessary to bind P-selectin [13, 14]. Because the N-terminal 42–62 amino acids of the mature PSGL-1 protein contains all the critical motifs necessary for the recognition of P-selectin [16, 17], we have designed recombinant soluble CD39 that incorporates these crucial 20 residues of the PSGL-1 protein that bind P-selectin, (rsol.CD39-PSGL-1). We show that rsol.CD39-PSGL-1 is able to significantly preserve renal function and histology. Hence, it represents a valid therapeutic strategy for kidney IRI that is unlikely to compromise haemostatic safety.
Materials and methods
Production of rsol.CD39-PSGL-1
We obtained CHO cells that stably express core 2 O-linked B1-6-N-acetylglucosaminyltransferase (C2GnT) and α1-3 fucosyltransferase activity (FucT-VII) (54) [18], a generous gift of Dr. Rodger McEver, (W.K. Warren Medical Research Institute, Oklahoma). These enzymes mediate the post-translational display of sialylated glycans that is critical for P-selectin recognition [17]. rsol.CD39-PSGL-1 secreted in the conditioned media was concentrated by immunoaffinity columns of anti-FLAG antibody (M2, Sigma-Aldrich Australia) covalently coupled to Sepharose and specifically bound rsol.CD39-PSGL-1 was eluted via competitive binding with FLAG Peptide according to the manufacturer’s instructions. Peak fractions were pooled and concentrated. solCD39 (without the PSGL-1 polypeptide tag) was also similarly expressed in CHO cells followed by FLAG-affinity purification.
P-selectin ELISA
The ability of rsol.CD39-PSGL-1 to bind P-selectin was assessed by ELISA wherein ELISA plates (MaxisorpTM, Nunc, ThermoFisher Scientific, Australia) were coated overnight at 4 °C with 20 μg/ml of recombinant human P-Selectin/CD62P Protein, CF (R&D Systems, Inc. USA; catalogue #ADP3) diluted in 0.2 M carbonate buffer pH 9.6. Following 3 washes with PBS + 0.05% Tween-20 (PBS-T), wells were blocked with 2% BSA in PBS-T for 1 h at room temperature (RT). After another wash in PBS-T, 0–20 μg rsol.CD39-PSGL-1 or 2% BSA (negative control) was added to the wells for 1 h at RT. After 3 washes in PBS-T, anti-FLAG antibody (mAb KM5–1C7; Walter and Eliza Hall Institute of Medical Research Melbourne) diluted 1:500 in 2%BSA/PBS was added and allowed to incubate for 1 h at RT and the plate was washed 3× in PBS-T. The secondary antibody, peroxidase-conjugated rabbit anti-mouse immunoglobulin (Dako Australia) diluted 1:2000 was then added for 45 min at RT. Following 3 washes in PBS-T the reaction was developed with o-Phenylenediamine dihydrochloride peroxidase substrate (Sigma-Aldrich Australia) dissolved in 0.05 M phosphate-citrate buffer, pH 5.0 for ~20 min and then stopped with 4 M H2SO4 and OD492nm was calculated using a spectrophotometer.
NTPDase activity of rsolCD39-PSGL-1
Purified, concentrated rsol.CD39-PSGL-1 was quantified by the Bradford method. ADPase activity was measured by incubating purified rsol.CD39-PSGL-1 in assay buffer (8 mM CaCl2, 50 mM imidazole, pH 7.4) and the reaction started by the addition of substrate (0.3 mM ADP) and terminated after 30 min with malachite green reagent and inorganic phosphate released from exogenous nucleotide was spectrophotometrically determined (OD620nm; [16]). Activity was defined as nmol Pi/mg/min. Apyrase (1 mg/ml containing 125 U, Sigma-Aldrich Australia) was included as a positive control, while a well containing assay buffer alone was included as a negative control.
SDS-PAGE and Coomassie stain
8.75 μg of rsol.CD39-PSGL-1 was incubated in citrated human plasma for 1–24 h at 37 °C. The proteins were then electrophoresed on a 10% SDS-polyacrylamide gel at 120 V. The gels were then stained for 60 min in Coomassie solution comprising 50% (v/v) methanol, 10% (v/v) acetic acid and 0.25% (w/v) Coomassie Brilliant Blue R-250 (Sigma-Aldrich Australia) at room temperature with shaking. The gel was then destained in 5% (v/v) methanol, 7.5% (v/v) acetic acid for 6 h and images were captured on a flatbed scanner.
Inhibition of platelet reactivity by rsol.CD39-PSGL-1
Platelet-rich plasma (PRP) from human volunteers was prepared by collecting blood with acid-citrate-dextrose anticoagulant followed by centrifugation at 200 g for 20 min at room temperature. PRP was incubated with PBS (vehicle control) or rsol.CD39-PSGL-1 (150–450 ng; purified from conditioned media) at 37 °C for 30 min. Platelet aggregation in the presence of agonists ADP and collagen were studied using the AggRAMTM System, Helena Laboratories, Beaumont, TX, USA, as published [19].
Flow cytometry to confirm localization of rsol.CD39-PSGL-1 on the platelet surface
Platelets isolated from PRP of WT and P-selectin−/− mice were stimulated with 20 U/ml of thrombin and incubated rsolCD39-PSGL-1-FITC. Platelets were gently washed by centrifugation and adherent rsolCD39-PSGL-1 will be quantified by flow cytometry using the BD FACS Canto flow cytometer.
Assessment of tail bleeding times after rsol.CD39-PSGL-1 administration
Mice were injected with saline or varying doses of rsol.CD39-PSGL-1 via the tail vein or orally administered clopidogrel (30 mg/kg; Plavix, Sanofi Winthrop, Paris, France) 2 h before, and tail transection bleed times and template tail bleed times were determined as described [20]. For template tail bleed times, mice were anaesthetized and a lateral incision 5 mm long and 2 mm deep, 1 cm from the tip of the mouse’s tail was made using a scalpel blade. A blotting tissue was placed against the wound site every 30 s for 15 min or until no further blood was detected on the tissue for two consecutive blotting periods.
Warm renal IRI model
10- to 14-week-old male mice were subjected to warm, unilateral kidney IRI surgery as described previously [19]. Briefly, following anaesthesia with xylazine ketamine, mice were placed on a 37 °C heating pad and their core body temperature was maintained for the duration of the surgery. A midline abdominal incision was performed to bluntly dissect the renal pedicles and remove the right kidney. The left kidney pedicle was then clamped using a microvascular clamp (Roboz, Rockville, MD) for 18 min and then removed. The surgical site was sutured and the mice were given 100 ml/kg warm normal saline delivered into the peritoneal cavity. Sham-operated mice were subjected to the same surgical procedures except clamping of the left kidney pedicle. All mice were allowed to recover on a heating pad and killed after 24 h of reperfusion, when blood and kidney specimens were harvested. Mice were administered saline (WT) or varying doses of rsol.CD39-PSGL-1 or clopidogrel as above, in a volume of 100 ml/kg, 30 min before IRI. n = 17 (WT); n = 13 (sham); n = 9–13 (0.2–0.5 μg/g rsol.CD39-PSGL-1); n = 5–7 (0.2–0.5 μg/g sol.CD39) and n = 7 (clopidogrel).
Assessment of renal function
Whole blood was collected via inferior vena cava puncture and serum creatinine (modified Jaffe rate reaction) and serum urea concentration were measured by the Department of Pathology of St. Vincent’s Hospital Melbourne (Olympus AU 2700, Integrated Science, Chatswood, NSW, Australia).
Renal histology and scoring
10% formalin-fixed and paraffin-embedded kidney tissue sections (4 μm) were stained with haematoxylin-eosin and assessed microscopically. The following semi-quantitative scale was used to evaluate the degree of tubular necrosis: 0, normal kidney; 1, minimal necrosis (<10% involvement); 2, mild necrosis (10–<25% involvement); 3, moderate necrosis (25–75% involvement); and 4, severe necrosis (>75% involvement) [20].
Ethical approval
Human plasma was collected from normal volunteer donors with approval from St.Vincent's Hospital Human Research Ethics Committee. All animal experiments were approved by the St Vincent’s Hospital Animal Ethics Committee and were conducted in compliance with the Australian code for the care and use of animals for scientific purposes.
Results and discussion
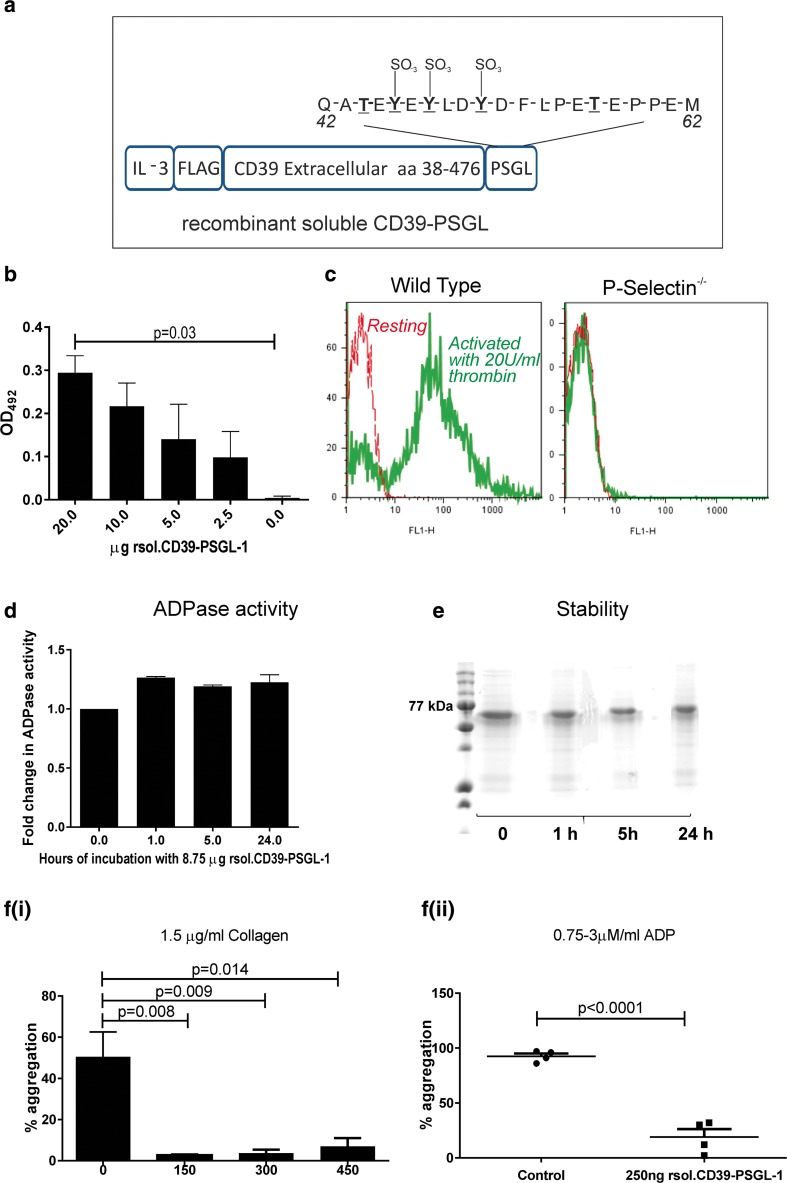
Figure 1a illustrates rsol.CD39-PSGL-1 that contains an interleukin-3 leader sequence for secretion, an N′-terminal FLAG-tag for affinity purification, followed by the extracellular region of CD39 fused to residues 42–62 of the mature PSGL-1 protein. As seen in Fig. 1b, rsol.CD39-PSGL-1 bound to recombinant P-selectin in a concentration-dependent manner in a solid phase ELISA with increasing amounts of protein binding to plates coated with 0–20 μg of P-selectin. To confirm binding of rsol.CD39-PSGL-1 to P-selectin, washed platelets were stimulated with thrombin (20 U/ml) and incubated with rsol.CD39-PSGL-1-FITC and analysed by flow cytometry (Fig. 1c). Significant binding of rsol.CD39-PSGL-1 was noted to activated platelets from wild type (WT) mice that expressed P-selectin when compared to non-activated WT platelets (no P-selectin upregulation) and platelets from P-selectin−/− mice. The ability of rsol.CD39-PSGL-1 to hydrolyse ADP (ADPase activity) was calculated to be 2.29 mmol/μg/min and was 0.36× that of an equimolar quantity of apyrase (which was determined to be 6.49 mmol/μg/min). Further, by incubating a fixed amount of rsol.CD39-PSGL-1 in citrated human plasma from 1 to 24 h at 37 °C, we determined that >90% ADPase activity was retained at 24 h (Fig. 1d). Lack of proteolysis was confirmed by Coomassie staining (Fig. 1e). To assess the biological activity of rsol.CD39-PSGL-1, an in vitro platelet aggregometry assay was performed. Platelet aggregation curves were determined with PRP, using ADP and collagen as agonists. rsol.CD39-PSGL-1 inhibited platelet aggregation after stimulation with 1.5 μg/ml collagen (Fig. 1f(i)) as well as 0.75–3 μM ADP (Fig. 1f(ii)). This confirms that rsol.CD39-PSGL-1 inhibited platelet aggregation in vitro.
Fig. 1.
Design and verification of recombinant soluble CD39-PSGL-1. a Illustration of the construct designed to produce rsol.CD39-PSGL-1. The construct contains an interleukin-3 leader sequence for secretion, an N′-terminal FLAG-tag for affinity purification, followed by the extracellular region of CD39, then a tag at the C′-terminal representing 20 amino acid residues (42–62) of PSGL-1 that contain three tyrosine residues (underlined) and two threonine residues which undergo posttranslational modification to display sialylated O-glycans. b The ability of rsol.CD39-PSGL-1 to bind P-selectin was assessed by ELISA whereby plates were coated P-selectin and varying concentrations of rsol.CD39-PSGL-1 were tested for binding. c Binding of rsol.CD39-PSGL-1-FITC to P-selectin by using platelets from P-selectin null mice and compared with wild-type mice that were activated with 20 U/ml thrombin; only activated platelets from wild-type mice bind rsol.CD39-PSGL-1 with no binding seen in platelets from P-selectin null mice. d 8.75 μg rsol.CD39-PSGL-1 was incubated for 1–24 h at 37 °C and fold change in ADPase activity was analysed. rsol.CD39-PSGL-1 retained activity at 24 h. Data is mean ± SEM (n = 3). e Lack of proteolysis was confirmed by SDS-PAGE and Coomassie stain. f(i) 150–450 ng rsol.CD39-PSGL-1 (10 μg/ml) purified from conditioned media was able to inhibit aggregation of platelets in a platelet rich plasma preparation after stimulation with 1.5 μg/ml collagen, and (ii) rsol.CD39-PSGL-1 inhibited platelet aggregation after stimulation with ADP (0.75–3 μM) confirming the presence of ADPase activity
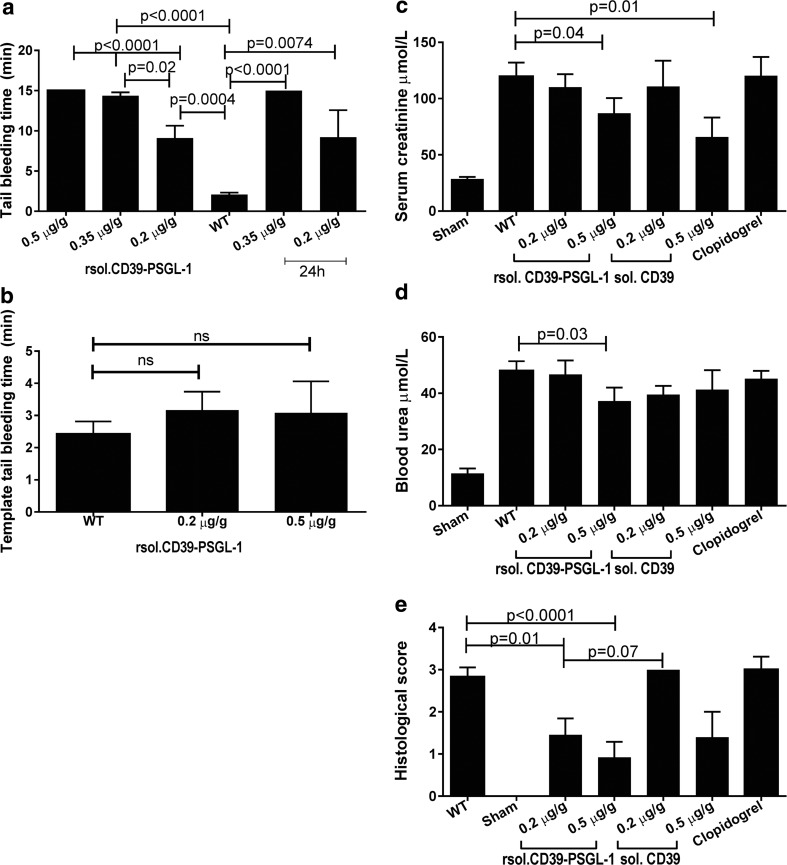
We injected mice with doses of 0.2–0.5 μg/g of rsol.CD39-PSGL-1 and assessed tail bleeding times via the tail tip transection and tail template bleeding methods, and also 24 h later via the tail tip transection method. This method revealed that doses of 0.2–0.5 μg/g of rsol.CD39-PSGL-1 prolonged tail bleeding times from 9 to 15 min when compared to bleeding times of ~2 min in saline-injected mice; similar bleeding times were maintained even 24 h after rsol.CD39-PSGL-1 was injected (Fig. 2a). However, the same doses of rsol.CD39-PSGL-1 did not significantly prolong template tail bleeding times (Fig. 2b); n = 3 (0.5 μg/g CD39-PSGL-1); n = 9(0.2 μg/g rsol.CD39-PSGL-1); n = 10 (WT); n = 4 (24 h, 0.2–0.5 μg/g).
Fig. 2.
Tail tip transection bleeding times are prolonged but template tail bleeding times are unchanged after injection with rsolCD39-PSGL-1, which also preserves renal histology after IRI, when compared to saline-injected WT mice. a Time to cessation of blood flow is increased in mice given doses ≥0.2 μg/g, even 24 h later. b Template tail bleeding times are unchanged in mice administered 0.2–0.5 μg/g. c Serum creatinine levels are significantly reduced in mice injected with 0.5 μg/g of rsol.CD39-PSGL-1 as well as soluble CD39, while d blood urea levels are significantly attenuated only in mice injected with 0.5 μg/g of rsol.CD39-PSGL-1; in both instances clopidogrel does not exert the same effect as 0.5 μg/g of CD39-PSGL-1. e Scoring of H&E stained kidney sections shows that 0.2–0.5 μg/g of rsol.CD39-PSGL-1 can significantly preserve renal histology, but soluble CD39 and clopidogrel do not have the same effect. Baseline creatinine and urea levels are evident in the sham cohort, which are subjected to the same surgical procedures except clamping of the left kidney pedicle; these mice do not show any histopathological changes. Data is mean ± SEM. a, b p values determined by one-way ANOVA followed by Tukey’s post hoc analysis. n = 3 (0.5 μg/g CD39-PSGL-1); n = 9(0.2 μg/g rsol.CD39-PSGL-1); n = 10 (WT); n = 4 (24 h 0.2–0.5 μg/g). c–e p values determined by one-way ANOVA followed by Fisher’s LSD or Tukey’s post hoc analysis; n = 17 (WT); n = 13 (sham); n = 9–13 (0.2–0.5 μg/g rsol.CD39-PSGL-1); n = 5–7 (0.2–0.5 μg/g sol.CD39) and n = 7 (clopidogrel)
When compared to sham-operated mice, WT mice subjected to 18 min of unilateral renal ischemia followed by 24 h of reperfusion mice exhibited ~4× increase in serum creatinine (Fig. 2c) and ~5× increase in urea (Fig. 2d). Mice that received 0.2 μg/g of either rsol.CD39-PSGL-1 or solCD39 did not show any reduction in creatinine or urea levels compared to untreated mice. However, a dose of 0.5 μg/g of rsol.CD39-PSGL-1 caused a significant 28 and 23% reduction in serum creatinine and blood urea nitrogen compared to untreated mice. While the 0.5 μg/g solCD39 caused a significant 45% decrease in serum creatinine, no benefit on blood urea nitrogen levels was observed. Notably, clopidogrel (30 mg/kg) did not exert the same protective effects as rsol.CD39-PSGL-1 on biochemical changes caused by kidney IRI; tubular necrosis was assessed semi-quantitatively by H&E staining of kidney sections. Sham animals had an average score of 0, as expected, while kidneys from WT mice scored an average of 2.8/4. rsol.CD39-PSGL-1 demonstrated dose-dependent attenuation of renal damage; doses of 0.2 and 0.5 μg/g resulted in renal damage scores of (mean ± SEM) 1.45 ± 0.39 and 0.92 ± 0.36, respectively (Fig. 2e). In contrast, comparable doses of neither solCD39 nor clopidogrel were able to achieve this degree of protection after kidney IRI.
It is well known that adenosine signalling improves outcome in kidney IRI and recently CD39 targeted to activated platelets was shown to be protective in a mouse model of thrombosis [4]. Here, we investigated whether targeting CD39 to the endothelium will mitigate renal damage sustained during IRI in the absence of any systemic effects.
We successfully purified and produced rsol.CD39-PSGL-1 that bound in a concentration-dependent manner to recombinant P-selectin in an ELISA as well as bound selectively to activated human platelets that expressed P-selectin. Consistent with the functionality of soluble CD39, the protein also inhibited platelet aggregation in response to 1.5 μg/ml collagen and up to 3 μM ADP as well as exhibited strong ADPase activity in a malachite green assay that was retained even 24 h after incubation in human plasma (Fig. 1). Evaluation of the effect of rsol.CD39-PSGL-1 on primary haemostasis revealed that rsol.CD39-PSGL-1 prolonged tail bleeding times at doses ≥0.2 μg/g similar to untargeted solCD39. The transection model, which involves amputation of a small section of the mouse tail, is believed to be a very severe model of assessing haemostatic changes in mice because it causes substantial vessel damage as vascular injury [21]. However, the tail template model, which closely resembles the skin bleeding times used to assess haemostatic changes in humans, shows that bleeding times were not altered following injection of the same dose, and is evidence that rsol.CD39-PSGL-1 is unlikely to have adversely altered systemic haemostasis if administered at doses of 0.2–0.5 μg/g in vivo (Fig. 2a, b). After kidney IRI both targeted and untargeted CD39 mediated significant reduction in serum creatinine at doses of 0.5 μg/g, while only mice injected with the same dose of targeted CD39 showed significant reduction of blood urea nitrogen levels. Remarkably, these effects were not observed in mice treated with clopidogrel, showing that antagonising ADP receptor P2Y12 is not sufficient to recapitulate the benefits of targeted CD39 in this model (Fig. 2c, d). Further, that significant amelioration of renal histological injury was noted at a lower dose of rsol.CD39-PSGL-1 as compared to non-targeted solCD39 highlights the overall benefits of endothelium-targeted CD39 activity (Fig. 2e).
While these data are promising, it should be noted that PSGL-1 is a homodimer of two disulphide-linked subunits of ∼120 kDa, and dimerization of PSGL-1 via the cysteine320 residue is essential for physiologic interactions with P-selectin. Complete antagonism of P-selectin adhesion to its ligand PSGL-1 can be achieved by the use of a single-chain Fv (ScFv) against PSGL-1, as developed by Swers et al. [22]. However, the data herein demonstrates the added benefit of rsol.CD39-PSGL-1 in specifically targeting adenosine signalling via CD39 to the renal endothelium, which preserves renal histology and minimises kidney dysfunction sustained during IRI.
Acknowledgements
This work was supported by grants awarded to HH Nandurkar from the NHMRC (344801) and NIH (1 R01 HL078651) and to SC Robson from the NIH (5 R01 HL094400).
Compliance with ethical standards
Conflict of interest
Maithili Sashindranath declares that she has no conflict of interest.
Karen M Dwyer declares that she has no conflict of interest.
Shala Dezfouli declares that she has no conflict of interest.
Carly Selan declares that she has no conflict of interest.
Sandra Crikis declares that she has no conflict of interest.
Bo Lu declares that he has no conflict of interest.
Yuping Yuan declares that she has no conflict of interest.
Michael J Hickey declares that he has no conflict of interest.
Karlheinz Peter declares that he has no conflict of interest.
Simon C Robson declares that he has no conflict of interest.
Peter J Cowan declares that he has no conflict of interest.
Harshal Nandurkar declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants . All animal experiments were approved by the St Vincent’s Hospital Animal Ethics Committee and were conducted in compliance with the Australian code for the care and use of animals for scientific purposes.
Statement of competing financial interests
No competing financial interests.
Footnotes
Peter J Cowan and Harshal H Nandurkar are equal senior authors.
References
- 1.Crikis S, et al. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10(12):2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu B, et al. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation. 2008;86(12):1707–1712. doi: 10.1097/TP.0b013e31819022bc. [DOI] [PubMed] [Google Scholar]
- 3.Rajakumar SV, et al. Deficiency or inhibition of CD73 protects in mild kidney ischemia-reperfusion injury. Transplantation. 2010;90(12):1260–1264. doi: 10.1097/TP.0b013e3182003d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohmann JD, et al. Delayed targeting of CD39 to activated platelet GPIIb/IIIa via a single-chain antibody: breaking the link between antithrombotic potency and bleeding? Blood. 2013;121(16):3067–3075. doi: 10.1182/blood-2012-08-449694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts V, et al. The CD39-adenosinergic axis in the pathogenesis of renal ischemia-reperfusion injury. Purinergic Signal. 2013;9(2):135–143. doi: 10.1007/s11302-012-9342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaczmarek E, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271(51):33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann H. 5'-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285(Pt 2):345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinsky DJ, et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. JClinInvest. 2002;109(8):1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai M, et al. Modulation of nucleoside [correction of nucleotide] triphosphate diphosphohydrolase-1 (NTPDase-1)cd39 in xenograft rejection. Mol Med. 1999;5(11):743–752. [PMC free article] [PubMed] [Google Scholar]
- 10.Imai M, et al. Recombinant adenoviral mediated CD39 gene transfer prolongs cardiac xenograft survival. Transplantation. 2000;70(6):864–870. doi: 10.1097/00007890-200009270-00003. [DOI] [PubMed] [Google Scholar]
- 11.Belayev L, et al. Neuroprotective effect of SolCD39, a novel platelet aggregation inhibitor, on transient middle cerebral artery occlusion in rats. Stroke. 2003;34(3):758–763. doi: 10.1161/01.STR.0000056169.45365.15. [DOI] [PubMed] [Google Scholar]
- 12.Gayle RB, 3rd, et al. Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J Clin Invest. 1998;101(9):1851–1859. doi: 10.1172/JCI1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEver RP, Cummings RD. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100(3):485–491. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Furie BC, Furie B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction. Thromb Haemost. 1999;81(1):1–7. [PubMed] [Google Scholar]
- 15.Vandendries ER, Furie BC, Furie B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. Thromb Haemost. 2004;92(3):459–466. doi: 10.1160/TH04-05-0306. [DOI] [PubMed] [Google Scholar]
- 16.Burch EE, et al. The N-terminal peptide of PSGL-1 can mediate adhesion to trauma-activated endothelium via P-selectin in vivo. Blood. 2002;100(2):531–538. doi: 10.1182/blood.V100.2.531. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, et al. Identification of N-terminal residues on P-selectin glycoprotein ligand-1 required for binding to P-selectin. J Biol Chem. 1998;273(12):7078–7087. doi: 10.1074/jbc.273.12.7078. [DOI] [PubMed] [Google Scholar]
- 18.Li F, et al. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271(6):3255–3264. doi: 10.1074/jbc.271.6.3255. [DOI] [PubMed] [Google Scholar]
- 19.Straub A, et al. Evidence of platelet activation at medically used hypothermia and mechanistic data indicating ADP as a key mediator and therapeutic target. Arterioscler Thromb Vasc Biol. 2011;31(7):1607–1616. doi: 10.1161/ATVBAHA.111.226373. [DOI] [PubMed] [Google Scholar]
- 20.Mountford JK, et al. The class II PI 3-kinase, PI3KC2alpha, links platelet internal membrane structure to shear-dependent adhesive function. Nat Commun. 2015;6:6535. doi: 10.1038/ncomms7535. [DOI] [PubMed] [Google Scholar]
- 21.Dejana E, et al. Bleeding time in laboratory animals. III—do tail bleeding times in rats only measure a platelet defect? (The aspirin puzzle) Thromb Res. 1979;15(1–2):199–207. doi: 10.1016/0049-3848(79)90065-3. [DOI] [PubMed] [Google Scholar]
- 22.Swers JS, et al. A high affinity human antibody antagonist of P-selectin mediated rolling. Biochem Biophys Res Commun. 2006;350(3):508–513. doi: 10.1016/j.bbrc.2006.08.197. [DOI] [PubMed] [Google Scholar]