Abstract
Background: Literature has documented the role of family in the outcome of chronic schizophrenia. In the light of this, family interventions (FIs) are becoming an integral component of treatment for psychosis. The First Episode of Psychosis (FEP) is the period when most of the changes in family atmosphere are observed; unfortunately, few studies on the relatives are available.
Objective: To explore burden of care and emotional distress at baseline and at 9-month follow-up and the levels of service satisfaction at follow-up in the two groups of relatives (experimental treatment EXP vs. treatment as usual TAU) recruited in the cluster-randomized controlled GET UP PIANO trial.
Methods: The experimental treatment was provided by routine public Community Mental Health Centers (Italian National Health Service) and consisted of Treatment as Usual plus evidence-based additional treatment (Cognitive Behavioral Therapy for psychosis for patients, Family Intervention for psychosis, and Case Management). TAU consisted of personalized outpatient psychopharmacological treatment, combined with non-specific supportive clinical management and informal support/educational sessions for families. The outcomes on relatives were assessed by the Involvement Evaluation Questionnaire (IEQ-EU), the General Health Questionnaire (GHQ-12), and the Verona Service Satisfaction Scale (VSSS-EU). Differences within and between groups were evaluated.
Results: At baseline, 75 TAU and 185 EXP caregivers were assessed. In the experimental group 92% of relatives participated in at least 1 family session. At follow-up both groups experienced improvement in all IEQ and GHQ items, but caregivers belonging to the EXP arm experienced a significantly greater change in 10 IEQ items (mainly pertaining to the “Tension” dimension) and in GHQ items. Due to the low sample size, a significant effectiveness was only observed for 2 IEQ items and 1 GHQ-12 item. With respect to VSSS data at follow-up, caregivers in the EXP arm experienced significantly greater satisfaction in 8 items, almost all pertaining to the dimensions “Relatives' Involvement” and “Professionals' Skills and Behavior.”
Conclusions: The Family intervention for psychosis delivered in the GET UP PIANO trial reduced family burden of illness and improved emotional distress and satisfaction with services. These results should encourage to promote FIs on caregivers of first-episode psychosis patients.
Keywords: early psychosis, relatives, outcomes, pragmatic trial, community psychiatry
Introduction
International literature has explored the role of family in the outcome of chronic schizophrenia, but only few studies are available on the relatives of people experiencing their first episode of psychosis (FEP). This is a period when most of the changes in family dynamics are observed (Addington et al., 2003; Koutra et al., 2014; Yesufu-Udechuku et al., 2015).
Schizophrenia impacts not only on the patients but also to those who care for them. The term caregiving burden since the 80s has been used to explore these consequences (Hatfield and Lefley, 1987) and contributed to highlight. Family members of persons with a severe mental disorder experience negative effects linked to these burdensome situations (Budd et al., 1998; Magliano et al., 2005).
With the shift from hospital-based to community-based care which occurred in most western countries, the relatives of mentally ill people have become an integral part of the care system. Thus, taking into account the carers' perspectives on mental health provision and on overall families' needs and support has become of crucial importance.
Research has shown that about one third of the relatives of schizophrenic patients suffer from emotional or behavioral distress and that patterns of familiar interpersonal interaction can worsen the course of schizophrenia (Magliano et al., 2000; Moller-Leimkuhler, 2006). Several studies have explored carers' needs and perceptions, coping styles, mental health and satisfaction. Follow-up studies on the burden on relatives of persons affected by psychosis treated in community-based routine mental health services are uncommon. Even less studies on the burden that develops in the early stages of psychosis, and on the impact of the interventions provided (Brown and Birtwistle, 1998; Magliano et al., 2000; Moller-Leimkuhler, 2006; Breitborde et al., 2011) have been conducted. These follow-up studies have not provided clear information on the patterns in family burden and thus, there is lack of consensus on burden in the medium long term and also on which specific factors could impact on it, and at which stages.
The GET UP (Genetics, Endophenotypes, Treatment: Understanding early Psychosis) PIANO (Psychosis: early Intervention and Assessment of Needs and Outcome) Trial (Ruggeri et al., 2012, 2015) was conducted in a large epidemiologically based cohort from Italian community mental health centers (CMHCs). It aimed to assess the feasibility and effectiveness of a multi-element psychosocial intervention for FEP patients and their families, compared with treatment as usual (TAU). Experimental treatment was administered as an adjunct to TAU, and included: (1) cognitive behavioral therapy for psychosis (CBTp) to patients; (2) psychosis-focused family intervention (FIp) to families; and (3) case management (CM) to both patients and relatives. The intervention was provided by CMHC staff, trained in the previous 6 months and supervised by experts.
It was hypothesized that in the patients add-on multi-component intervention could favor: (1) greater improvements in symptoms, as measured by the PANSS; and (2) reduction in days of hospital admission over the 9-month follow-up. Secondary hypotheses were that the intervention could improve subjective burden of psychotic symptoms (auditory hallucinations and delusions), social functioning and emotional well-being and produce lower service disengagement rates (Ruggeri et al., 2015).
The hypotheses regarding the relatives were that the multi-element experimental intervention at the 9 month follow-up could: (1) reduce burden of the key relative, as measured using the IEQ; and (2) improve service satisfaction, as measured using the VSSS Relatives scale (Ruggeri and Dall'Agnola, 1993), at follow-up.
In the present paper we aim to provide comprehensive descriptives on the effect on relatives of the 9 month multi-element psychosocial intervention for FEP patients and their families in routine MH services versus treatment as usual (TAU). Specifically we address: (a) changes in the relatives' burden of care and emotional distress and (b) differences in the levels of service satisfaction reached at the end of the follow-up. Testing these hypotheses seems to be of particular relevance in a cultural context, such as Italy, where the majority of the patients continue to live with their families even for long time after the mental illness onset, with potential severe consequences on the everyday life of those caring families.
Methods
Study design
The assignment units (clusters) in the GET UP cluster were the CMHCs and the units of observation and analysis were patients and their families (Ruggeri et al., 2012, 2015). Participation was offered to all CMHCs serving two northern Italian regions (Veneto and Emilia-Romagna) and the urban areas of Florence, Milan, and Bolzano, covering an area of 9,951,306 inhabitants. Of 126 CMHCs, 117 (92.8%, covering 9,304,093 inhabitants) participated.
Service organization context and participating sites
MH care in Italy is organized in catchment areas, where one or more CMHCs provide outpatient care, daycare, and rehabilitation to nearly 100,000 inhabitants. It is delivered by the National Health Service through the Departments of Mental Health (DMH).
Randomization
Stratified randomization of CMHCs took into account catchment area size, and its geographical characteristics (urban/mixed versus rural context) and was performed to balance differences in their characteristics. Also organizational constraints (such as affiliation to the same DMH) were taken into account. One CMHC each in the intervention and TAU arms withdrew consent to participate and were excluded.
Participants
All CMHCs were asked to refer potential psychosis cases at first contact during the index period to the study team. Based on the WHO 10-country study (Jablensky et al., 1992), the inclusion criteria to ascertain FEP patients were:
age 18–54 years
residence in catchment areas of CMHCs
presence of at least 1 of the following: hallucinations, delusions, qualitative speech disorder, qualitative psychomotor disorder, bizarre, or grossly inappropriate behavior, or 2 of the following: loss of interest, initiative, and drive; social withdrawal; episodic severe excitement; purposeless destructiveness; overwhelming fear; or marked self-neglect, per the WHO Screening Schedule for Psychosis (World Health Organization, 1992)
first lifetime contact with CMHCs, prompted by these symptoms
Exclusion criteria were: (a) anti-psychotic medication (>3 months) prescribed for an identical or similar mental disorder; (b) mental disorders due to general medical condition; (c) moderate-severe mental retardation per a clinical functional assessment; and (d) psychiatric diagnosis other than ICD-10 for psychosis.
All eligible patients, identified as those who reached the clinical stabilization, were invited to provide written informed consent to be assessed and informed of the nature, scope, and possible consequences of the trial and that they could withdraw consent at any time. Patients were asked to give consent for family member assessments; family members who agreed to participate provided written informed consent.
The trial received approval by the ethics committees of the coordinating center (Azienda Ospedaliera Universitaria Integrata di Verona) and each participating unit and was registered with ClinicalTrials.gov (NCT01436331).
Diagnostic ascertainment of patients
Since FEP is generally a phase of high diagnostic instability, the specific ICD-10 codes for psychosis (F1x.4; F1x.5; F1x.7; F20–29; F30.2, F31.2, F31.5, F31.6, F32.3, F33.3) were assigned at 9 months. The best-estimate ICD-10 diagnosis was made by consensus of a panel of clinicians by considering all available information on the time interval from patient's intake needed to apply the Item Group Checklist (IGC) of the Schedule for Clinical Assessment in Neuropsychiatry (SCAN, World Health Organization, 1992).
Treatments
Experimental treatment (TAU+CBT+FI+CM)
The experimental treatment package was provided by routine public Community Mental Health Centers (CMHCs) which operate within the Italian National Health Service and consisted of standard care (treatment as usual, TAU) plus evidence-based additional treatment. Specifically, the multi-element psychosocial intervention, adjunctive to TAU, comprised: (i) Cognitive Behavioral Treatment for psychosis (CBTp) to patients; (ii) psychosis-focused Family Intervention (FIp) to individual families; and (iii) Case Management (CM) to both parties. FIp was based on the model proposed by Leff et al. (1985) and further developed by Kuipers et al. (2002). It included an optimal number of 10–15 sessions over 9 months, with each individual family: 6 sessions in the first 3 months, and at least 1 session/month in the 6 months afterwards. Every patient/family had a dedicated CM, who coordinated all planned interventions (Burns and Firn, 2002).
Treatment as usual (TAU)
Treatment as usual (TAU) was also provided by routine public Community Mental Health Centers (CMHCs) involved in the Trial. In Italy standard care for FEP patients typically consisted of personalized outpatient psychopharmacological treatment, combined with non-specific supportive clinical management and non-specific informal support/educational sessions for families.
Outcome measures
The outcomes on relatives were assessed by the Involvement Evaluation Questionnaire, European Version (IEQ-EU), the 12-item General Health Questionnaire (GHQ-12), and the Verona Service Satisfaction Scale, European Version (VSSS-EU).
Specifically, the IEQ-EU is an 81-item self-rated questionnaire completed by the caregiver (van Wijngaarden et al., 2000). The questionnaire consists of seven distinct sections which assess several burden related aspects in the foregoing 4 weeks. All items are scored on a 3 or 5 point Likert scale, and can be summarized into four distinct scales: (I) “tension” (9 items)—strained interpersonal atmosphere between patient and caregiver, such as quarrels; (II) “supervision” (5 items)—caregiver's tasks of guarding the patient, for instance to prevent suicide or to supervise the intake of medicine; (III) “worrying” (6 items)—caregiver's concern about the patient's safety, future, and health; (IV) “urging” (8 items), the need to stimulate the patient to undertake activities. The other six sections of the IEQ include items which assess background information on patient, caregiver and mutual relationship. These sections are: (a) general information on patient, caregiver and household composition, time spent together (15 items); (b) costs (8 items); (c) the General Health Questionnaire to assess caregiver's distress, 12 items version (GHQ-12) (Goldberg and Williams, 1988); (d) caregiver's use of professional help (3 items); (e) consequences for patient's children (11 items); and (f) an open question for comments and additions.
Satisfaction with mental health services was assessed using the Verona Service Satisfaction Scale (VSSSR; Ruggeri and Dall'Agnola, 1993; Ruggeri et al., 1994, 1996, 2007) which is designed for self-administration and can be completed without prior training in 20–30 min. The VSSS has been implemented both in the version for Patients and for Relatives (Ruggeri et al., 2007). The VSSS Relatives consists of 54 items, which conceptually cover seven dimensions: “Overall satisfaction,” “Professionals” skills, and “behavior,” “Information,” “Access,” “Efficacy,” “Types of intervention,” and “Relative's involvement.” Relatives were asked to give overall rating about their experience of the mental health services they have attended. Satisfaction ratings are on a 5-point Likert scale (1 = terrible, 2 = mostly unsatisfactory, 3 = mixed, 4 = mostly satisfactory, 5 = excellent). The items are presented with alternate directionality to reduce stereotypic responses.
Statistical analyses
Descriptives were calculated [mean (sd) for continuous and n (%) for categorical variables, respectively]. Comparisons between characteristics and outcome of relatives assigned to the two groups (TAU and EXP) were performed by using Fisher's exact tests for categorical items and independent samples t-tests for continuous scores. Changes between baseline and follow-up within each group were evaluated by using repeated measures t-tests and the difference between groups was explored by independent t-tests. All tests were bilateral at p < 0.05. No correction for multiple comparisons was performed due to the explorative nature of the study. All analyses were executed by SPSS for Windows (version 22).
Results
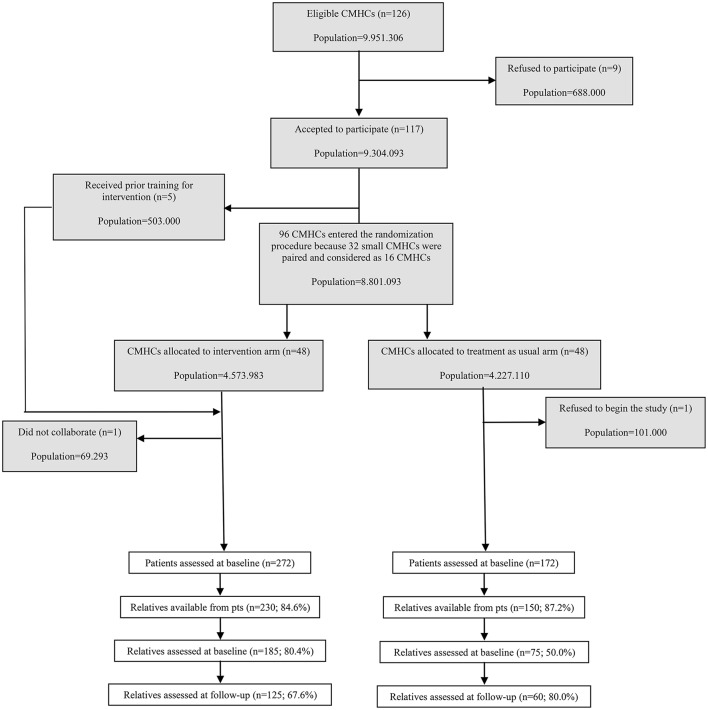
In the experimental arm out of the 272 patients enrolled, 16 did not have a relative; in 6 cases patients did not give the permission to contact the relative; in 7 cases the relatives did not want to undergo into Family Intervention and in 13 cases the patient refused to enter in the CBT intervention, and thus the relative was also excluded. In the TAU arm, out of the 172 patients enrolled, 10 did not have a relative; in 12 cases patients did not give the permission to contact the relative. Thus, it has been possible to ask the permission to be assessed to 230 relatives in the experimental arm and to 150 relatives in the TAU arm. Of these, 185 subjects in the experimental arm (out of 230 available; 80%) and 75 in the TAU arm (out of 150 available; 50%) accepted to complete the trial's baseline assessments (Figure 1).
Figure 1.
Trial profile for relatives.
Relatives in the two groups did not differ in any socio-demographic characteristics. No other between-group differences were observed at baseline in any variable concerning cohabitation with the patient (see Table 1). It is noteworthy that the majority of the relatives were parents of the affected patient, and that the largest majority lived with the patient and were used to stay with the patient all days and for several hours.
Table 1.
Socio-demographics of relatives assessed at baseline and cohabitation with patient variables.
| Baseline | |||
|---|---|---|---|
| Treatment as usual group (n = 75) | Experimental treatment group (n = 185) | Test and significance of difference | |
| Gender, n (%) | |||
| Male | 28 (37.3%) | 69 (37.3%) | χ2 = 0.00, df = 1, p = 0.980 |
| Female | 47 (62.7%) | 115 (62.7%) | |
| Age, mean (sd) | 50.7 (10.5) | 49.6 (11.2) | t = 0.73, df = 258, p = 0.466 |
| Education, n (%) | (1 missing) | ||
| Low level | 40 (54.1%) | 81 (43.8%) | χ2 = 2.24, df = 1, p = 0.135 |
| High level | 34 (45.9%) | 104 (56.2%) | |
| Marital status, n (%) | |||
| Unmarried | 7 (9.3%) | 12 (6.5%) | χ2 = 2.65, df = 2, p = 0.265 |
| Married | 53 (70.7%) | 148 (80.0%) | |
| Widowed, separated, divorced | 15 (20.0%) | 25 (13.5%) | |
| Living status, n (%) | (2 missing) | (5 missing) | |
| Alone | 1 (1.4%) | 4 (2.2%) | χ2 = 1.04, df = 4, p = 0.904 |
| With partner and/or children With parents and/or siblings With other relatives | 63 (86.3%)3 (4.1%)3 (4.1%) | 157 (87.2%)6 (3.3%)4 (2.2%) | |
| Other | 3 (4.1%) | 9 (5.0%) | |
| Relationship with patient, n (%) | (2 missing) | ||
| Mother/Father | 47 (62.7%) | 121 (66.1%) | χ2 = 1.52, df = 4, p = 0.821 |
| Daughter/Son | 3 (4.0%) | 6 (3.3%) | |
| Sister/Brother | 9 (12.0%) | 14 (7.6%) | |
| Wife/Husband Friend/Other | 15 (20.0%)1 (1.3%) | 38 (20.8%)4 (2.2%) | |
| Patient's gender, n (%) | (1 missing | ||
| Male | 47 (62.7%) | 113 (61.4%) | χ2 = 0.04, df = 1, p = 0.851 |
| Female | 28 (37.3%) | 71 (38.6%) | |
| Patient's age, mean (sd) | 30.2 (8.9) | (2 missing) 28.2 (9.9) | t = 1.52, df = 256, p = 0.131 |
| Years from the beginning of mental disorder, n (%) ≤ 1 1–5 5–10 >10 | (4 missing) 35 (49.3%) 26 (36.6%)3 (4.2%)7 (9.9%) | (12 missing) 97 (56.1%)60 (34.7%)8 (4.6%)8 (4.6%) | χ2 = 2.74, df = 3, p = 0.433 |
| Is patient receiving help for mental disorder now? n (%) | |||
| Yes | 73 (94.7%) | 183 (98.9%) | χ2 = 0.89, df = 1, p = 0.347 |
| No | 2 (5.3%) | 2 (1.1%) | |
| Relative lives with patient, n (%) | (1 missing) | ||
| No | 9 (12.0%%) | 14 (7.6%) | χ2 = 1.24, df = 1, p = 0.266 |
| Yes | 66 (88.0%) | 169 (92.4%) | |
| Days per week in the last 4 weeks, n (%) | (2 missing) | ||
| Never | 9 (12.0%) | 12 (6.6%) | χ2 = 4.16, df = 2, p = 0.125 |
| Some days | 3 (4.0%) | 18 (9.8%) | |
| All days | 63 (84.0%) | 153 (83.6%) | |
| Hours per week in the last 4 weeks, n (%) | (5 missing) | ||
| 0–4 5–8 9–16 17–32 >32 | 2 (2.7%)7 (9.3%)7 (9.3%)8 (10.7%)51 (68.0%) | 7 (3.9%)10 (5.6%)21 (11.7%)16 (8.8%)126 (70.0%) | χ2 = 1.83, df = 4, p = 0.766 |
Engagement of the relatives in the Family intervention was good: as shown in Table 2, in the experimental group 170 out of 185 relatives assessed at BL (91.9%) participated in at least 1 family session. Most of them (n = 154; 90.6%) received ≥5 FI sessions; of these 112 (72.7%) received ≥10 sessions.
Table 2.
Intervention provision (Family intervention and non-specific interventions) in the relatives assessed at BL.
| Period between BL and FU | ||
|---|---|---|
| Treatment as usual group (n = 75) | Experimental treatment group (n = 185) | |
|
Family intervention sessions, n
(%) 0 1–4 5–9 10–19 20+ |
————– | 15 (8.1%) 16 (8.6%) 42 (22.7%) 94 (50.8%) 18 (9.8%) |
| Families receiving non-specific interventions, n (%) | (5 missing) 21 (30.0%) | (12 missing) 15 (8.7%) |
| Types of non-specific interventions, n [mean (sd)] | (3 missing) | (3 missing) |
| Psychological support | 8 [4.2 (2.9)] | 11 [3.5 (2.4)] |
| Non-specific psychoeducation | 4 [9.7 (5.9)] | – |
| Systemic psychotherapy | 2 [6.0 (4.2)] | 1 [5.0 (.)] |
| Other | 4 [6.5 (6.4)] | – |
At follow-up 75 relatives could not be traced and thus could not be assessed: 60 subjects belonged to the experimental arm (32.4% of the cohort) and 15 to the TAU arm (20.0% of the cohort). There were no significant differences in demographics and outcome variables at baseline between completers and non-completers. Both groups had similar baseline levels of burden of care. At follow-up both groups experienced a general trend to improvement in most IEQ items.
However, as shown in Table 3, caregivers belonging to the experimental arm at the 9 month follow-up had significantly greater improvement in burden caused by a series of aspects of everyday life with the patient: “Encouraged to eat,” “Ensured medicine,” “Guarded dangerous acts,” “Guarded taking drugs,” “Disturbed sleep,” “Atmosphere strained?,” “Caused quarrel,” “Annoyed Behavior,” “Others annoyed,” and “Burden” (see Table 3). The majority of these aspects pertains to the “Tension” dimension. The effectiveness of the treatment reached significance only for “Disturbed sleep” and “Atmosphere strained?.”
Table 3.
Mean scores (sd) of burden of care (IEQ-EU) for relatives assessed at baseline and at 9-month follow-up (dimensions: S, Supervision; T, Tension; U, Urging; W, Worrying).
| IEQ-EU items | Treatment as usual group | Experimental treatment group | Δ (FU-BL) TAU vs. EXP | ||||
|---|---|---|---|---|---|---|---|
| BL (n = 60) | FU (n = 60) | p-value paired t-test | BL (n = 125) | FU (n = 125) | p-value paired t-test | p-value t-test | |
| Encouraged proper care (U) | 2.33 (1.44) | 1.82 (1.18) | 0.012** | 2.09 (1.23) | 1.82 (1.12) | 0.029* | 0.306 |
| Helped proper care (U) | 1.67 (1.22) | 1.41 (0.88) | 0.150 | 1.45 (1.01) | 1.32 (0.84) | 0.204 | 0.542 |
| Encouraged to eat (U) | 1.52 (0.97) | 1.30 (0.46) | 0.102 | 1.63 (1.07) | 1.33 (0.78) | 0.006** | 0.696 |
| Encouraged activity (U) | 2.82 (1.32) | 2.35 (1.25) | 0.002** | 2.53 (1.14) | 2.24 (1.18) | 0.021* | 0.380 |
| Accompanied activity (U) | 1.76 (1.20) | 1.32 (0.84) | 0.014** | 1.74 (1.11) | 1.27 (0.65) | 0.000** | 0.856 |
| Ensured medicine (U) | 2.78 (1.74) | 2.46 (1.76) | 0.182 | 2.90 (1.79) | 2.05 (1.64) | 0.000** | 0.121 |
| Guarded dangerous acts (S) | 1.51 (1.17) | 1.27 (0.90) | 0.219 | 1.56 (1.15) | 1.24 (0.82) | 0.002** | 0.646 |
| Guarded self-harm (S) | 1.61 (1.18) | 1.29 (0.92) | 0.038* | 1.66 (1.20) | 1.17 (0.71) | 0.000** | 0.370 |
| Ensured sleep (S) | 2.12 (1.38) | 1.73 (1.27) | 0.007** | 2.35 (1.46) | 1.64 (1.00) | 0.000** | 0.182 |
| Guarded alcohol (S) | 1.78 (1.35) | 1.43 (1.10) | 0.048* | 1.60 (1.25) | 1.48 (1.03) | 0.304 | 0.272 |
| Guarded taking drugs (S) | 1.49 (1.22) | 1.29 (0.90) | 0.222 | 1.58 (1.29) | 1.36 (0.98) | 0.034* | 0.890 |
| Carried out tasks (U) | 1.78 (1.08) | 1.49 (0.89) | 0.068 | 1.77 (1.09) | 1.53 (0.96) | 0.067 | 0.829 |
| Get up in the morning (U) | 2.08 (1.26) | 2.08 (1.31) | 1.000 | 2.04 (1.26) | 2.22 (1.40) | 0.181 | 0.483 |
| Disturbed sleep (T, S) | 1.25 (0.56) | 1.33 (0.95) | 0.591 | 1.48 (0.96) | 1.20 (0.59) | 0.004** | 0.041a |
| Atmosphere strained (T) | 1.75 (0.84) | 1.76 (0.91) | 0.868 | 1.90 (0.99) | 1.59 (0.69) | 0.001** | 0.045a |
| Caused quarrel (T) | 1.43 (0.79) | 1.51 (0.94) | 0.498 | 1.54 (0.89) | 1.36 (0.66) | 0.032* | 0.084 |
| Behavior annoyed (T) | 1.80 (0.88) | 1.74 (0.94) | 0.679 | 1.74 (0.96) | 1.55 (0.77) | 0.041* | 0.446 |
| Others annoyed (T) | 1.20 (0.45) | 1.16 (0.42) | 0.598 | 1.33 (0.71) | 1.12 (0.47) | 0.000** | 0.078 |
| You felt threatened (T) | 1.08 (0.28) | 1.02 (0.14) | 0.083 | 1.13 (0.46) | 1.05 (0.26) | 0.083 | 0.833 |
| Moving out behavior (T) | 1.16 (0.51) | 1.16 (0.47) | 1.000 | 1.26 (0.76) | 1.21 (0.76) | 0.433 | 0.636 |
| Pursue own activities | 2.92 (1.44) | 3.06 (1.53) | 0.566 | 3.05 (1.46) | 3.32 (1.53) | 0.169 | 0.714 |
| Worried about safety (W) | 2.32 (1.50) | 1.70 (0.97) | 0.001** | 2.59 (1.45) | 1.85 (1.31) | 0.000** | 0.643 |
| Worried treatment received (W) | 2.56 (1.40) | 2.04 (1.29) | 0.016* | 2.58 (1.34) | 1.87 (1.19) | 0.000** | 0.483 |
| Worried general health (W) | 3.37 (1.36) | 2.57 (1.39) | 0.000** | 3.50 (1.25) | 2.52 (1.44) | 0.000** | 0.491 |
| Worried manage financially (W) | 2.80 (1.55) | 2.22 (1.28) | 0.010** | 2.25 (1.44) | 2.05 (1.30) | 0.142 | 0.124 |
| Worried relative/friend's future (W) | 3.76 (1.21) | 3.02 (1.35) | 0.000** | 3.31 (1.34) | 2.64 (1.35) | 0.000** | 0.760 |
| Worried own future (T) | 2.82 (1.60) | 2.59 (1.43) | 0.274 | 2.51 (1.39) | 2.24 (1.30) | 0.068 | 0.898 |
| Burden (T, W) | 1.75 (0.91) | 1.58 (0.99) | 0.197 | 2.02 (1.06) | 1.64 (1.02) | 0.000** | 0.236 |
p < 0.05,
p < 0.01.
Cohen's d = 0.34.
Emotional distress at baseline, as measured by GHQ, was similar in both groups. As shown in Table 4, at follow-up both groups experienced a general trend for improvement in emotional distress. However, caregivers belonging to the experimental arm had a significantly greater improvement in the majority of GHQ items, with the only exception of “Feeling reasonably happy” (Table 4) where improvement was equally significant in the two groups. The effectiveness of the treatment reached significance only for “Under strain.”
Table 4.
Mean scores (sd) of emotional distress (GHQ-12) for relatives assessed at baseline and at 9-month follow-up.
| GHQ-12 items | Treatment as usual group | Experimental treatment group | Δ (FU-BL) TAU vs. EXP | ||||
|---|---|---|---|---|---|---|---|
| BL (n = 60) | FU (n = 60) | p-value paired t-test | BL (n = 125) | FU (n = 125) | p-value paired t-test | p-value t-test | |
| Concentrate on what doing | 2.26 (0.72) | 2.18 (0.52) | 0.420 | 2.33 (0.76) | 2.10 (0.48) | 0.004** | 0.264 |
| Lost much sleep | 2.41 (1.02) | 2.12 (0.97) | 0.050 | 2.54 (1.02) | 1.90 (0.87) | 0.000** | 0.056 |
| Useful part in things | 1.57 (0.57) | 1.88 (0.65) | 0.004** | 1.86 (0.77) | 1.95 (0.55) | 0.304 | 0.129 |
| Making decisions | 1.84 (0.54) | 1.94 (0.54) | 0.280 | 1.96 (0.62) | 1.99 (0.43) | 0.608 | 0.602 |
| Under strain | 2.61 (1.00) | 2.41 (1.00) | 0.176 | 2.88 (0.96) | 2.19 (0.87) | 0.000** | 0.007**a |
| Overcome difficulties | 2.20 (1.06) | 1.94 (0.99) | 0.108 | 2.27 (1.02) | 1.84 (0.80) | 0.000** | 0.359 |
| Enjoy normal activities | 2.29 (0.78) | 2.14 (0.53) | 0.146 | 2.42 (0.71) | 2.09 (0.41) | 0.000** | 0.180 |
| Face up to problems | 2.14 (0.57) | 2.12 (0.47) | 0.811 | 2.17 (0.64) | 1.99 (0.36) | 0.013** | 0.183 |
| Unhappy or depressed | 2.33 (1.07) | 2.14 (1.02) | 0.215 | 2.43 (1.06) | 1.91 (0.97) | 0.000** | 0.063 |
| Losing confidence | 1.65 (0.87) | 1.55 (0.81) | 0.389 | 1.58 (0.88) | 1.45 (0.80) | 0.198 | 0.848 |
| Worthless person | 1.33 (0.65) | 1.43 (0.78) | 0.471 | 1.27 (0.63) | 1.25 (0.63) | 0.807 | 0.409 |
| Reasonable happy | 2.59 (0.83) | 2.22 (0.67) | 0.001** | 2.63 (0.81) | 2.12 (0.61) | 0.000** | 0.336 |
*p < 0.05,
p < 0.01.
Cohen's d = 0.46.
Caregivers in the experimental arm experienced significantly greater levels of service satisfaction after the 9 month treatment in “Ability of psychiatrists to listen and understand patients,” “Recommendations to the closest relative,” “Relatives' knowledge to problems,” “How information is given on diagnosis and prognosis,” “Ability of psychiatrists to listen and understand relatives,” “Thoroughness of nurses,” “Helping relatives,” and “Continuity of care” (see Table 5). All these aspects pertain to two dimensions (“Relatives' Involvement” and “Professionals' Skills and Behavior”), with the only exception of “How information is given on diagnosis and prognosis.”
Table 5.
Mean scores (sd) and n (%) of service satisfaction (VSSS-EU) for relatives assessed at 9-month follow-up (dimensions: A, Access; E, Efficacy; I, Information; OS, Overall Satisfaction; PSB, Professionals' Skills and Behavior; RI, Relatives' Involvement; TI, Types of Intervention).
| VSSS-EU items | Treatment as usual group | Experimental treatment group | |
|---|---|---|---|
| FU (n = 60) | FU (n = 125) | p-value t-test or Fisher's exact test | |
| Effectiveness of service in helping (E) | 4.15 (0.74) | 4.17 (0.66) | 0.867 |
| Behavior of secretary staff (PSB) | 4.33 (0.82) | 4.34 (0.70) | 0.925 |
| Professional competence of psychiatrists/psychologists (PSB) | 4.33 (0.71) | 4.36 (0.65) | 0.822 |
| Comfort facilities (A) | 3.97 (0.72) | 4.01 (0.78) | 0.725 |
| Ability of psychiatrists/psychologists to listen and understand patient (PSB) | 4.20 (0.64) | 4.41 (0.70) | 0.049* |
| Manners of psychiatrists/psychologists (PSB) | 4.37 (0.84) | 4.46 (0.63) | 0.441 |
| Punctuality of professionals (PSB) | 4.15 (0.82) | 4.14 (0.82) | 0.928 |
| Cost of service (A) | 4.46 (0.68) | 4.45 (0.70) | 0.972 |
| Service effective in preventing relapse (E) | 4.20 (0.71) | 4.14 (0.79) | 0.622 |
| Respect for rights (PSB) | 4.47 (0.75) | 4.41 (0.68) | 0.583 |
| Help received (OS) | 4.19 (0.63) | 4.21 (0.72) | 0.878 |
| Explanation procedures (I) | 3.92 (0.95) | 4.10 (0.75) | 0.163 |
| Service effective in relieving symptoms (E) | 4.11 (0.75) | 4.11 (0.73) | 0.985 |
| Response of service to urgent needs (TI) | 4.04 (0.77) | 4.11 (0.84) | 0.627 |
| Arrangements for after hour emergencies (TI) | 3.85 (0.82) | 3.93 (0.94) | 0.673 |
| Thoroughness of psychiatrists/psychologists (PSB) | 4.09 (0.81) | 4.20 (0.74) | 0.381 |
| Referring to GP or other professionals (PSB) | 4.08 (0.89) | 4.04 (0.77) | 0.775 |
| Cooperation between service providers (PSB) | 4.20 (0.80) | 4.20 (0.76) | 0.965 |
| Publicity or information on services (I) | 3.70 (1.05) | 3.85 (0.80) | 0.293 |
| Kinds of service offered (OS) | 4.02 (0.78) | 4.11 (0.74) | 0.434 |
| Service received (OS) | 4.23 (0.73) | 4.24 (0.76) | 0.949 |
| Professional competence of nurses (PSB) | 4.18 (0.74) | 4.32 (0.64) | 0.210 |
| Recommendations to closest relative (RI) | 3.88 (1.08) | 4.23 (0.76) | 0.013** |
| Effectiveness of service for your knowledge of problems (E) | 3.93 (0.90) | 4.06 (0.83) | 0.352 |
| Manners of nurses (PSB) | 4.25 (0.84) | 4.37 (0.69) | 0.342 |
| Effectiveness of service for improving relationship with relative (E) | 3.88 (0.99) | 4.04 (0.80) | 0.271 |
| Effectiveness of service for relatives' knowledge of problems (RI) | 3.82 (1.05) | 4.14 (0.74) | 0.023* |
| Nurses knowledge about you (PSB) | 3.84 (0.84) | 3.89 (0.78) | 0.721 |
| How information is given on diagnosis and prognosis (I) | 3.67 (1.02) | 3.95 (0.76) | 0.048* |
| Ability of psychiatrists/psychologists to listen and understand relative (RI) | 3.86 (1.02) | 4.25 (0.94) | 0.012** |
| Effectiveness of service for relationships out the family (E) | 3.80 (0.96) | 3.99 (0.83) | 0.171 |
| How information is given to relative (RI) | 3.64 (1.04) | 3.82 (0.89) | 0.237 |
| Instructions on what to do between appointments (PSB) | 3.93 (0.98) | 4.00 (0.70) | 0.589 |
| Effectiveness of service for your self-care (E) | 3.80 (1.03) | 3.84 (0.86) | 0.808 |
| Thoroughness of nurses (PSB) | 3.96 (0.91) | 4.25 (0.60) | 0.017* |
| Effectiveness of service for helping relative (RI) | 3.73 (1.15) | 4.05 (0.87) | 0.043* |
| Ability of nurses and social workers to listen and understand patient (PSB) | 3.94 (0.93) | 4.19 (0.72) | 0.063 |
| Effectiveness of service for your work (E) | 3.71 (1.03) | 3.86 (0.83) | 0.309 |
| Help on side effects from medications (TI) | 3.81 (0.91) | 3.84 (0.88) | 0.878 |
| Continuity of care (PSB) | 4.05 (0.89) | 4.38 (0.65) | 0.007** |
p < 0.05,
p < 0.01.
Discussion
Relatives of persons affected by psychosis play a crucial role in their pathways to care, being the first to request for help in the majority of the cases (Del Vecchio et al., 2015; Jansen et al., 2015). Several studies have also shown that relatives often feel despair, fears, concerns regarding professional support (Tennakoon et al., 2000; Faridi et al., 2009; Boydell et al., 2014; Lavis et al., 2015; Hickman et al., 2016; Koutra et al., 2014). This paper contributes to shed some light on the potentiality that interventions specific for the FEP have to reduce burden of care in an Italian context that is characterized for the majority of psychotic patients by a close link with they families, who continue to take care of them also for long time after the onset.
Data provided by the GET UP Trial allow to draw interesting conclusions on the role of relatives in the care of their loved ones and on the potentiality that specific forms of individual Family interventions can have already in the early phases of psychosis. The multi-element experimental intervention tested in the GET UP Trial, consisting of CBTp for the patients, Family intervention and Case management is effective in decreasing relatives' burden in several specific aspects of burden mainly related to the “Tension” dimension of the IEQ. The study has also shown that emotional distress is present in the relatives already in the first months after psychosis onset, and proved that the experimental multi-element intervention tested is effective in decreasing that emotional distress. Due to the multi-element treatment design of the study, it is not possible to disentangle the effectiveness that might be attributed to the family intervention. However, in the GET UP Trial, acceptability and engagement in family intervention was very good included a large number of families: in the experimental group 92% of relatives participated in at least 1 family session with the majority that could attend up to 10 sessions (defined as optimal standard for the 9 month treatment).
Caregivers in the experimental arm showed significantly greater satisfaction in 8 items, all pertaining to the dimensions “Relatives' Involvement” and “Professionals' Skills and Behavior,” with the only exception of “How information is given on diagnosis and prognosis,” related to the domain “Information given by the professionals.” This finding is of interest also in the light of literature findings showing that relatives often feel that their role and contribution is undervalued by the clinicians (Iyer et al., 2011; McCann et al., 2012).
Overall, the Family Intervention for psychosis delivered in the GET UP PIANO trial reduced family burden of illness and improved emotional distress and satisfaction with services. It should however be said that this improvement is uneven and that not all aspects of everyday interaction with the patient and with the service have been more positively affected in the experimental group.
Results presented in this paper have some methodological limitations. The relatively high number of relatives that did not give consent to the baseline assessment and the relatively high proportion of relatives that were non completers at follow-up is a limitation of these data even if the lack of significant differences in the sociodemographic characteristics between the two groups hampers this limitation. Moreover, the majority of the differences between changes within groups did not result significant due to the low sample size. Finally, financial constraints have compelled the GET UP trial researchers to plan a follow-up period of only 9 months: Thus, the short duration of the intervention did not allow to fully exploit the effects of treatment in improving burden and emotional distress in the relatives.
Among the strengths of this paper we first enlist the fact that 90% of CMHCs asked to participate accepted and completed the study, thus favoring high representativeness of the services and subjects included in the study. A further strength is that relatives belonging to experimental and TAU arm had similar baseline socio-demographic characteristics. Moreover, as most study's relatives live with the patients and stay with them for several hours/day proves that the trial results provide a realistic picture of problems and potentiality to benefit of treatments regarding those relatives which have the most relevant need of help.
In conclusion, data provided in this paper prove the higher effectiveness over TAU of a specific evidence-based multi-element treatment including family intervention in improving family burden and emotional distress. These data further strengthen previous literature on this issue (Jeppesen et al., 2005; Bird et al., 2010; Gleeson et al., 2010; Onwumere et al., 2011; Lee et al., 2014; Nordentoft et al., 2015) and encourage to promote specific forms of family interventions on caregivers of first-episode psychosis patients, and implement further studies that target their needs in the different stages of psychosis.
Ethics statement
This study is conducted according to globally accepted standards of good clinical practice, in agreement with the Declaration of Helsinki and in keeping with local regulations.
GET UP PIANO investigators ensure that all professionals involved in the trial are adequately qualified and informed about the protocol, the study interventions, and their trial-related duties and functions. The coordinating center maintains a list of all appropriately qualified persons involved in the study.
Ethics committee approval
Formal ethical approval for conducting the trial has been sought and obtained by the Coordinating Center's Ethics Committee (Comitato Etico per la Sperimentazione, Azienda Ospedaliera di Verona, http://www.ospedaliverona.it/Istituzionale/Comitati-Etici/Sperimentazione), which has approved the study protocol, the information sheets (patient and family versions), and the informed consent sheets (patient and familiar versions) on May 6th 2009 (Prot. N. 20406/CE, Date 14/05/2009) and by the Ethics Committee of each participating unit.
Informed consent form and information sheet
Eligible participants are asked to participate only after receiving a detailed explanation of the nature, scope, and possible consequences of the trial. Participants receive an informed consent document including both information about the study and the consent form to sign. This document contains all the elements required by the Guidelines of Good Clinical Practice and any additional elements required by local regulations. The document is in a language understandable to the participants and specified who informs the participant. The person informing the participant is a psychiatrist or a psychologist. After reading the informed consent document, the patient, or his/her legal representative, gives consent in writing. The patient's consent is confirmed at the time of the consent by the personally dated signature of the participant and by the personally dated signature of the person conducting the informed consent discussion.
According to the Guideline of Good Clinical Practice, participants enrolled in the trial with the consent of the participants' legally acceptable representative are informed about the trial to the extent compatible with the participants' understanding and, if capable, the participant is asked to sign and personally date the written informed consent.
Participating patients are asked to give consent for the involvement of their family members in the study and those providing consent receive an informed consent document that includes both information about the study and the consent form that is given to family members. The staff member or the researcher informing family members is a psychiatrist or a psychologist. After reading the informed consent document, the participant gives consent in writing.
Author contributions
MR conceived the project, coordinated all its phases and wrote the paper. AL collaborated in conceiving the project, coordinated the data collection, and had an important role in writing the paper. PS, MM, SS, STor, AF coordinated the data collection and revised the final draft. CB planned and realized the methodological protocol of the project, coordinated the realization of the on-line system for data collection, planned, and executed statistical analyses and contributed in writing the manuscript. DC realized the on-line system for data collection, merged data from all centers, checked data quality and prepared the dataset for statistical analyses. FP, EL, STos, KDS, CCo, STom, CCr, GP collaborated in data collection and revised the final draft.
Funding
Ministry of Health, Italy—Ricerca Sanitaria Finalizzata, Code H61J08000200001.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the patients and their family members who participated in this study.
References
- Addington J., Coldham E. L., Jones B., Ko T., Addington D. (2003). The first episode of psychosis: the experience of relatives. Acta Psychiatr. Scand. 108, 285–289. 10.1034/j.1600-0447.2003.00153.x [DOI] [PubMed] [Google Scholar]
- Bird V., Premkumar P., Kendall T., Whittington C., Mitchell J., Kuipers E. (2010). Early intervention services, cognitive-behavioural therapy and family intervention in early psychosis: systematic review. Br. J. Psychiatry 197, 350–356. 10.1192/bjp.bp.109.074526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boydell J., Onwumere J., Dutta R., Bhavsar V., Hill N., Morgan C., et al. (2014). Caregiving in first-episode psychosis: social characteristics associated with perceived “burden” and associations with compulsory treatment. Early Interv. Psychiatry 8, 122–129. 10.1111/eip.12041 [DOI] [PubMed] [Google Scholar]
- Breitborde N. J., Moreno F. A., Mai-Dixon N., Peterson R., Durst L., Bernstein B., et al. (2011). Multifamily group psychoeducation and cognitive remediation for first-episode psychosis: a randomized controlled trial. BMC Psychiatry 11:9. 10.1186/1471-244X-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Birtwistle J. (1998). People with schizophrenia and their families. Fifteen-year outcome. Br. J. Psychiatry 173, 139–144. 10.1192/bjp.173.2.139 [DOI] [PubMed] [Google Scholar]
- Budd R. J., Oles G., Hughes I. C. T. (1998). The relationship between coping style and burden in the carers of relatives with schizophrenia. Acta Psychiatr. Scand. 98, 304–309. 10.1111/j.1600-0447.1998.tb10088.x [DOI] [PubMed] [Google Scholar]
- Burns T., Firn M. (2002). Assertive Outreach in Mental Health. A Manual for Practitioners. Oxford: Oxford University Press. [Google Scholar]
- Del Vecchio V., Luciano M., Sampogna G., De Rosa C., Giacco D., Tarricone I., et al. (2015). The role of relatives in pathways to care of patients with a first episode of psychosis. Int. J. Soc. Psychiatry 61, 631–637. 10.1177/0020764014568129 [DOI] [PubMed] [Google Scholar]
- Faridi K., Pawliuk N., King S., Joober R., Malla A. K. (2009). Prevalence of psychotic and non-psychotic disorders in relatives of patients with a first episode psychosis. Schizophr. Res. 114, 57–63. 10.1016/j.schres.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Gleeson J. F., Cotton S. M., Alvarez-Jimenez M., Wade D., Crisp K., Newman B., et al. (2010). Family outcomes from a randomized control trial of relapse prevention therapy in first-episode psychosis. J. Clin. Psychiatry 71, 475–483. 10.4088/JCP.08m04672yel [DOI] [PubMed] [Google Scholar]
- Goldberg D., Williams P. A. (1988). Users Guide to General Health Questionnaire. Windsor: NEER-Nelson. [Google Scholar]
- Hatfield A. B., Lefley H. P. (1987). Families of the Mentally Ill: Coping and Adaptation. New York, NY: Guilford Press. [Google Scholar]
- Hickman G., Newton E., Fenton K., Thompson J., Boden Z. V., Larkin M. (2016). The experiential impact of hospitalisation: parents' accounts of caring for young people with early psychosis. Clin. Child Psychol. Psychiatry 21, 145–155. 10.1177/1359104515581716 [DOI] [PubMed] [Google Scholar]
- Iyer S. N., Loohuis H., Pawliuk N., Joober R., Malla A. K. (2011). Concerns reported by family members of individuals with first-episode psychosis. Early Interv. Psychiatry 5, 163–167. 10.1111/j.1751-7893.2011.00265.x [DOI] [PubMed] [Google Scholar]
- Jablensky A., Sartorius N., Ernberg G., Anker M., Korten A., Cooper J. E., et al. (1992). Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol. Med. 20, 1–97. 10.1192/bjp.146.6.594 [DOI] [PubMed] [Google Scholar]
- Jansen J. E., Wøldike P. M., Haahr U. H., Simonsen E. (2015). Service user perspectives on the experience of illness and pathway to care in first-episode psychosis: a qualitative study within the TOP project. Psychiatr. Q. 86, 83–94. 10.1007/s11126-014-9332-4 [DOI] [PubMed] [Google Scholar]
- Jeppesen P., Petersen L., Thorup A., Abel M. B., Oehlenschlaeger J., Christensen T. Ø., et al. (2005). Integrated treatment of first-episode psychosis: effect of treatment on family burden: OPUS trial. Br. J. Psychiatry Suppl. 48, 85–90. 10.1192/bjp.187.48.s85 [DOI] [PubMed] [Google Scholar]
- Koutra K., Vgontzas A. N., Lionis C., Triliva S. (2014). Family functioning in first-episode psychosis: a systematic review of the literature. Soc. Psychiatry Psychiatr. Epidemiol. 49, 1023–1036. 10.1007/s00127-013-0816-6 [DOI] [PubMed] [Google Scholar]
- Kuipers E., Leff J., Lam D. (2002). Family Work for Schizophrenia: A Practical Guide. London: Gaskell. [Google Scholar]
- Lavis A., Lester H., Everard L., Freemantle N., Amos T., Fowler D., et al. (2015). Layers of listening: qualitative analysis of the impact of early intervention services for first-episode psychosis on carers' experiences. Br. J. Psychiatry 207, 135–142. 10.1192/bjp.bp.114.146415 [DOI] [PubMed] [Google Scholar]
- Lee G., Barrowclough C., Lobban F. (2014). Positive affect in the family environment protects against relapse in first-episode psychosis. Soc. Psychiatry Psychiatr. Epidemiol. 49, 367–376. 10.1007/s00127-013-0768-x [DOI] [PubMed] [Google Scholar]
- Leff J., Kuipers L., Berkowitz R., Sturgeon D. (1985). A controlled trial of social intervention in the families of schizophrenic patients: two year follow-up. Br. J. Psychiatry 146, 594–600. 10.1192/bjp.146.6.594 [DOI] [PubMed] [Google Scholar]
- Magliano L., Fadden G., Economou M., Held T., Xavier M., Guarneri M., et al. (2000). Family burden and coping strategies in schizophrenia: 1-year follow-up data from the BIOMED I study. Soc Psychiatry Psychiatr Epidemiol. 35, 109–115. 10.1007/s001270050192 [DOI] [PubMed] [Google Scholar]
- Magliano L., Fiorillo A., De Rosa C., Malangone C., Maj M. (2005). The national mental health project working group. Family burden in long-term diseases: a comparative study in schizophrenia vs. physical disorders. Soc. Sci. Med. 61, 313–322. 10.1016/j.socscimed.2004.11.064 [DOI] [PubMed] [Google Scholar]
- McCann T. V., Lubman D. I., Clark E. (2012). Primary caregivers' satisfaction with clinicians' response to them as informal carers of young people with first-episode psychosis: a qualitative study. J. Clin. Nurs. 21, 224–231. 10.1111/j.1365-2702.2011.03836.x [DOI] [PubMed] [Google Scholar]
- Moller-Leimkuhler A. M. (2006). Multivariate prediction of relatives' stress outcome one year after first hospitalization of schizophrenic and depressed patients. Eur. Arch. Psychiatry Clin. Neurosci. 256, 122–130. 10.1007/s00406-005-0619-1 [DOI] [PubMed] [Google Scholar]
- Nordentoft M., Melau M., Iversen T., Petersen L., Jeppesen P., Thorup A., et al. (2015). From research to practice: how OPUS treatment was accepted and implemented throughout Denmark. Early Interv. Psychiatry 9, 156–162. 10.1111/eip.12108 [DOI] [PubMed] [Google Scholar]
- Onwumere J., Bebbington P., Kuipers E. (2011). Family interventions in early psychosis: specificity and effectiveness. Epidemiol. Psychiatr. Sci. 20, 113–119. 10.1017/S2045796011000187 [DOI] [PubMed] [Google Scholar]
- Ruggeri M., Bonetto C., Lasalvia A., De Girolamo G., Fioritti A., Rucci P., et al. (2012). A multi-element psychosocial intervention for early psychosis (GET UP PIANO TRIAL) conducted in a catchment area of 10 million inhabitants: study protocol for a pragmatic cluster randomized controlled trial. Trials 13, 73. 10.1186/1745-6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri M., Bonetto C., Lasalvia A., Fioritti A., De Girolamo G., Santonastaso P., et al. (2015). Feasibility and effectiveness of a multi-element psychosocial intervention for first-episode psychosis: results from the cluster-randomized controlled GET UP PIANO trial in a catchment area of 10 million inhabitants. Schizophr. Bull. 41, 1192–1203. 10.1093/schbul/sbv058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri M., Dall'Agnola R. (1993). The development and use of the Verona Expectations for Care Scale (VECS) and the Verona Service Satisfaction Scale (VSSS) for measuring expectations and satisfaction with community-based psychiatric services in patients, relatives and professionals. Psychol. Med. 23, 511–523. 10.1017/S0033291700028609 [DOI] [PubMed] [Google Scholar]
- Ruggeri M., Dall'Agnola R., Agostini C., Bisoffi G. (1994). Acceptability, sensitivity and content validity of VECS and VSSS in measuring expectations and satisfaction in psychiatric patients and their relatives. Soc. Psychiatry Psychiatr. Epidemiol. 29, 265–276. 10.1007/BF00802049 [DOI] [PubMed] [Google Scholar]
- Ruggeri M., Dall'Agnola R., Greenfield T., Bisoffi G. (1996). Factor analysis of the Verona Service Satisfaction Scale-82 and development of reduced versions. Int. J. Methods Psychiatr. Res. 6, 23–38. [Google Scholar]
- Ruggeri M., Lasalvia A., Salvi G., Cristofalo D., Bonetto C., Tansella M. (2007). Applications and usefulness of routine measurement of patients' satisfaction with community-based mental health care. Acta Psychiatr. Scand. Suppl. 437, 53–65. 10.1111/j.1600-0447.2007.01093.x [DOI] [PubMed] [Google Scholar]
- Tennakoon L., Fannon D., Doku V., O'Ceallaigh S., Soni W., Santamaria M., et al. (2000). Experience of caregiving: relatives of people experiencing a first episode of psychosis. Br. J. Psychiatry 177, 529–533. 10.1192/bjp.177.6.529 [DOI] [PubMed] [Google Scholar]
- van Wijngaarden B., Schene A., Koeter M., Vázquez-Barquero J. L., Knudsen H. C., Lasalvia A., et al. (2000). Caregiving in Schizophrenia: development, internal consistency and reliability of the Involvement Evaluation Questionnaire-European Version. EPSILON Study 4. European psychiatric services: inputs linked to outcome domains and needs. Br. J. Psychiatry 177(Suppl. 39), 21–27. 10.1192/bjp.177.39.s21 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1992). Schedules for Clinical Assessment in Neuropsychiatry. Geneva: World Health Organization. [Google Scholar]
- Yesufu-Udechuku A., Harrison B., Mayo-Wilson E., Young N., Woodhams P., Shiers D., et al. (2015). Interventions to improve the experience of caring for people with severe mental illness: systematic review and meta-analysis. Br. J. Psychiatry 206, 268–274. 10.1192/bjp.bp.114.147561 [DOI] [PubMed] [Google Scholar]