Abstract
A one-pot three-component double-click process for preparing tumor-targeting agents for cancer radiotherapy is described here. By utilizing DOTA (or NOTA) containing tetrazines and the TCO-substituted aldehyde, the two click reactions, the tetrazine ligation (an inverse electron-demand Diels-Alder cycloaddition) and the RIKEN click (a rapid 6π-azaelectrocyclization), could simultaneously proceed under mild conditions to afford covalent attachment of the metal chelator DOTA or NOTA to biomolecules such as to albumin and anti-IGSF4 antibody without altering their activities. Subsequently, radiolabeling of DOTA- or NOTA-attached albumin and anti-IGSF4 antibody (an anti-tumor-targeting antibody) with [67Cu], a β−-emitting radionuclide, could be achieved in a highly efficient manner via a simple chelation with DOTA proving to be a more superior chelator than NOTA. Our work provides a new and operationally simple method for introducing the [67Cu] isotope even in large quantities to biomolecules, thereby representing an important process for preparations of clinically relevant tumor-targeting agents for radiotherapy.
Introduction
Radiogenic therapies represent an important approach to treatment of cancers. Carbon-ion based radiations1 have been one of the most common cancer therapeutic methods. To improve the therapeutic effect of radiations, sensitizers using nano-materials such as gold nanoparticle, magnetic nanoparticles, and quantum dots have been developed recently2–4. Unfortunately, such usage have been restricted to treatment of stomach cancer and bowel cancer. More critically, access to radiations targeting specifically to cancer cells remains a huge challenge. On the other hand, radioisotopes (RI) have emerged as power radio-therapeutic agents and have been widely utilized in clinical practices. Radionuclide such as isotope [89Sr] has been employed for metastatic bone cancers5 and isotope [131I] is used as radio-therapeutic medicine for thyroid cancers6. More importantly, radiolabeled biomolecules have become more useful as tumor-targeting drugs for specific radiations. For example, the [90Y]-labeled anti-CD20 antibody has been developed for clinical usage in the treatment of malignant lymphomas.
Consequently, recent efforts have been devoted to development of radiolabeled tumor-targeting biomolecules, and particularly, in evolving new and efficient synthetic methods for incorporating radionuclides into biomolecules. Some simple and well-known radiolabeling methods would involve assembly of metal chelating moieties and subsequent introduction of a radioisotopic label. More specifically, amidations of lysine residues using activated esters such as succinimidyl ester7, or Michael additions of thiols to maleimides8 have been made available to attach a metal chelator onto peptides and antibodies. Recently, click chemistry such as Cu(I)-accelerated Huisgen [3 + 2] cycloadditions9, 10, strain-promoted [3 + 2] cycloadditions11, and inverse electron demand Diels-Alder reactions12 have been used for chemoselective and high yielding methods for radiolabelling. However, while selective and efficient introduction of radioactive tags to complex and highly functionalized bioactive molecules could be achieved using click reactions, efficient and regioselective introduction of radiolabels still presents a challenge. In addition, these click methods require key functional groups such as azides, alkynes, tetrazines, and trans-alkenes be chemically and/or genetically pre-installed within biomolecules13–16. Therefore, a direct radiolabeling via click reactions without overt structural modifications of biomolecules should be more ideal.
In pursuant of such ideal click process, in which the labeling can be performed simply by mixing a native biomolecule with the probe solution under mild conditions, our lab reported a direct reaction of lysine residue on the side chain of peptides via a rapid 6π-azaelectrocyclization (RIKEN click reaction)17–23. Fluorescence, positron emitter labels and biofunctional molecules are efficiently and conveniently introduced into the amino groups of the proteins and on the cell surfaces via a reaction involving unsaturated aldehyde probe (such as compound 1 in Fig. 2) at low concentrations over a short period of time at room temperature. Although our RIKEN click method is not bioorthogonal with respect to the natural primary amino groups, the mild reaction conditions yield the preferential and selective labeling of the most exposed and densely expressed amines24–28. RIKEN click process hardly proceeds with internal lysines in a tertiary protein or the N-terminal amines; however, the lysine residues at the protein surface react rapidly. Bioconjugation therefore occurred preferentially at the surface positions. RIKEN click method thus minimizes indiscriminate amino modification or interference with the native protein functions while introducing new functionalities to solvent-exposed residues. The dihydropyridine electrocyclization products, which preserved the cationic charges of the original lysine residues, contribute to the retention of the native protein activity. This is entirely different from the conventional NHS-ester reaction, which generally proceeds under high reagent concentrations (~10−2 M) and long reaction times (a few to several hours), hence indiscriminately modifies the key lysines, resulting in killing native activity of biomolecules.
Figure 2.
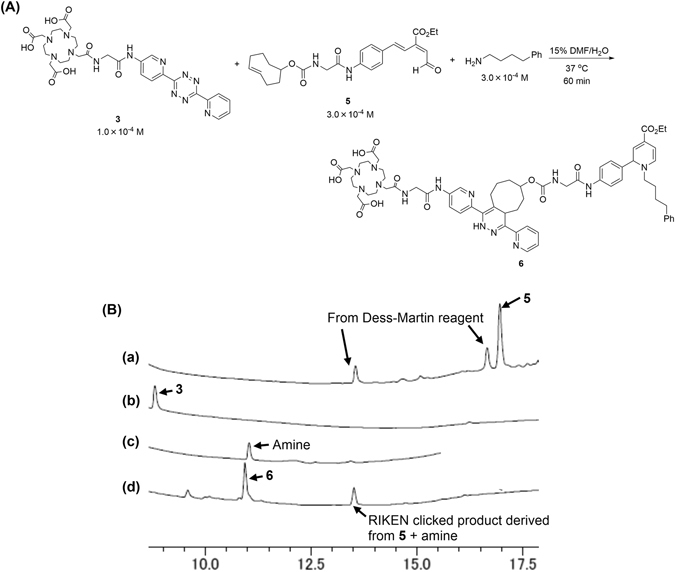
One-pot three-component double-click labeling with 4-phenyl-1-butylamine as a model primary amine. (A) Scheme and conditions. (B) Reverse phase HPLC analysis. Charts indicate: (a) TCO-substituted aldehyde 5, (b) DOTA-substituted tetrazine 3, (c) 4-phenyl-1-butylamine, and (d) reaction mixture. In chart (a), a peak at 16.8 min corresponded to aldehyde 5 and other two peaks at 13.6 and 16.4 min are derived from Dess-Martin reagent during oxidation of the alcohol precursor synthesizing to TCO-aldehyde 5. In chart (d), two peaks at 10.9 and 13.5 min corresponded to three-component coupling product 6 (m/z 1182.8 calcd for C60H71N13O13 [M + H]+) and RIKEN clicked product derived from three-fold excess 5 and 4-phenyl-1-butylamine (m/z 584.3 calcd for C35H42N3O5 [M − H]+). DMF = N,N-Dimethylformamide.
As examples of RIKEN click reaction applied to biomolecule labeling, we succeeded in preparing the [68Ga]-labeled somatostatin and N-glycoconjugates via introduction of a DOTA chelating motif (DOTA: 1,4,7,10-tetraazadodecane-1,4,7,10-tetraacetic acid) using the RIKEN click process with DOTA containing aldehyde 1, followed by chelation with the [68Ga] metal radioisotope (Fig. 1a)17–19. This radiolabeling enabled in vivo visualization of their kinetics for the first time. However, due to the difficulty in synthesizing and handling of 1, a more general application of RIKEN click reaction for radiolabeling remains elusive.
Figure 1.
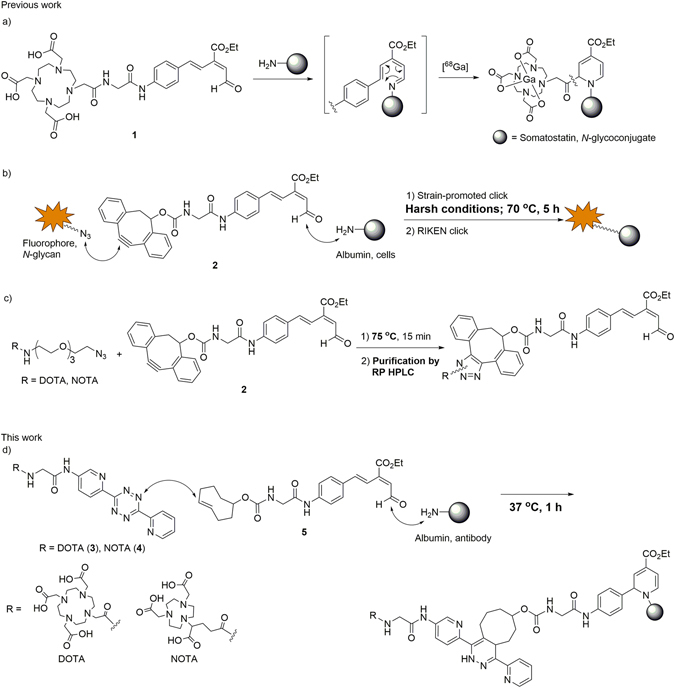
Radiolabeling using the RIKEN click reaction. DOTA: 1,4,7,10-tetraazadodecane-1,4,7,10-tetraacetic acid; NOTA: 1,4,7-triazacyclononane-1,4,7-triacetic acid; TCO: trans-cyclooctene.
To develop a facile preparation of the tag-substituted aldehyde, we synthesized aldehyde 2 substituted with a dibenzocyclooctyne (DIBO) motif based on Boons’ report (Fig. 1b)29. Strain-promoted click reaction using aldehyde 2 allowed incorporations of reporter groups such as fluorophores or N-glycans, and the ultimate introduction into proteins30–33 and live cells34, 35 through the ensuing RIKEN click reaction. However, heating at 70 °C30–33 and/or prolonged reaction time (5 h)35 were required for the strain-promoted click reaction. Furthermore, during our preliminary trials of incorporating DOTA, and purification of the click product was also necessary because of low efficiency (Fig. 1c). Thus, to develop a facile and near-quantitative entry to radiolabelled biomolecules, we envisioned DOTA (or NOTA) containing tetrazine 3 (or 4) and the TCO-substituted aldehyde 5 (NOTA: 1,4,7-triazacyclononane-1,4,7-triacetic acid, tetrazine: 3,6-Di-(2-pyridyl)-s-tetrazine, TCO: trans-cyclooctene) could be implemented in a one-pot three-component double-click process to radiolabel proteins and antibodies such as albumin and anti-IGSF4 (Immunoglobulin superfamily member 4) (Fig. 1d). We wish to report herein a new and practical method for introducing radiolabels to proteins and antibodies that could serve as tumor-targeting radio-therapeutics.
Results and Discussion
One-pot three-component labeling using both the tetrazine ligation and RIKEN click reaction
To identify a more reactive “click” reaction that can be employed in conjunction with our RIKEN click reaction, we were inspired by the tetrazine ligation, which is an inverse electro-demand Diels-Alder reaction that was developed by Fox36. We expected that the tetrazine ligation could be complete under mild conditions similar to those for the RIKEN click. More specifically, we thought that both click reactions could be carried out simultaneously in one–pot. Toward this goal, we synthesized DOTA-substituted tetrazine 3 and NOTA-substituted tetrazine 4 as metal chelating motifs, and the TCO-substituted aldehyde 5 as the RIKEN click partner (see Supporting Information).
To evaluate reactivity and compatibility of those click components, 1.0 × 10−4 M of the DOTA-substituted tetrazine 3, 3.0 × 10−4 M of the TCO-substituted aldehyde 5, and 3.0 × 10−4 M of 4-phenyl-1-butylamine (serving as a model primary amine of lysines in biomolecules) were reacted by simply mixing them in 15% DMF-containing water and heating at 37 °C (Fig. 2). After 1 h, reverse phase HPLC analysis showed that all of these starting materials were clearly consumed and that the desired double-click product 6 was detected at t = 10.9 min, accompanied by the RIKEN clicked product between excess amount of 5 and amine at t = 13.5 min (Fig. 2d). This result suggests that tetrazine ligation was efficiently completed under comparably mild conditions as those adopted for the RIKEN click reaction, thereby rendering a one-pot three-component coupling labeling feasible.
One-pot three-component click labeling of albumin and antibody
Labeling of proteins and antibodies without the loss of functions represents one of the major challenges. In our previous report on RIKEN click labeling of DOTA (Fig. 1a), affinity of the DOTA-labeled anti-GFP antibody retained with that of intact antibody17. Given that we had some success with the DOTA-attached anti-GFP antibody for the RIKEN click reaction (Fig. 1a), which retained its activity17, we investigated our new one-pot three-component double-click reaction on both human serum albumin and anti-IGSF4 as models.
Based on conditions described above, a one-pot three-component double-click reaction was conducted on albumin using the DOTA containing tetrazine 3 or NOTA containing tetrazine 4 and the TCO-substituted aldehyde 5 in 5% DMF-containing aqueous solution at 37 °C (pH 7) for 60 min (Fig. 3A). The resulting product was analyzed by MALDI-TOF-MS and the number of the attached molecules (1,119 of MW increase for 1 molecule of 3 + 5) was determined by difference of the molecular weight from that of intact albumin. When the one-pot three-component click reaction was carried out with 5.0 × 10−5 M of 3, 1.5 × 10−4 M of 5, and 1.0 × 10−5 M of albumin, 2 molecules of DOTA were introduced to albumin (Fig. 3B-b). To further optimize the reaction, when 1.0 × 10−4 M of 3 and 3.0 × 10−4 M of 5 were treated with albumin, attachment of 4 molecules of DOTA was predominantly observed (Fig. 3B-c). In the case of attaching NOTA to albumin, the one-pot double click reaction under the same conditions as shown in Fig. 3B-c lead to approximately 3 molecules of NOTA-attached albumin were obtained (see Supporting Information). Thus, the number of DOTA motif introduced to albumin by the one-pot three-component procedure could be precisely controlled through adjusting the concentration of the respective click partner. This phenomenon is consistent with those previously reported reactivity of RIKEN click reaction in terms of the efficiency17.
Figure 3.
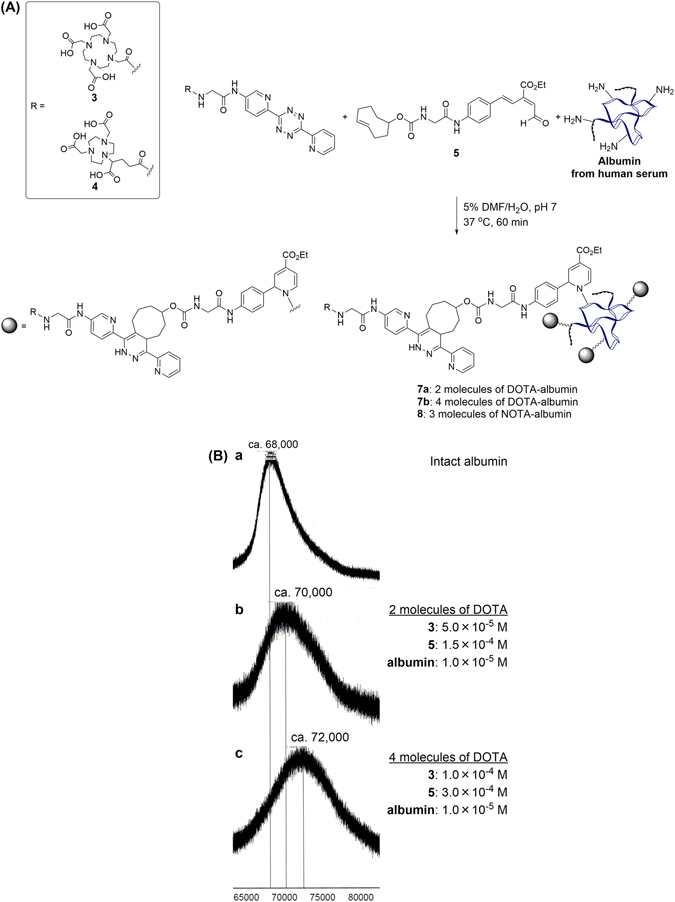
(A) One-pot three-component click labeling of albumin using the tetrazine ligation and RIKEN click reaction. (B) MALDI-TOF-MS analysis of intact albumin and each product obtained using the one-pot three-component click labeling. Spectra show: (a) Intact albumin. (b) DOTA-labeled albumin performed by using 3 and 5 at concentrations of 5.0 × 10−5 M and 1.5 × 10−4 M, and (c) 1.0 × 10−4 M and 3.0 × 10−4 M, for 60 min. DMF = N,N-Dimethylformamide.
Encouraged by the success of this one-pot double-click process using albumin, anti-IGSF4 antibody, a potential tumor-targeting agent, was attached with DOTA and NOTA using the conditions established in Fig. 3. Thus, one-pot three-component labeling was performed by treating 1.0 × 10−4 M of 3, 3.0 × 10−4 M of 5 and 1.0 × 10−5 M of anti-IGSF4 antibody in 5% DMF-containing aqueous solution at 37 °C (pH 7) for 60 min, similar to those applied in Fig. 3B-c (Fig. 4a). Based on the radioactivity of [67Cu] incorporated into DOTA-labeled anti-IGSF4 antibody (vide infra, see Fig. 5), approximately 3 DOTA molecules were incorporated for each antibody under the conditions. The antigen recognizing activity of DOTA- and NOTA-attached anti-IGSF4 antibodies 9a and 9b were measured by enzyme-linked immunosorbent assay (ELISA), and were found to be same as that of the intact anti-IGSF4 antibody (Fig. 4b). Thus, our new one-pot three-component click process was not obstructive to the antibody activity. As previously found for RIKEN click reaction17, the labeling might preferentially proceed at sterically non-hindered position of proteins without inhibiting the activity, such as the lysines at Fc moiety of antibody.
Figure 4.
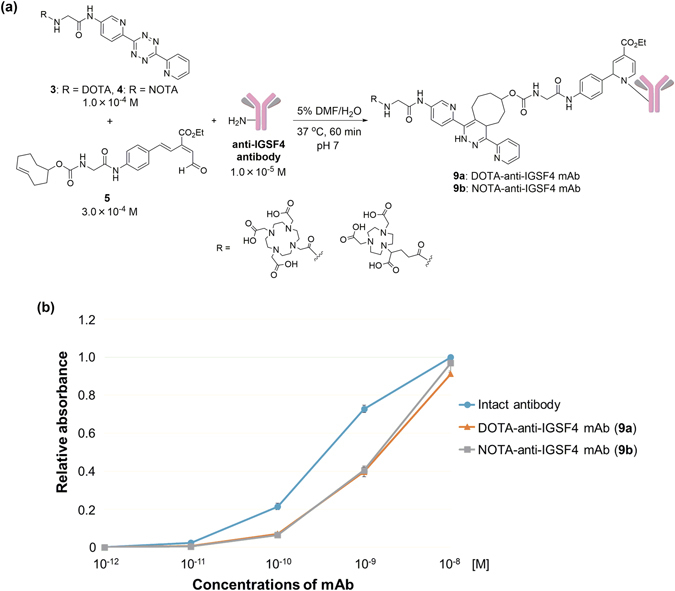
(a) One-pot three-component click labeling of anti-IGSF4 antibody as cancer-targeting agent. (b) Affinities of intact and labeled anti-IGSF4 antibodies to IGSF4 analyzed by ELISA. DMF = N,N-Dimethylformamide.
Figure 5.
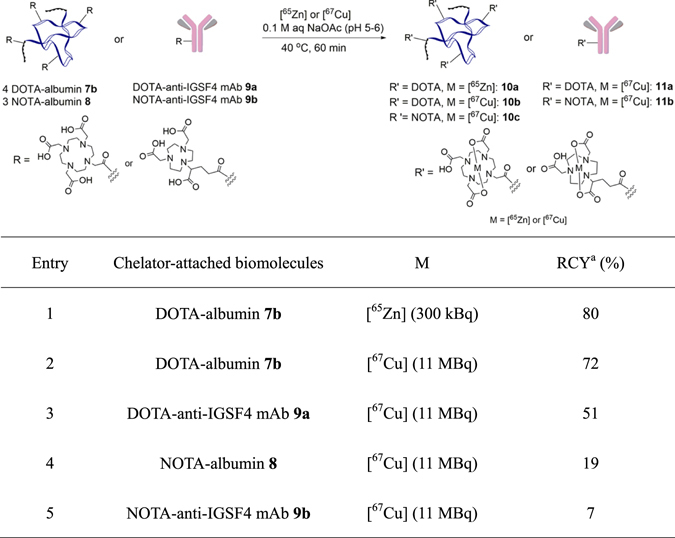
Radiolabelings of DOTA or NOTA-attached albumins and anti-IGSF4 antibody. aRadiochemical yield. Specific activity of 67Cu and 65Zn was 110 MBq μg−1 and 125 MBq μg−1, respectively.
Radiolabeling of DOTA or NOTA-attached albumin and anti-IGSF4 antibody
With DOTA/NOTA-labeled albumin and anti-IGSF4 antibody efficiently and non-invasively prepared via the one-pot three-component click labeling, we pursued radiolabeling of them with [67Cu] (a β−emitting radionuclide) as an application of labeling of tumor-targeting agents. The use of [67Cu] as a RI has received much attention as a highly useful radionuclide for cancer radiotherapy37.
The radioisotope of [67Cu] was produced in the [70Zn(d,αn)][67Cu] reaction (see details in Experimental Section and Supporting Information). The specific radioactivity of [67Cu] with 110 MBq μg−1 was obtained38. [67Cu]-Labeling of DOTA- and NOTA-linked albumins and anti-IGSF4 antibodies were then performed with pH being approximately 5–6 using optimized conditions reported by Chen39. Prior to labeling with [67Cu], radiolabeling of DOTA/NOTA-linked albumin and anti-IGSF4 antibody with [65Zn]40, 41 was initially evaluated as a model metallic radionuclide (Entry 1 in Fig. 5). When 1.0 × 10−5 M of DOTA-linked albumin 7b (4 DOTA are labeled, see Fig. 3B-c) was treated with 300 kBq of [65Zn] in aqueous sodium acetate at 40 °C for 1 h, the chelation to the DOTA motif was successful with a radiochemical yield (RCY) of 80% (10a, Entry 1). With this success in hand, labeling with [67Cu] was performed. As shown in Entry 2, treatment of DOTA-linked albumin 7b with 11 MBq of [67Cu] afforded [67Cu]-labeled albumin 10b in 72% RCY. [67Cu]-Labeling of DOTA-linked anti-IGSF4 antibody 9a was similarly successful to afford [67Cu]-labeled anti-IGSF4 antibody 11a in a practical RCY of 51% (Entry 3). To probe the selective chelation of these radioactive metals to DOTA-linked albumin or anti-IGSF4 antibody, albumin and anti-IGSF4 antibody without the DOTA motif were subjected to the same reaction conditions. Neither [65Zn] nor [67Cu] was found to be incorporated, thereby suggesting that the labeling of these metal radionuclides could occur only with albumin or antibody linked to the DOTA chelator (see Supporting Information). Given that chelation of Cu2+ metal ion is dependent upon the amount of DOTA linked to antibodies, it could be estimated that approximately 3 molecules of DOTA were found introduced onto antibodies based on RCY of 72% and 51% for [67Cu]-labeled DOTA-linked albumin 10b and labeled antibody 11a, respectively (See also labeling analysis in Fig. 4).
Lastly, [67Cu]-labelings of NOTA-linked albumin and anti-IGSF4 antibody 8 and 9b were evaluated under same conditions employed for DOTA-attached systems. Unfortunately, [67Cu]-labeled NOTA-albumin 10c and anti-IGSF4 antibody 11b were obtained in just 19% and 7% of RCY (Entries 4 and 5), which are much lower those of DOTA-attached systems 7b and 9a. The low efficiency in the metal chelation is likely due to the structural rigidity of the NOTA chelator42 that usually require a much high temperature and a longer incubation time43. Our studies suggest that DOTA is a more suitable metal chelator than NOTA for radiolabeling of unstable biomolecules such as antibodies at low temperatures.
Conclusion
In summary, we have developed a one-pot three-component double-click process for preparing tumor-targeting agents for cancer radiotherapy. Specifically, by employing DOTA- or NOTA containing tetrazines and the TCO-substituted aldehyde, the two click reactions, the tetrazine ligation and the RIKEN click reaction, could simultaneously take place to covalently attach DOTA or NOTA to biomolecules without disrupting their activities or destabilizing them. Subsequently, concise preparations of [67Cu]-labeled albumin and anti-IGSF4 antibody (anti-tumor-targeting antibody) could be achieved with DOTA being a more superior chelator to the Cu2+ metal than NOTA. Our work provides a novel and practical method for introducing the [67Cu] metal isotope, a β−-emitting radionuclide, even in large quantities to biomolecules that can serve as new cancer radio-therapeutics. Efforts are underway to explore potential clinical applications of this efficient access to tumor-targeting agents.
Methods
All other commercially available reagents were used without further purification. Distilled water was purchased from nacalai teshque. DMF was purchased from Wako Pure Chemicals Industries Ltd. Syntheses of DOTA-tetrazine 3, NOTA-tetrazine 4, and TCO-aldehyde 5 were described in Supporting Information. DMF = N,N-dimethylformamide, PBS = phosphate buffer saline, HEK293 cells = human embryonic kidney 293 cells.
One-pot three-component double-click reaction for attaching DOTA to albumin (7a)
Aqueous solution of albumin (1 × 10−4 M, 20 μL) was diluted with distilled water (170 μL), then aqueous solution of DOTA-tetrazine 3 (2 × 10−3 M, 5 μL) and TCO-aldehyde 5 in DMF (6 × 10−3 M, 5 μL) were added and the mixture was heated to 37 °C. After 1 h, the reaction mixture was transferred into Amicon® 10 K, centrifuge with 14k rpm was performed for 12 min. To the filter was added 10% DMF-containing water (100 μL) and centrifuge with 14k rpm was done for 12 min. Then, to the filter was added distilled water (300 μL) and centrifuge with 14k rpm was done for 12 min. This wash was repeated more than 2 times. The residue on filter was collected and diluted with water to give 2 × 10−5 M of 7a in water as stock solution.
One-pot three-component doubl-click reaction for attaching DOTA to albumin (7b)
According to the procedure of one-pot three-component double click labeling for preparation of 7a, the labeling was carried out using distilled water (160 μL), aqueous solution of albumin (1 × 10−4 M, 20 μL), aqueous solution of the DOTA containing tetrazine 3 (2 × 10−3 M, 10 μL) and TCO-substituted aldehyde 5 in DMF (6 × 10−3 M, 10 μL). The stock solution of 7b (2 × 10−5 M) in water was prepared for the subsequent radiolabeling.
One-pot three-component click reaction for attaching DOTA to anti-IGSF4 antibody (9a)
According to the protocol of one-pot three-component double click labeling of albumin with DOTA (preparation of 7b), the labeling of anti-IGSF4 antibody with DOTA was carried out. The anti-IGSF4 mouse monoclonal antibody was raised against the extracellular domain of IGSF4 produced by HEK293 cells (Health Science Research Resources Bank, Osaka, Japan). Anti-IGSF4 antibody in PBS (3 × 10−5 M) was centrifuged with Amicon® with 14k rpm for 12 min and diluted with distilled water to give anti-IGSF4 antibody in water (2 × 10−5 M) prior to use. The labeling was performed with the antibody in water. The DOTA-attached anti-IGSF4 antibody 9a in water (2 × 10−5 M for each) were stocked for radiolabeling.
One-pot three-component click reaction for attaching NOTA to anti-IGSF4 antibody (9b)
According to the same protocol of one-pot three-component double click labeling of anti-IGSF4 antibody with DOTA (preparation of 9a), the labeling of anti-IGSF4 antibody with NOTA was carried out. The labeling was performed with the antibody in water. The NOTA-attached anti-IGSF4 antibody 9b in water (4 × 10−6 M for each) were stocked for radiolabeling.
[67Cu] production
The radioisotope of [67Cu] was produced in the [70Zn(d,αn)][67Cu] reaction. A schematic of the [67Cu] production chamber is shown in Supporting Information. The 24-MeV deuteron beam was extracted from the RIKEN AVF cyclotron, and the beam intensity was 4.0 μA. Zinc-70-enriched oxide powder ([70Zn]O) was pressed for 3 min at 2.0 × 103 kg cm−2 to form a disk of 10-mm diameter and 3.4 × 102 mg cm−2 thickness. The isotopic composition of the [70Zn]O target was 96.87% [70Zn], 1.55% [68Zn], 0.09% [67Zn], 0.55% [66Zn], and 0.94% [64Zn]. The [70Zn]O disk target was placed on a tantalum beam stopper and covered with a 10-μm aluminum foil (see figures in Supporting Information). During the irradiation, the [70Zn]O target was cooled with circulating helium gas (30 L/min) and water (1.5 L/min) at the upstream (aluminum cover) and downstream (tantalum plate) of the beam, respectively. The beam axis was continuously rotated in 3-mm diameter at 2 Hz to avoid a local heating of the [70Zn]O target using a beam wobbling electromagnet on the beam line of the AVF cyclotron. After the irradiation for 10 h, [67Cu] was separated from the target material and by-product radioisotopes such as [67Ga], [69mZn] and [71Zn] through the two-step chromatographic separation with the Eichrom Cu resin and the Dowex 1X8 anion-exchange resin.[16] 4 MBq of [67Cu] was finally prepared in 300 μL of 0.1 M CH3COOH. The radionuclidic purity of the [67Cu] solution was evaluated to be >99.9% by γ-ray spectrometry with a germanium semiconductor detector (ORTEC GEM-25185-P). A typical γ-ray spectrum of the purified [67Cu] used for radiolabeling is provided in Supporting Information. The chemical purity of the solution was evaluated with an inductively coupled plasma mass spectrometer (Agilent Technologies 7700x). Among the elements having atomic number Z ≥ 20, Cu (2.1 ppm) and Br (1.0 ppm) were only detected with concentrations of >1 ppm. The specific radioactivity of [67Cu] was then 110 MBq μg−1.
Radiolabeling of DOTA-attached albumin (7b) with [67Cu]
To the stock solution of 7b (2 × 10−5 M, 20 μL) was added [67Cu] (11 MBq) in 0.1 M of aqueous sodium acetate (pH 5–6, 20 μL) and the mixture was heated at 40 °C for 1 h. The reaction mixture was transferred into Amicon® 10 K and 0.1 M of aqueous acetic acid (pH 5–6) was fully added. Centrifuge was performed with 14k rpm for 12 min. 0.1 M of aqueous acetic acid (pH 5–6, 450 μL) was added and centrifuge was done with 14k rpm for 12 min. This wash was repeated one more time. γ-Ray doses of the residue and filtrate were measured using germanium semiconductor detector.
ELISA to test affinity of DOTA/NOTA-linked anti-IGSF4 antibodies (9a/b)
The wells of ELISA plate (Nunc-Immuno Plate, Thermo Fisher Scientific, Waltham, MA, USA) were coated with 100 ng/mL IGSF4 protein in coating buffer (50 mM carbonate buffer, pH 9.6) and incubated overnight at 4 °C. After two washes with washing buffer (phosphate buffered saline (PBS) containing 0.05% (v/v) Tween-20), the wells were blocked with 1% BSA in PBS for 1 h at RT. Anti-IGSF4 antibodies conjugated with DOTA and NOTA were serially diluted with PBS, added to the wells, and incubated for 1 h at RT. After four washes with buffer, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Merck Millipore, Darmstadt, Germany) in PBS was added to the wells and incubated for 1 h at RT. After six washes with washing buffer, color development was performed by incubation with TMB solution (ScyTek, Logan, UT, USA) for 10 min at RT and was stopped by addition of TMB Stop Buffer (ScyTek). Finally, the absorbance of 450 nm was detected using a microplate reader (PerkinElmer, Waltham, MA, USA).
Electronic supplementary material
Acknowledgements
This work was supported by the JSPS KAKENHI Grant Numbers JP16H03287, JP16K13104, and JP15H05843 in Middle Molecular Strategy, and by RIKEN Incentive Research Projects 2016. This work was also performed with the support of the Russian Government Program for Competitive Growth, granted to Kazan Federal University. A part of this work was performed at the RI Beam Factory operated by the RIKEN Nishina Center and CNS, University of Tokyo. We are grateful to Dr. Y. Komori, Dr. K. Takahashi, and Dr. S. Shibata (RIKEN) for their helps to produce the 67Cu radiotracer, and to Prof. R. Hsung (University of Wisconsin-Madison) for carefully reading the manuscript and for giving valuable comments.
Author Contributions
K.T. directed the research. K.F. designed and synthesized the labeling probes. S.Y. and H.H. prepared the [67Cu] and [65Zn] isotopes. K.F., S.Y., H.H., O.K., and K.T. conducted the radiolabeling experiment. T.I., Y.K., and Y.M. prepared IGSF-4 antibody and carried out ELISA of DOTA/NOTA-labeled antibodies. K.F. and K.T. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02123-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ishikawa H, et al. Carbon-ion radiation therapy for prostate cancer. Int J Urol. 2012;19:296–305. doi: 10.1111/j.1442-2042.2012.02961.x. [DOI] [PubMed] [Google Scholar]
- 2.Hainfeld JF, Slatkin D, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004;49(18):309–315. doi: 10.1088/0031-9155/49/18/N03. [DOI] [PubMed] [Google Scholar]
- 3.Klaus M-H, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2007;81:53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 4.Juzenas P, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008;60:1600–1614. doi: 10.1016/j.addr.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baziotis N, et al. Strontium-89 chloride in the treatment of bone metastases from breast cancer. Oncology. 1998;55:377–381. doi: 10.1159/000011881. [DOI] [PubMed] [Google Scholar]
- 6.Rosario PW, Mourao GF, Calsolari MR. Can the follow-up of patients with papillary thyroid carcinoma of low and intermediate risk and excellent response to initial therapy be simplified using second-generation thyroglobulin assays? Clin. Endocrinol. 2016;85(4):596–601. doi: 10.1111/cen.13053. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, et al. Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FRGD2. J. Nucl. Med. 2006;47:113–121. [PMC free article] [PubMed] [Google Scholar]
- 8.Cai W, Zhang X, Wu Y, Chen X. A Thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide, and synthesis of RGD peptide-based tracer for PET imaging of αvβ3 integrin expression. J. Nucl. Med. 2006;47:1172–1180. [PMC free article] [PubMed] [Google Scholar]
- 9.Marik J, Sutcliffe JL. Click for PET: rapid preparation of [18F]fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006;47:6681–6684. doi: 10.1016/j.tetlet.2006.06.176. [DOI] [Google Scholar]
- 10.Hausner SH, et al. In vivo positron emission tomography (PET) imaging with an αvβ6 specific peptide radiolabeled using 18F-“click” chemistry: evaluation and comparison with the corresponding 4-[18F]fluorobenzoyl- and 2-[18F]fluoropropionyl-peptides. J. Med. Chem. 2008;51:5901–5904. doi: 10.1021/jm800608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell-Verduyn LS, et al. Strain-promoted copper-free “click” chemistry for 18F radiolabeling of bombesin. Angew. Chem. Int. Ed. 2011;50:11117–11120. doi: 10.1002/anie.201105547. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, et al. Tetrazine-trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem. Commun. 2010;46:8043–8045. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeglis BM, et al. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels-Alder click chemistry. Bioconjugate Chem. 2011;22:2048–2059. doi: 10.1021/bc200288d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.New K, Brechbiel MW. Growing applications of “click chemistry” for bioconjugation in contemporary biomedical research. Cancer Biother. Radiopharm. 2009;24(3):299–302. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Struthers H, Spingler B, Mindt TL, Schibli R. “Click-to-chelate”: design and incorporation of triazole-containing metal-chelating systems into biomolecules of diagnostic and therapeutic interest. Chem. Eur. J. 2008;14(20):6173–6183. doi: 10.1002/chem.200702024. [DOI] [PubMed] [Google Scholar]
- 16.Cook BE, et al. Pretargeted PET imaging using a site-specifically labeled immunoconjugate. Bioconjugate Chem. 2016;27(8):1789–1795. doi: 10.1021/acs.bioconjchem.6b00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka K, et al. A submicrogram-scale protocol for biomolecule-based PET imaging by rapid 6π-azaelectrocyclization: visualization of sialic acid dependent circulatory residence of glycoproteins. Angew. Chem. Int. Ed. 2008;47:102–105. doi: 10.1002/anie.200702989. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, et al. Noninvasive imaging of dendrimer-type N-glycan clusters: in vivo dynamics dependence on oligosaccharide structure. Angew. Chem. Int. Ed. 2010;49:8195–8200. doi: 10.1002/anie.201000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukase K, Tanaka K. Bio-imaging and cancer targeting with glycoproteins and N-glycans. Curr. Opin. Chem. Biol. 2012;16:614–621. doi: 10.1016/j.cbpa.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, et al. Significant acceleration of 6π-azaelectrocyclization resulting from a remarkable substituent effect and formal synthesis of the ocular age pigment A2-E by a new method for substituted pyridine synthesis. J. Org. Chem. 2001;66:3099–3110. doi: 10.1021/jo005779+. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Katsumura S. Highly stereoselective asymmetric 6π-azaelectrocyclization utilizing the novel 7-alkyl substituted cis-1-amino-2-indanols: Formal synthesis of 20-epiuleine. J. Am. Chem. Soc. 2002;124:9660–9661. doi: 10.1021/ja026464+. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Kobayashi K, Mori H, Katsumura S. Development of highly stereoselective asymmetric 6π-azaelectrocyclization of conformationally flexible linear 1-azatrienes from determination of multifunctional chiral amines, 7-alkyl cis-1-amino-2-indanols, to application as a new synthetic strategy: formal synthesis of 20-epiuleine. J. Org. Chem. 2004;69:5906–5925. doi: 10.1021/jo049381f. [DOI] [PubMed] [Google Scholar]
- 23.Fujiki, K. & Tanaka, K. e-EROS Encyclopedia of Reagents for Organic Synthesis (Wiley) in press (2017).
- 24.Tung CL, Wong CTT, Fung EYM, Li X. Traceless and Chemoselective Amine Bioconjugation via Phthalimidine Formation in Native Protein Modification. Org. Lett. 2016;18:2600–2603. doi: 10.1021/acs.orglett.6b00983. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald JI, Munch HK, Moore T, Francis MB. One-step site-specific modification of native proteins with 2-pyridinecarboxyaldehydes. Nat. Chem. Biol. 2015;11:326–331. doi: 10.1038/nchembio.1792. [DOI] [PubMed] [Google Scholar]
- 26.Yano Y, et al. Selective amine labeling of cell surface proteins guided by coiled-coil assembly. Biopolymers. 2016;106:484–490. doi: 10.1002/bip.22715. [DOI] [PubMed] [Google Scholar]
- 27.Larda ST, Pichugin D, Prosser RS. Site-Specific Labeling of Protein Lysine Residues and N-Terminal Amino Groups with Indoles and Indole-Derivatives. Bioconjugate Chem. 2015;26:2376–2383. doi: 10.1021/acs.bioconjchem.5b00457. [DOI] [PubMed] [Google Scholar]
- 28.Asano S, Patterson JT, Gaj T, Barbas CF., III Site-selective labeling of a lysine residue in human serum albumin. Angew. Chem. Int. Ed. 2014;53:11783–11786. doi: 10.1002/anie.201405924. [DOI] [PubMed] [Google Scholar]
- 29.Ning X, Guo J, Wolfert MA, Boons G-J. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast Huisgen cycloadditions. Angew. Chem. Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogura A, et al. Visualizing trimming dependence of biodistribution and kinetics with homo- and heterogeneous N-glycoclusters on fluorescent albumin. Sci. Rep. 2016;6:21797. doi: 10.1038/srep21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogura A, et al. Glycan multivalency effects toward albumin enable N-glycan-dependent tumor targeting. Bioorg. Med. Chem. Lett. 2016;26:2251–2254. doi: 10.1016/j.bmcl.2016.03.046. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Fukase K. PET (positron emission tomography) imaging of biomolecules using metal-DOTA complexes: a new collaborative challenge by chemists, biologists, and physicians for future diagnostics and exploration of in vivo dynamics. Org. Biomol. Chem. 2008;6:815–828. doi: 10.1039/b718157b. [DOI] [PubMed] [Google Scholar]
- 33.Latypova L, et al. Sequential double “clicks” toward structurally well-defined heterogeneous N-Glycoclusters: the importance of cluster heterogeneity on pattern recognition in vivo. Adv. Sci. 2016;4:1600394. doi: 10.1002/advs.201600394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka K, et al. A cascading reaction sequence involving ligand-directed azaelectrocyclization and autooxidation-induced fluorescence recovery enables visualization of target proteins on the surfaces of live cells. Org. Biomol. Chem. 2014;12:1412–1418. doi: 10.1039/c3ob42267d. [DOI] [PubMed] [Google Scholar]
- 35.Ogura A, Tanaka K. Azaelectrocyclization on cell surface: convenient and general approach to chemical biology research. Tetrahedron. 2015;71:4518–4521. doi: 10.1016/j.tet.2015.02.063. [DOI] [Google Scholar]
- 36.Blackman ML, Royzen M, Fox JM. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novak-Hofer I, Schubiger AP. Copper-67 as a therapeutic nuclide for radioimmunotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2002;29(6):821–830. doi: 10.1007/s00259-001-0724-y. [DOI] [PubMed] [Google Scholar]
- 38.Yano, S. et al. Production of 67Cu using the 70Zn(d,αn)67Cu reaction, RIKEN Accel. Prog. Rep. 49, in press.
- 39.Chen X, et al. MicroPET and autoradiographic imaging of breast cancer αv-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjugate Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 40.Salgueiro MJ, et al. Bioavailability, biodistribution, and toxicity of BioZn-AAS: a new zinc source. comparative studies in rats. Nutrition. 2000;16(9):762–766. doi: 10.1016/S0899-9007(00)00379-8. [DOI] [PubMed] [Google Scholar]
- 41.Takeda A, Tamano H, Enomoto S, Oku N. Zinc-65 imaging of rat brain tumors. Cancer Res. 2001;61:5065–5069. [PubMed] [Google Scholar]
- 42.Velikyan I, Maecke H, Langstrom B. Convenient preparation of 68Ga-based PET-radiopharmaceuticals at room temperature. Bioconjugate Chem. 2008;19:569–573. doi: 10.1021/bc700341x. [DOI] [PubMed] [Google Scholar]
- 43.Gai Y, et al. New bifunctional chelator p-SCN-PhPr-NE3TA for copper-64: synthesis, peptidomimetic conjugation, radiolabeling, and evaluation for PET imaging. Inorg. Chem. 2016;55:6892–6901. doi: 10.1021/acs.inorgchem.6b00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


