Abstract
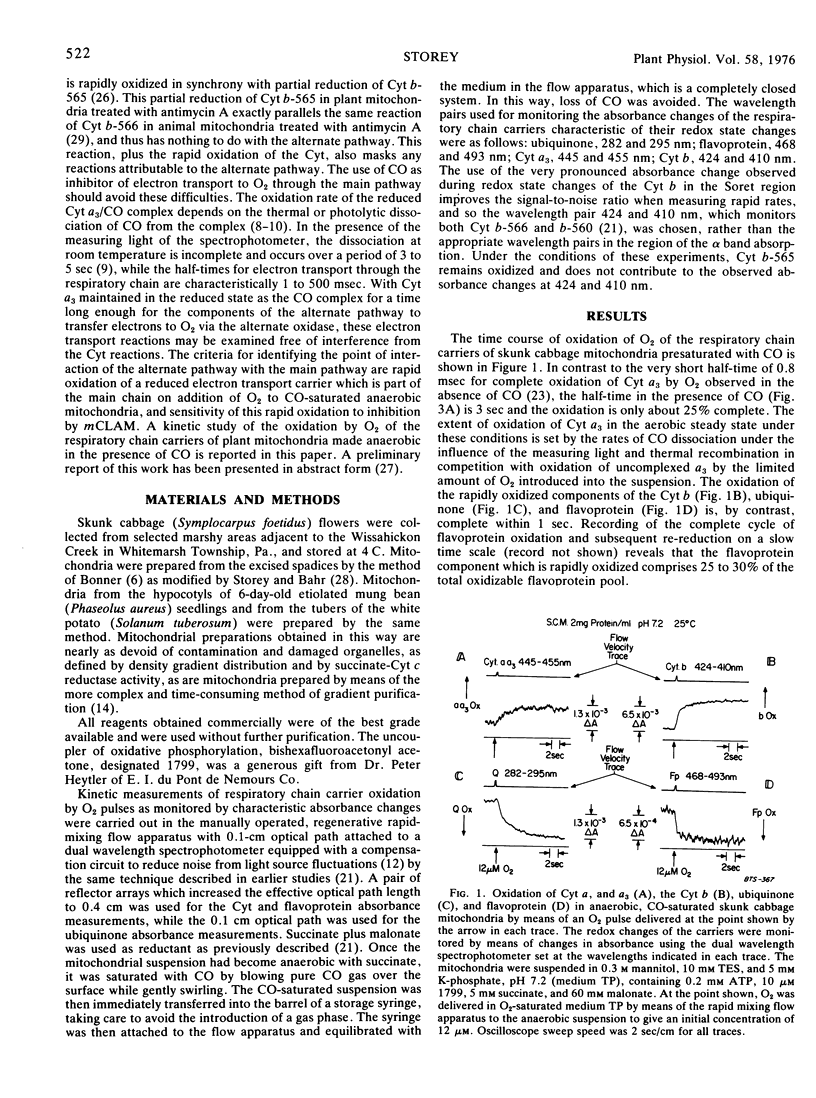
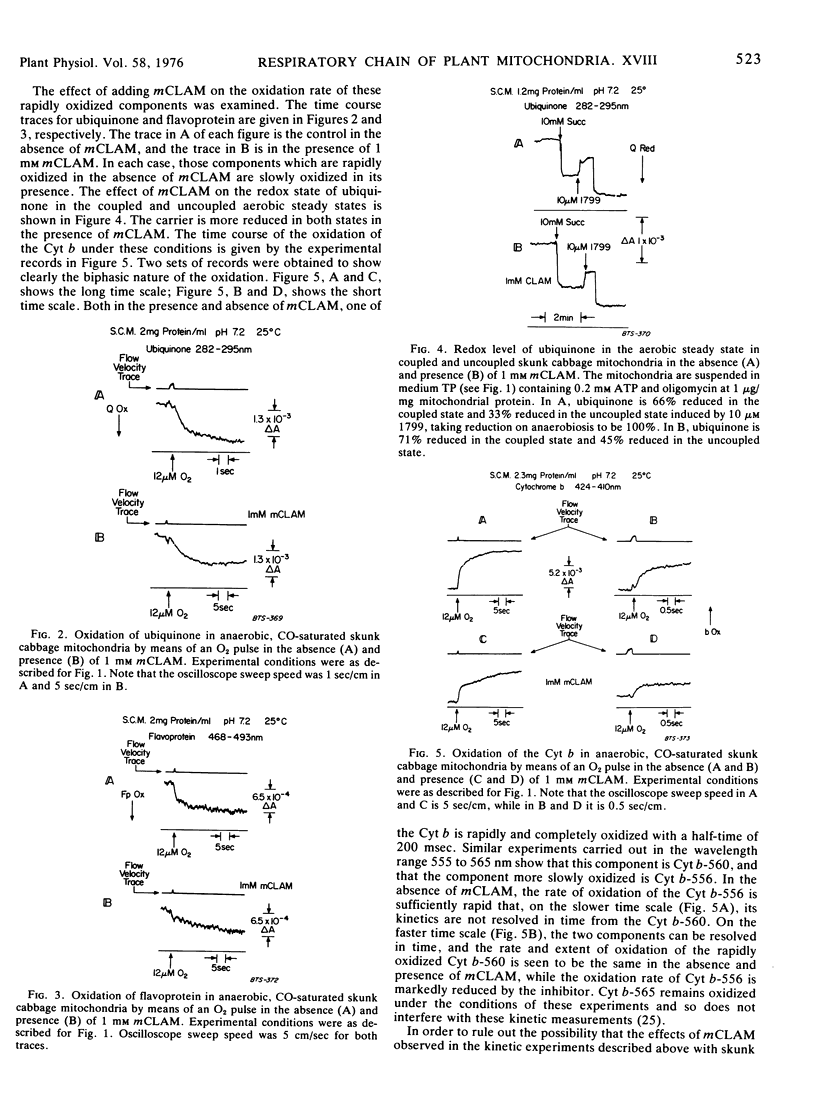
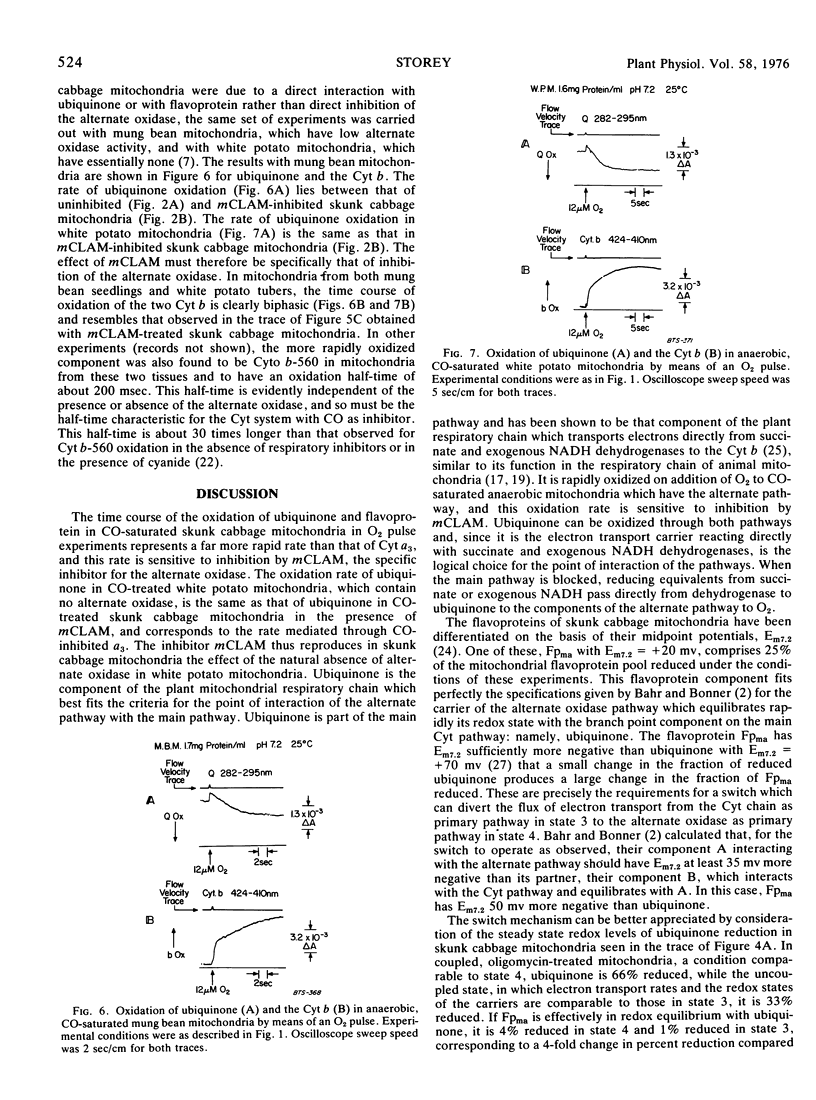
Oxidation of the respiratory chain carriers of anaerobic, CO-saturated skunk cabbage (Symplocarpus foetidus) mitochondria, by means of an O2 pulse, proceeds primarily through the cyanide-insensitive alternate oxidase, since the oxidation of cytochromes a and a3 takes place with a half-time of 3 seconds, corresponding to the rate of dissociation of CO from reduced cytochrome a3. Ubiquinone and part of the flavoprotein are oxidized within 1 second under these conditions, and this rapid rate of oxidation is strongly inhibited by m-chlorobenzhydroxamic acid (mCLAM), a specific inhibitor of the alternate oxidase of plant mitochondria. The rate of ubiquinone oxidation under these conditions in white potato (Solanum tuberosum) mitochondria, which have no alternate oxidase, is the same as that in skunk cabbage mitochondria treated with mCLAM. Ubiquinone, thus identified as the carrier common to both the cytochrome and alternate oxidase pathways, is linked to the alternate oxidase by a flavoprotein of midpoint potential 50 millivolts more negative with which it is in equilibrium. This arrangement provides a switch for diverting electron transport primarily through the cytochrome pathway under state 3 conditions and primarily through the alternate oxidase pathway under state 4 conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem. 1973 May 25;248(10):3441–3445. [PubMed] [Google Scholar]
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- Bendall D. S., Bonner W. D. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971 Feb;47(2):236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. The carbon monoxide compounds of the cytochrome oxidases. I. Difference spectra. J Biol Chem. 1953 May;202(1):383–396. [PubMed] [Google Scholar]
- CHANCE B. The carbon monoxide compounds of the cytochrome oxidases. II. Photodissociation spectra. J Biol Chem. 1953 May;202(1):397–406. [PubMed] [Google Scholar]
- Chance B., Erecińska M. Flow flash kinetics of the cytochrome a 3 -oxygen reaction in coupled and uncoupled mitochondria using the liquid dye laser. Arch Biochem Biophys. 1971 Apr;143(2):675–687. doi: 10.1016/0003-9861(71)90249-9. [DOI] [PubMed] [Google Scholar]
- Chance B., Hackett D. P. The Electron Transfer System of Skunk Cabbage Mitochondria. Plant Physiol. 1959 Jan;34(1):33–49. doi: 10.1104/pp.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Erecinska M., Storey B. T. The Respiratory Chain of Plant Mitochondria: VII. Kinetics of Flavoprotein Oxidation in Skunk Cabbage Mitochondria. Plant Physiol. 1970 Oct;46(4):618–624. doi: 10.1104/pp.46.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E., Norling B., Persson B., Ernster L. Studies with ubiquinone-depleted submitochondrial particles. Effects of extraction and reincorporation of ubiquinone on the kinetics of succinate dehydrogenase. Eur J Biochem. 1970 Nov;16(3):508–513. doi: 10.1111/j.1432-1033.1970.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria. III. Oxidation Rates of the Cytochromes c and b in Mung Bean Mitochondria Reduced With Succinate. Plant Physiol. 1969 Mar;44(3):413–421. doi: 10.1104/pp.44.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The Respiratory Chain of Plant Mitochondria: XVII. Flavoprotein-Cytochrome b(562) Interaction in Antimycin-treated Skunk Cabbage Mitochondria. Plant Physiol. 1974 Jun;53(6):846–850. doi: 10.1104/pp.53.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. IV. Oxidation rates of the respiratory carriers of mung bean mitochondria in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):447–454. doi: 10.1104/pp.45.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. V. Reaction of reduced cytochromes a and a3 in mung bean mitochondria with oxygen in the presence of cyanide. Plant Physiol. 1970 Apr;45(4):455–460. doi: 10.1104/pp.45.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria: x. Oxidation-reduction potentials of the flavoproteins of skunk cabbage mitochondria. Plant Physiol. 1971 Oct;48(4):493–497. doi: 10.1104/pp.48.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]


