Abstract
The genus Monascus was described by van Tieghem (1884) to accommodate M. ruber and M. mucoroides, two species with non-ostiolate ascomata. Species delimitation in the genus is still mainly based on phenotypic characters, and taxonomic studies that include sequence data are limited. The genus is of economic importance. Species are used in fermented Asian foods as food colourants (e.g. ‘red rice’ (ang-kak, angka)) and found as spoilage organisms, and recently Monascus was found to be essential in the lifecycle of stingless bees. In this study, a polyphasic approach was applied combining morphological characters, ITS, LSU, β-tubulin, calmodulin and RNA polymerase II second largest subunit sequences and extrolite data, to delimit species and to study phylogenetic relationships in Monascus. Furthermore, 30 Monascus isolates from honey, pollen and nests of stingless bees in Brazil were included. Based on this polyphasic approach, the genus Monascus is resolved in nine species, including three new species associated with stingless bees (M. flavipigmentosus sp. nov., M. mellicola sp. nov., M. recifensis sp. nov., M. argentinensis, M. floridanus, M. lunisporas, M. pallens, M. purpureus, M. ruber), and split in two new sections (section Floridani sect. nov., section Rubri sect. nov.). Phylogenetic analysis showed that the xerophile Monascus eremophilus does not belong in Monascus and monophyly in Monascus is restored with the transfer of M. eremophilus to Penicillium (P. eremophilum comb. nov.). A list of accepted and excluded Monascus and Basipetospora species is given, together with information on (ex-)types cultures and barcode sequence data.
Key words: Aspergillaceae, Extrolites, Fungal ecology, Phylogeny, Taxonomy
Taxonomic novelties: New sections: Monascus section Floridani R.N. Barbosa & Houbraken, Monascus section Rubri R.N. Barbosa & Houbraken
New species: Monascus flavipigmentosus R.N. Barbosa, Souza-Motta, N.T. Oliveira & Houbraken; Monascus mellicola R.N. Barbosa, Souza-Motta, N.T. Oliveira & Houbraken; Monascus recifensis R.N. Barbosa, Souza-Motta, N.T. Oliveira & Houbraken
New combination: Penicillium eremophilum (A.D. Hocking & Pitt) Houbraken, Leong & Vinnere-Pettersson
Introduction
Van Tieghem (1884) introduced the genus Monascus for species that produce non-ostiolate ascomata and introduced two species, M. ruber and M. mucoroides. The position of Monascus (and the Monascaceae) has been the subject of discussion in various papers and it was often placed outside the order Eurotiales (Benny and Kimbrough, 1980, Arx, 1987, Stchigel and Guarro, 2007), but phylogenetic analyses confidentially places this genus in Aspergillaceae (Eurotiales) (Berbee et al., 1995, Ogawa et al., 1997, Ogawa and Sugiyama, 2000, Peterson, 2008, Houbraken and Samson, 2011, Vinnere-Pettersson et al., 2011). The genus Basipetospora was found to be the anamorph of Monascus and is characterized by the production of aleurioconidia in a basipetal manner from undifferentiated conidiogenous cells that progressively shorten (retrogression, Cole & Samson 1979). The conidia have a truncated base and resemble chlamydospores. These features set this genus apart from the phylogenetically related genera Aspergillus and Penicillium.
After the description of the genus, more than 20 species have been introduced and many of them are considered to be synonyms (Shao et al. 2011). Classification of Monascus has primary been based on macro- and microscopic features, such as the pigmentation of the cleistothecial walls and conidia and growth rates on agar media. Hawksworth & Pitt (1983) revised the genus based on physiological and morphological characteristics and reduced the number of accepted species to three: M. pilosus, M. ruber and M. purpureus. Since that study, ten new species were introduced: M. albidulus, M. argentinensis, M. aurantiacus, M. eremophilus, M. floridanus, M. fumeus, M. lunisporas, M. pallens, M. rutilus and M. sanguineus (Barnard and Cannon, 1987, Hocking and Pitt, 1988, Cannon et al., 1995, Udagawa and Baba, 1998, Stchigel et al., 2004, Li & Guo 2004). With the description of those species, the genus became morphologically and physiologically more diverse, suggesting a large genetic diversity. For example, Monascus ruber grows rapidly on agar media, M. lunisporas and M. pallens grow restrictedly and M. eremophilus is a strict xerophile and only grows on low water activity media. The phenotype-based identification schemes in Monascus were difficult to match with the results obtained by ITS, partial LSU and/or β-tubulin gene sequencing (Park and Jong, 2003, Park et al., 2004). Nowadays, species can be delimited on the genotype, for example based on the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) concept. The application of this concept in Monascus has yet not been performed and the results of such an analysis will give insight on the species boundaries.
The genus Monascus has economic importance in several areas, and several species have been widely used for over years in the production of yellow and red food colourants and Asian fermented foods, particularly red rice (ang-kak, angka, ‘red kojic rice’). Red rice is of particular interest because of its health promoting effects (Lee and Pan, 2011, Lee and Pan, 2012, Hsu and Pan, 2012, Shi and Pan, 2012) and indeed, production of compounds with antibacterial properties and cholesterol-lowering statins of the monacolin K-type (= mevinolin = lovastatin) are reported in the species M. pilosus, M. pubigerus, M. purpureus, M. ruber and M. vitreus (Negishi et al. 1986, Jůzlova et al. 1996, Vendruscolo et al. 2014). However, Monascus species such as M. anka, M. aurantiacus, M. kaoliang, M. pilosus, M. purpureus, M. ruber and M. sanguineus have been reported to produce the mycotoxin citrinin (Blanc et al., 1995, Dietrich et al., 1999, Wang et al., 2003, Wang et al., 2005, Pisareva et al., 2005, Shimizu et al., 2005, Huang et al., 2007, Pattangul et al., 2008, Kim et al., 2010, Li et al., 2012, Li et al., 2015), and the presence of this mycotoxin in food, including red rice, should be avoided. Among these reports on citrinin production by Monascus species, Wang et al. (2005) also reported on citrinin production by M. floridanus, M. lunisporas and M. pallens, but this has not been confirmed by any other authors working on citrinin and Monascus. Besides their beneficial properties for human, Monascus species can also cause spoilage, for example of silage, bakery (tortillas), pasteurized products (olives) and dried prunes (M. eremophilus). Species are also rarely associated with human infections, and an invasive gastric infection case was linked to the consumption of Monascus contaminated dried and salted fish (Moreau, 1971, Iriart et al., 2010, Samson et al., 2010).
Specific fungi and other micro-organisms live in close association with social and solitary bees. This association is mandatory, and investigations on the biology, ecology and evolution have been undertaken (Wynns 2015). Recently, a study described a symbiosis between Scaptotrigona postica bees and a fungus (Menezes et al. 2015). The fungus was identified by morphology and ITS sequencing as being closely related to M. ruber and M. pilosus. The study showed that the Monascus biomass on the food inside the brood cells is essential for the larvae of the S. postica bees, and without the consumption of this biomass, only a few larvae can continue their life cycle.
Monascus was one of the predominant genera during the study of fungi associated with honey, pollen and nests of Melipona scutellaris bees living in the Atlantic Forest in Pernambuco, Brazil. The phylogenetic relationship of those strains with other species of the genus was determined by the analysis of ITS, LSU, β-tubulin (BenA), calmodulin (CaM) and RNA polymerase II second largest subunit (RPB2) sequences. Furthermore, three new species from honey, pollen and the inside of the nest are described based on a polyphasic approach combining sequence data, macro- and microscopic characters and extrolites.
Materials and methods
Fungal isolation
Samples were collected from honey, pollen and inside nests of Melipona scutellaris bees in the Brazilian Tropical Forest in Pernambuco state (8°7ʹ30ʺS, 34°52ʹ30ʺW and 8°4′36″S, 34°57′34″W) between January and June 2014. For the honey and pollen samples, 25 g of each specimen was suspended in 225 mL peptone water (0.1 %) and decimal dilutions were made until 10−3. Subsequently, 0.1 mL of each dilution was spread plated on the agar media dichloran 18 % glycerol agar (DG18) and malt extract agar supplemented with chloramphenicol. The plates were incubated at 25 °C for 7–14 d in darkness. For collection of the samples inside nests, a sterile cotton swab was used to sample the surface of the pollen and honey pots, and brood cells. The swab was soaked in 3 mL peptone water (0.1 %) and vortexed vigorously. The samples were subsequently analysed as described above. All fungal colonies were isolated and purified prior identification.
Cultivation and morphological analyses
Molecular characterization
Genomic DNA of 7 d old cultures was extracted using the UltraClean Microbial DNA kit (MoBio Laboratories, Solana Beach, CA, USA) and processed according to the manufacturer's instructions. Polymerase chain reaction (PCR) amplification of the ITS region (ITS1, 5.8S rDNA and ITS2) was performed using the primers V9G and LS266 and a part of the Large SubUnit (LSU) rDNA was amplified using the primers LR0R and LR5. Partial β-tubulin fragments were generated using the primer combination Bt2a and Bt2b, for calmodulin the primers Cmd5 and Cmd6 were used and for RPB2 the primers RPB2-5F and RPB2-7CR. Details on the primer sequences, PCR mixtures and conditions are previously described (Samson et al., 2010, Houbraken et al., 2012).
The PCR products were sequenced in both directions with the same primers using the BigDye® Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems Life Technologies, Carlsbad, CA, USA) and were purified with Sephadex, according to the manufacturers' recommendations. Contigs were assembled using the forward and reverse sequence with the SeqMan v. 10.0.1 program. Newly generated sequences were deposited in GenBank. Sequence datasets were generated by combining the newly generated sequences with sequences from GenBank (Table 1). The sequences were aligned using MAFFT (Katoh et al. 2005) and were manually optimized using MEGA 5 (Tamura et al. 2011). The most suitable substitution model was determined using FindModel (Posada & Crandall 1998). Phylogenetic trees were constructed using maximum likelihood (ML) analysis in RAxML-VI-HPC v. 7.0.3 (Stamatakis 2006) using the GTRGAMMA substitution model and 1 000 bootstrap replicates. Bayesian inference (BI) in MrBayes v.3.2.1 (Ronquist et al. 2012) was performed using Markov Chain Monte Carlo (MCMC) algorithm and the best scoring substitution model is indicated in the results section. Trees were visualized in FigTree v. 1.1.2 (Rambaut 2009) and edited in Adobe Illustrator v.CS5.1. Individual alignments were concatenated by using Mesquite v3.04 (Maddison & Maddison 2016). The quality of final alignment was evaluated using Transitive Consistence Score (TCS) by the T-Coffee web server (Chang et al. 2015).
Table 1.
Strains and sequences used in the morphological and molecular study.
| Species | Strain numbers | Substrate; location | GenBank accession no. |
||||
|---|---|---|---|---|---|---|---|
| ITS | BenA | LSU | CaM | RPB2 | |||
| Leiothecium ellipsoideum | CBS 607.74T = ATCC 32453 | Soil, between rocks; Pelopennesos, Greece | KF732839 | KY709178 | FJ358285 | KY611939 | JN121541 |
| Monascus argentinensis | CBS 109402T = DTO 138-C5 = FMR 7393 | Soil sample; Tucumán province, Argentina | JF922046 | KY709174 | KY645974 | KY611935 | JN121423 |
| M.eremophilus | CBS 123361T = DTO 122-C7 = FRR 3338 | Mouldy prunes; New South Wales, Australia | GU733347 | KY709170 | KY645973 | KY611931 | KY611970 |
| M.flavipigmentosus | URM 7536T = CBS 142366 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511751 | KY709168 | KY511781 | KY611929 | KY611968 |
| M.flavipigmentosus | URM 7535 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511752 | KY709169 | KY511782 | KY611930 | KY611969 |
| M.flavipigmentosus | URM 7534 | Pollen of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511750 | KY709167 | KY511780 | KY611928 | KY611967 |
| M.floridanus | CBS 142228T = DTO 360-E7 = CGMCC 3.5843 = IMI 282587 = UAMH 4180 | Sand pine roots; USA | KY635848 | KY709172 | KY635856 | KY611933 | KY611972 |
| M.lunisporas | CBS 142230T = DTO 360-E9 = CGMCC 3.7951 = ATCC 204397 | Mouldy feed for race horses; Japan | KY635847 | KY709171 | KY635855 | KY611932 | KY611971 |
| M.mellicola | URM 7510T = CBS 142364 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511726 | KY709143 | KY511756 | KY611904 | KY611943 |
| M.mellicola | URM 7507 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511723 | KY709140 | KY511753 | KY611901 | KY611940 |
| M.mellicola | URM 7508 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511724 | KY709141 | KY511754 | KY611902 | KY611941 |
| M.mellicola | URM 7509 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511725 | KY709142 | KY511755 | KY611903 | KY611942 |
| M.mellicola | URM 7511 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511727 | KY709144 | KY511757 | KY611905 | KY611944 |
| M.mellicola | URM 7512 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511728 | KY709145 | KY511758 | KY611906 | KY611945 |
| M.mellicola | URM 7513 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511729 | KY709146 | KY511759 | KY611907 | KY611946 |
| M.mellicola | URM 7514 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511730 | KY709147 | KY511760 | KY611908 | KY611947 |
| M.mellicola | URM 7515 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511731 | KY709148 | KY511761 | KY611909 | KY611948 |
| M.mellicola | URM 7516 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511732 | KY709149 | KY511762 | KY611910 | KY611949 |
| M.mellicola | URM 7517 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511733 | KY709150 | KY511763 | KY611911 | KY611950 |
| M.mellicola | URM 7518 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511734 | KY709151 | KY511764 | KY611912 | KY611951 |
| M.mellicola | URM 7519 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511735 | KY709152 | KY511765 | KY611913 | KY611952 |
| M.mellicola | URM 7520 | Pollen; Recife, Pernambuco, Brazil | KY511736 | KY709153 | KY511766 | KY611914 | KY611953 |
| M.mellicola | URM 7521 | Honey of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511737 | KY709154 | KY511767 | KY611915 | KY611954 |
| M.mellicola | URM 7522 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511738 | KY709155 | KY511768 | KY611916 | KY611955 |
| M.pallens | CBS 142229T = DTO 360-E8 = CGMCC 3.5844 = ATCC 200612 = IMI 356820 | River sediment; Iraq | KY635849 | KY709173 | KY635857 | KY611934 | KY611973 |
| M.pilosus | CBS 286.34T = DTO 165-B1 = ATCC 16363 = FRR 2194 = IFO 4480 | Fermented grain, Sorghum vulgare; Japan | KY635852 | JF922085 | KY635860 | KY849968 | KY849967 |
| M.purpureus | CBS 109.07T = DTO 364-D8 = ATCC 16365 = IFO 4513 = IMI 210765 = NRRL 1596 | Fermented rice grain (‘ang-quac’); Java, Indonesia | KY635851 | KY709176 | KY635859 | KY611937 | JN121422 |
| M.recifensis | URM 7524T = CBS 142365 | Pollen of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511740 | KY709157 | KY511770 | KY611918 | KY611957 |
| M.recifensis | URM 7523 | Pollen of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511739 | KY709156 | KY511769 | KY611917 | KY611956 |
| M.ruber | URM 7525 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511741 | KY709158 | KY511771 | KY611919 | KY611958 |
| M.ruber | URM 7526 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511742 | KY709159 | KY511772 | KY611920 | KY611959 |
| M.ruber | URM 7527 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511743 | KY709160 | KY511773 | KY611921 | KY611960 |
| M.ruber | URM 7528 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511744 | KY709161 | KY511774 | KY611922 | KY611961 |
| M.ruber | URM 7529 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511745 | KY709162 | KY511775 | KY611923 | KY611962 |
| M.ruber | URM 7530 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511746 | KY709163 | KY511776 | KY611924 | KY611963 |
| M.ruber | URM 7531 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511747 | KY709164 | KY511777 | KY611925 | KY611964 |
| M.ruber | URM 7532 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511748 | KY709165 | KY511778 | KY611926 | KY611965 |
| M.ruber | URM 7533 | Inside nest of Melipona scutellaris; Recife, Pernambuco, Brazil | KY511749 | KY709166 | KY511779 | KY611927 | KY611966 |
| M.ruber | CBS 135.60NT = DTO 359-E8 = ATCC 15670 = IFO 8451 = IMI 081596 | Soil; India | KY635850 | KY709175 | KY635858 | KY611936 | KY611974 |
| M.sanguineus | IMI 356821T = ATCC 200613 | River sediment; Iraq | JF922055 | JF922088 | AF364968 | KY611938 | n/a |
| Penicillium polonicum | CBS 222.28T = IBT 12821 = IMI 291194 = NRRL 995 | Soil, Poland | AF033475 | AF001206 | JN939272 | KU896848 | JN985417 |
| P.verrucosum | CBS 603.74NT = IMI 200310 = ATCC 48957 = FRR 965 = IBT 4733 = NRRL 965 | Unknown source, Belgium | AB479317 | AF001205 | AB479285 | DQ911138 | JN121539 |
| Talaromyces purpurogenus | CBS 286.36 = IMI 091926 | Unknown source; Japan | JX315671 | JX315639 | KY635863 | KF741947 | JX315709 |
| T.ruber | CBS 132704 = IBT 10703 | Aircraft fuel tank; UK | NR111780 | JX315629 | KY635864 | KF741938 | JX315700 |
| Xerochrysium dermatitidis | CBS 132.31T = IMI 096729 = UAMH 802 | Skin, man; Italy | KY635853 | n/a | KY635861 | n/a | JN121443 |
| Xeromyces bisporus | CBS 236.71T = IMI 063718 | Mouldy stick of liquorice; New South Wales, Australia | KY635854 | JF922089 | KY635862 | 7419877121 | JN121612 |
Abbreviations: T = type strain; NT = neotype strain; URM, URM Culture Collection (www.ufpe.br/micoteca), Brazil; CBS, Culture collection of the Westerdijk Fungal Biodiversity Institute (formerly known as Centraalbureau voor Schimmelcultures), The Netherlands; DTO, Internal culture collection at Westerdijk Fungal Biodiversity Institute.
Sequence from genome sequenced strain; n/a: no sequence available.
Extrolite analysis
Extrolites were extracted from fungal strains grown on CYA, YES, MEA, OA at 25 °C for 14 d and PDA and DG18 at 25 °C for 20 d. Three agar plugs of each culture were extracted as previously described (Smedsgaard, 1997, Houbraken et al., 2012). After extraction, the liquid was transferred to a clean screw-cap vial and evaporated to dryness. Prior analysis, the dried extracts were re-dissolved in methanol by ultrasonication and filtered through a 0.45 μm filter. The extracts were analysed by ultra-high performance liquid chromatography with diode-array detection (UHPLC-DAD) (Houbraken et al. 2012). The detected eluted compounds were identified by comparing the retention time, retention index and UV spectra measured at 200–600 nm. The UV spectra were compared to a database of UV spectra and data from literature (Nielsen et al., 2011, Klitgaard et al., 2014).
Results
Phylogeny and GCPSR
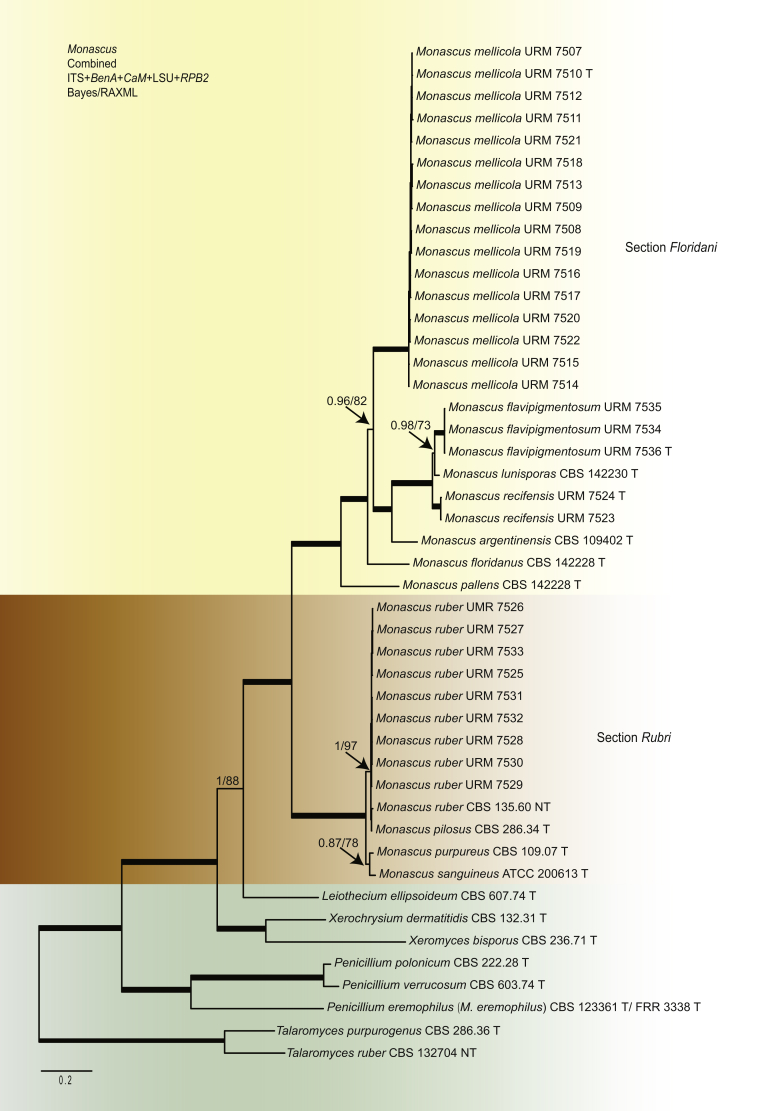
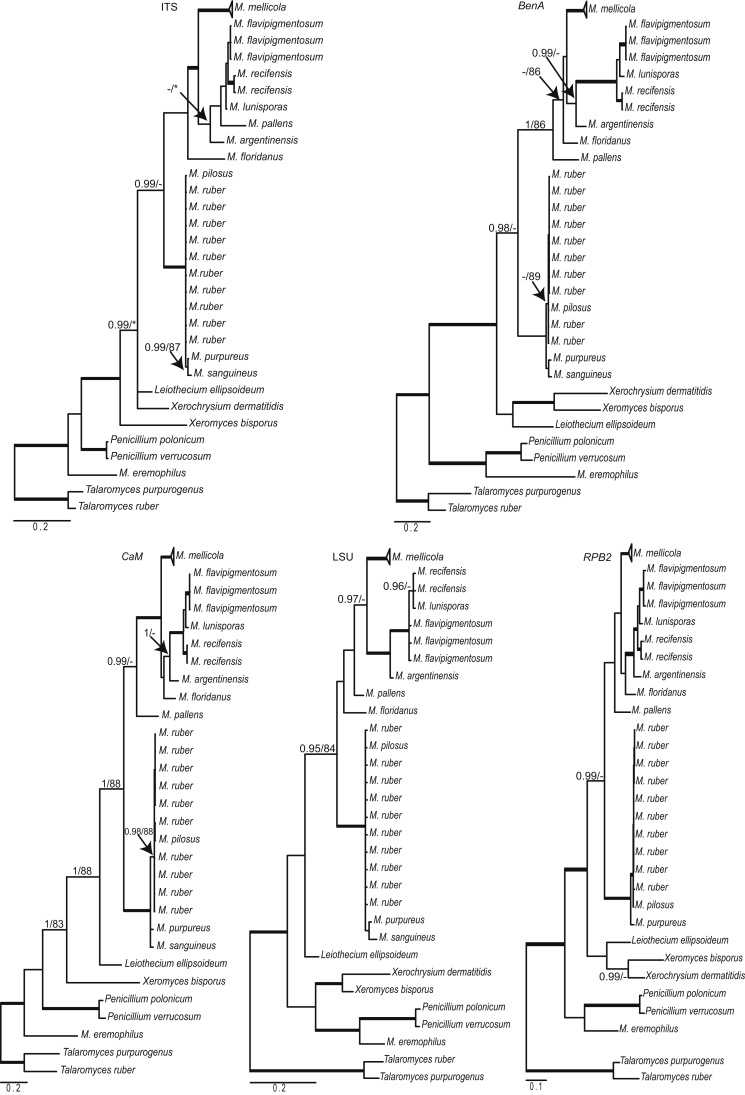
Monascus eremophilus is positioned outside the main Monascus clade and proved to be related to Penicillium species (100 % bs, 1.00 pp) (Fig. 1). Our analysis revealed two well-supported groups in Monascus, referred here to as the M. floridanus- and M. ruber-clades. Seven well-supported lineages are present in the M. floridanus-clade and these lineages are treated as separate species. Four are known species (M. lunisporas, M. argentinensis, M. floridanus, M. pallens), and three are proposed as newly described below (Monascus mellicola, M. recifensis and M. flavipigmentosus). Monascus mellicola is phylogenetically distinct and is with moderate bootstrap and posterior probability support (82 % bs, 0.96 pp) related to M. argentinensis, M. lunisporas, M. recifensis and M. flavipigmentosus. The latter three species are resolved as close relatives in a distinct, well-supported clade. In our concatenate phylogenetic analysis these species are separated in three well-supported groups, with M. lunisporas and M. flavipigmentosus being sister species and M. recifensis taking a basal position. Similar clustering was obtained in the single gene analyses; however, the species were unresolved in the LSU phylogram (Fig. 2).
Fig. 1.
Concatenated phylogeny of the ITS, BenA, CaM, LSU and RPB2 gene regions showing the relationship in Monascus. Branches with posterior probability values of 1.00 and >95 % are thickened.
Fig. 2.
Single gene phylogenetic trees of the ITS, BenA, CaM, LSU and RPB2 gene regions of species from Monascus. Branches with posterior probability values of 1.00 and >95 % are thickened.
The (neo)type strains of M. pilosus (CBS 286.34T), M. purpureus (CBS 109.07T), M. ruber (CBS 135.60NT) and M. sanguineus (ATCC 200613T) are located in the M. ruber-clade. Two lineages are present within the M. ruber-clade (Fig. 1). The (neo)type strains of M. pilosus (CBS 286.34T) and M. ruber (CBS 135.60NT) are together on a well-supported branch (97 % bs; 1.00 pp), and the branch containing the types of M. purpureus (CBS 109.07T) and M. sanguineus (ATCC 200613T) has weak statistical support (78 % bs; <0.95 pp). In our single gene analyses, M. pilosus (CBS 286.34T) and M. ruber (CBS 135.60NT) always cluster together with high (ITS: 99 % bs, 1.00 pp; LSU: 98 % bs, 1.00 pp; CaM: 88 % bs, 0.98 pp) or moderate (RPB2: 96 % bs, <0.95 pp, BenA 89 % bs, <0.95 pp) statistical support. The branch with M. purpureus (CBS 109.07T) and M. sanguineus (ATCC 200613T) is well supported in the ITS phylogram (87 % bs, 0.99 pp), and no support was found in the BenA, CaM and LSU analyses (<70 %, <0.95 pp). Following the GCPSR concept, we keep two lineages in the M. ruber-clade. Monascus pilosus and M. sanguineus are treated here as synonym of M. ruber and M. purpureus, respectively.
Morphology
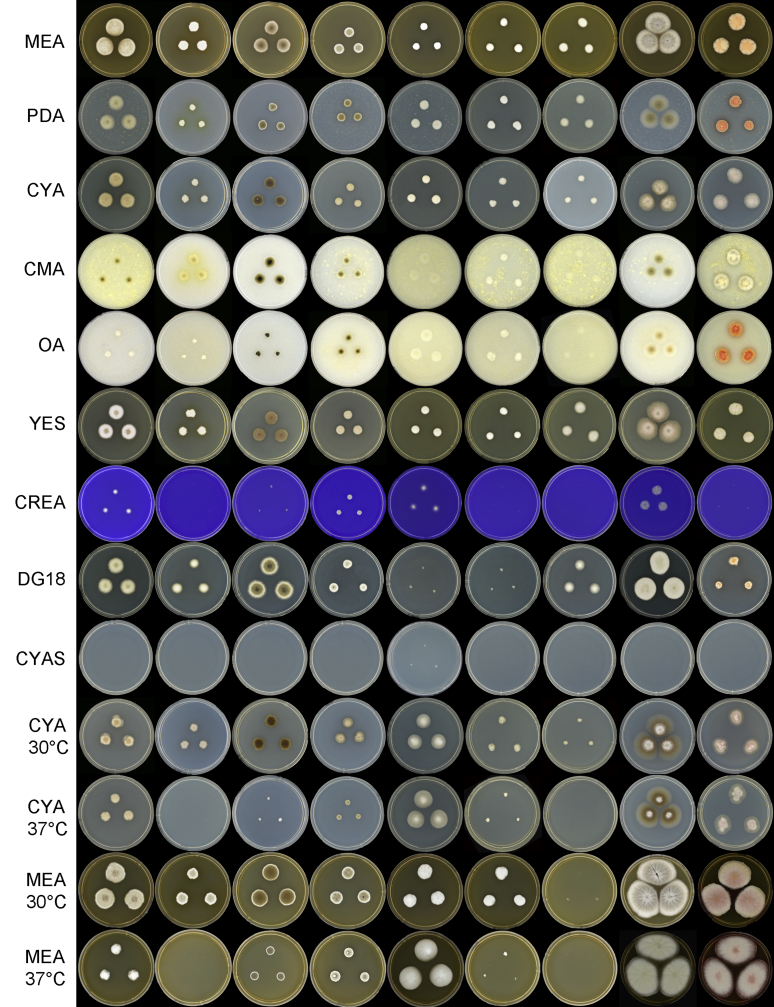
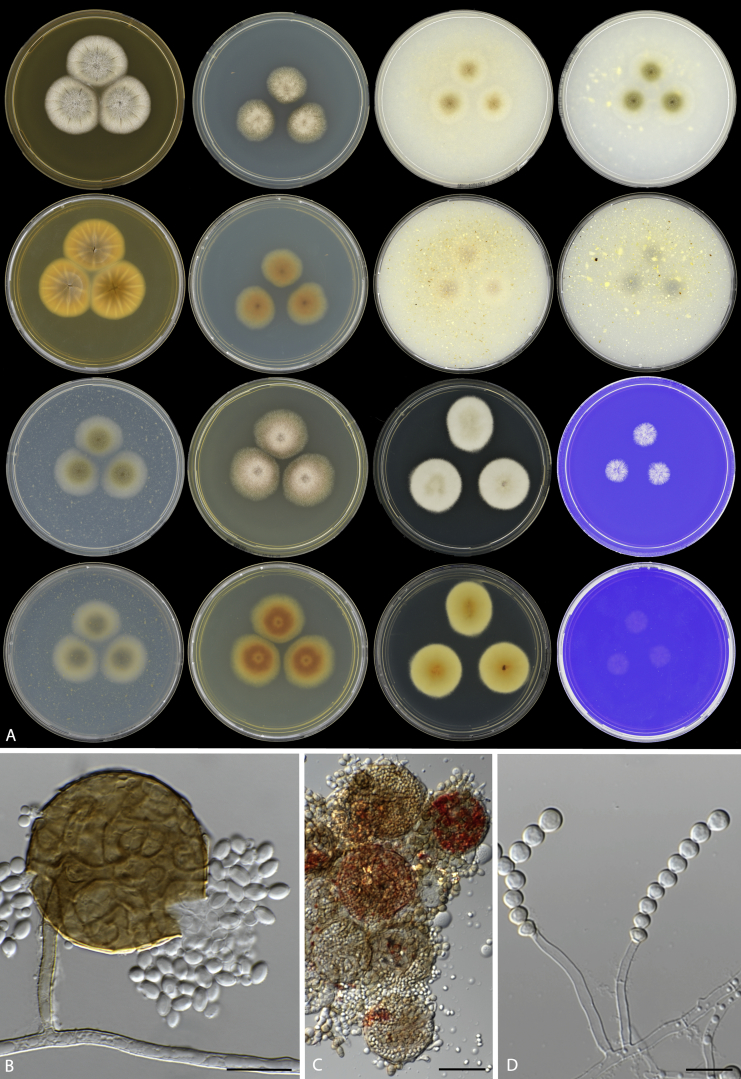
Fig. 3.
Cultural characters of Monascus species on different agar media and incubation conditions. Left to right: M. lunisporas, M. flavipigmentosus, M. recifensis, M. mellicola, M. pallens, M. floridanus, M. argentinensis, M. ruber and M. purpureus.
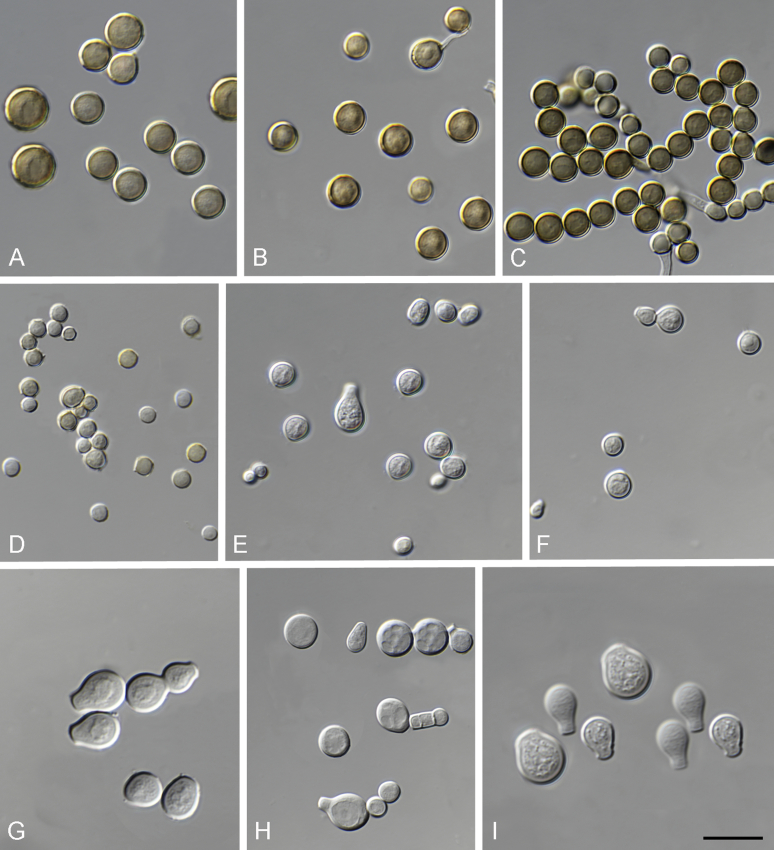
Fig. 4.
Conidial shapes and colours of Monascus species. A.M. lunisporas. B.M. flavipigmentosus. C.M. recifensis. D.M. mellicola. E.M. pallens. F.M. floridanus. G.M. argentinensis. H.M. ruber. I.M. purpureus. Scale bars = 10 μm.
Extrolites
Identification of Monascus isolates associated with Melipona scutellaris
Fig. 5.
Monascus ruber URM 7525 isolated during the course of this study. A. Colonies from left to right (first row) MEA, CYA, OA, CMA; (second row) MEA reverse, CYA reverse, OA reverse, CMA reverse; (third row) PDA, YES, DG18, CREA; (forth row) PDA reverse, YES reverse, DG18 reverse, CREA reverse. B–C. Typical ascoma and ascospores. D. Conidiophores with conidia chain. Scale bars = 10 μm.
Discussion
Monascus belongs to the order Eurotiales, and this genus is characterized by the production of stalked cleistothecial ascomata that are non-ostiolate and have hyaline to brown walls. The ascomatal cavity is filled with unicellular ascospores. Asexual reproduction takes place on basipetospora-type conidiophores. These conidiophores are erect, variable in length, and the conidia are hyaline to brown and produced singly or in short basipetal chains (up to 15–20 conidia). Phenotypic identification of Monascus species largely depends on shape, size and pigmentation of the cleistothecia and ascospores (Hawksworth & Pitt 1983). No cleistothecia and only the basipetospora-state was observed in the two newly described species M. mellicola and M. recifensis; however, these species do phylogenetically belong to the Monascus clade. They produce a basipetospora-state, which is the characteristic asexual stage of this genus. Following the latest International Code of Nomenclature for algae, fungi and plants (McNeill et al. 2012), in respect to the principle of priority, and that nomenclature has economic and social implications, particularly for old, important genera, we give priority to Monascus over Basipetospora, even when no sexual state is observed in those species. This is in line with the recommendations of Rossman et al. (2016), who also recommended giving priority to the name Monascus over Basipetospora.
In the last years numerous new genera have been proposed primary based on phylogenetic data and sometimes with only a few distinctive morphological features. Phenotypic and phylogenetic analysis revealed two well-supported clades in Monascus. Following the guidelines proposed by Vellinga et al. (2015), these differences would justify splitting Monascus into two separate genera. On the other hand, Monascus species do share various characters, such as similar basipetospora-type conidiophores and stalked cleistothecia. The majority of Monascus species produce indole alkaloids (possibly gypsetins) and this study shows that various Monascus species are also associated with stingless bees, indicating that they are also ecologically related. We therefore give preference to introduce two new sections instead of two small genera. A sectional classification system is commonly applied in genera related to Monascus, such as Penicillium, Aspergillus and Talaromyces and this is in line with that approach (Gams et al., 1985, Houbraken and Samson, 2011, Yilmaz et al., 2014). The two sections have few extrolites in common (Table 5). The Rubri section contains species that produce mevinolins, citrinin and other yellow and red azaphilone pigments, including the red pigments (rubropunctamine, PP-V, PP-R etc.) that are colouring red rice, while the species in section Floridani do not produce any of these bioactive extrolites at all. Isolates in each species in section Floridani produce species specific combinations of extrolites, and few are in common between those species. One example is the red compound “GULLA”, which was detected in both M. mellicola and M. recifensis, but the latter species produce several extrolites that are not produced by M. mellicola, including secalonic acid D, asterric acid, questin, (−)-bisdechlorogeodin and some red anthraquinone extrolites not related to the azaphilones produced by M. purpureus and M. ruber. Strains of M. flavipigmentosus produce a high number of unique as yet not structure elucidated extrolites, including some yellow coloured extrolites (Y1 and Y2) and an anthraquinone (Table 5). The red extrolite “GULLA” has previously been found in Penicillium species, including Penicillium oxalicum and P. mononematosum (Frisvad, personal communication).
Table 5.
Retention index and absorption maxima for extrolites dectected in Monascus (the UV spectra of the unknown compounds are shown in the Supplementary data).
| Extrolite | Retention index | Absorption maxima (nm) | Extrolite by section |
|---|---|---|---|
| Anthraquinone X (= atrochrysone?) | 1207 | 220, 261, 282sh, 427 | Floridani |
| Anthraquinone Z | 948 | 223, 271, 298, 433 | Floridani |
| Asterric acid | 921 | 207, 220sh, 252, 317 | Floridani |
| Asterric acid derivative | 825 | 207, 220sh, 252, 317 | Floridani |
| (−)-bisdechlorogeodin | 868 | 203, 224sh, 278, 336sh | Floridani |
| Citrinadin-like | 783, 821, 830 | 200, 227sh, 246, 265sh, 325 | Floridani |
| Citrinin | 907 | 221, 242sh, 328, 415sh | Rubri |
| Curvularin | 881 | 200, 223, 270, 301 | Floridani |
| Dehydrocurvularin | 861 | 202, 225, 283, 334sh | Floridani |
| ENDI | 745 | End-absorption | Floridani |
| GULLA | 1007 | 202, 258, 286, 328, 369, 428 | Floridani |
| Indole alkaloid (= gypsetin-like) | 967 | 224, 278, 288, 295 | Floridani, Rubri |
| JOPS | 1098 | 208, 248, 275, 353 | Floridani |
| Metabolite M series | 845, 854, 865, 881, 906, 946 | 291, 242sh, 283, 318 | Floridani |
| Metabolite N series | 917, 1048 | 203, 236, 251sh, 326, 381 | Floridani |
| Metabolite O series | 905, 982, 993 | 202, 226sh, 254, 272sh, 335 | Floridani |
| Metabolite Y series | 1097 (Y1), 1273 (Y2) | 200, 228sh, 274, 375 | Floridani |
| Methyl asterrate | 934 | 200, 227sh, 246, 265sh, 325 | Floridani |
| Mevinolin | 1232 | 230sh, 240, 250sh | Rubri |
| Mevinolin, open acid form | 1121 | 230sh, 240, 250sh | Rubri |
| Monascin | 1251 | 230, 282, 397 | Rubri |
| Rubratoxin-like (Nonadrides, provisionally identified as rubratoxins) | 1033, 1066 | 215sh, 263 | Floridani |
| Orthosporin-like | 721 | 241sh, 248273, 282, 324 | Floridani |
| Physcion-like (anthraquinone W) | 1079 | 221, 250sh, 264, 282, 331, 440 | Floridani |
| PP-V | 943 | 250, 296, 420, 524 | Rubri |
| PP-R | 981 | 252, 306, 417, 524 | Rubri |
| Questin | 958 | 223, 247sh, 280, 428 | Floridani |
| Red anthraquinone series | 1316, 1326, 1387, 1412, 1422 | 227, 268, 330, 442 | Floridani |
| Rubratoxin-like | 1198 | 202, 251 | Floridani, Rubri |
| Rubropunctamine | 1417 | 218, 250, 279, 298sh, 447sh, 475, 512sh | Rubri |
| Rubropunctatin | 1252 | 218sh, 235, 279, 394475sh, 521 | Rubri |
| Secalonic acid D | 1104 | 200, 215sh, 258, 331, 388sh | Floridani |
| Shamixanthone-like | 1121 | 201, 228, 263, 301, 366 | Floridani |
| Sulochrin | 873 | 203, 224sh, 278, 324sh | Floridani |
| Xanthomonascin A | 1143 | 230, 282, 397 | Rubri |
Asterric acid, methyl asterrate and (−)-bisdechlorogeodin are all part of the geodin biosynthetic family; sh: shoulder.
Several morphological features are shared between Monascus species; however, there are also various characters that can be used for identification (Table 2, Table 3). For example, the conidial size can differ between species. All species except two (M. mellicola, M. recifensis) produce a sexual state and the size and shape of the ascospores can differ among species. The species also differ in their growth rates, and for example M. flavipigmentosus, M. pallens and M. floridanus grow more restrictedly on agar media than M. ruber and M. purpureus. Most species do not produce soluble pigments; however, the production of red (soluble) pigments is a character of M. purpureus and M. ruber (Hawksworth & Pitt 1983) and M. flavipigmentosus produces yellow pigments on CMA and PDA (and old cultures on DG18). Also the growth rate at 37 °C is diagnostic. M. pallens, M. ruber and M. purpureus grow equally or even faster at 37 °C than at 30 °C. On the other hand, M. floridanus and M. mellicola and M. recifensis grow slowly at 37 °C, and M. argentinensis and M. flavipigmentosus did not grow at this temperature at all.
Table 2.
Growth rate comparison of Monascus species after 7 d (in mm) and most important colony characters.
| Species | CYA | MEA | DG18 | CYAS | OA | CREA | YES | CMA | PDA | MEA 30 °C | CYA 30 °C | MEA 37 °C | CYA 37 °C | Colour mycelium on MEA | Soluble pigments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monascusargentinensis | 8–9 | 11–13 | 13–15 | ng | 10–11 | ng | 14–15 | 9–10 | 10–11 | ng | 5–6 | ng | ng | White | Absent |
| M. flavipigmentosus | 7–10 | 10–12 | 8–10 | ng | 4–5 | ng | 10–11 | 10–12 | 6–8 | 10–11 | 9–10 | 0–2 | 0–3 | White | Yellow |
| M. floridanus | 9–10 | 9–10 | 3–5 | ng | 10–11 | ng | 9–10 | 9–10 | 10–11 | 10–11 | 8–10 | 2–4 | 3–4 | White | Absent |
| M. lunisporas | 15–17 | 24–25 | 20–22 | ng | 14–15 | 3–5 | 20–23 | 19–20 | 18–20 | 24–25 | 15–17 | 9–10 | 12–13 | Brownish | Absent |
| M. mellicola | 8–10 | 11–12 | 7–10 | ng | 9–10 | 5–7 | 10–11 | 9–10 | 9–10 | 21–22 | 11–12 | 11–12 | 5–7 | White | Absent |
| M. pallens | 10–11 | 8–10 | 3–4 | 3–4 | 14–15 | 9–10 | 10–11 | 9–11 | 12–13 | 11–15 | 17–18 | 26–30 | 21–22 | White | Absent |
| M. purpureus | 19–20 | 20–22 | 3–5 | ng | 16–20 | ng | 13–18 | 18–20 | 11–15 | 39–40 | 20–21 | 52–55 | 20–21 | Red to orange | Orange |
| M. recifensis | 12–14 | 16–18 | 20–21 | ng | 3–5 | 1–2 | 14–15 | 10–12 | 10–11 | 19–20 | 10–11 | 9–10 | 3–4 | White to brownish | Absent |
| M. ruber | 17–19 | 25–26 | 18–20 | ng | 18–20 | 8–10 | 17–28 | 15–20 | 26–30 | 47–48 | 35–37 | 49–50 | 35–40 | White | Absent |
Table 3.
Most important micromorphological characters for species recognition.
| Species | Colour and size (μm) ascomata on PDA | Shape ascospores on PDA | Size ascospores (μm) | Shape and colour conidia | Size of conidia (μm) | Number of conidia per phialide |
|---|---|---|---|---|---|---|
| Monascusargentinensis* | Dark olivaceous-brown, 20–75 | Ellipsoidal to subglobose | 3–4 × 2.5–3 | Globose to obovoid or obpyriform | Globose, 5–15; obpyriform, 7–15 × 5–9 | Single or formed in short chains |
| M. flavipigmentosus | Hyaline to brown, 40–60 | Lunate | 4–5 × 1.7–2.5 | Globose to subglobose, hyaline to brown | 5.5–7.5 | Single or formed in short chains |
| M. floridanus* | Dark brown, 22–58 | Ellipsoidal | 3.5–4.5 × 2–3 | Globose to obovoid or obpyriform, pale brown | 4–9 × 3.5–9 | Single or formed in short chains (up to 6?) |
| M. lunisporas* | Brown, 25–60 | Lunate | 6–7 × 2–2.5 | Globose to obpyriform, hyaline to brown | Globose, 6–11; obpyriform, 5–7 × 7–10 | Single or formed in short chains |
| M. mellicola | – | – | – | Globose to subglobose, hyaline to brown | 2.5–5.0 × 3.5–5.0 | Single or up to 17 conidia |
| M. pallens* | Hyaline, 23–38 | Ellipsoidal | 3.5–4 × 2.5–3 | Usually pyriform, hyaline | 3.5–10 (–13) × 2.5–8 | Short terminal or intercalary basipetal |
| M. purpureus** | Hyaline, (25–) 45 × 60 (–70) | Ellipsoidal | (5.5–) 6–7 × 4–5 | Globose to obpyriform | 8–11 × 8–10 | Single or in short chains |
| M. recifensis | – | – | – | Globose to subglobose, hyaline to brown | 4.0–7.0 | Single or in short chains |
| M. ruber** | Brown, 30–50 (–60) | Ellipsoidal | 5–6 (–7.5) × (3.5–) 4–5 | Globose to obpyriform | 10–18 × 8–14 | Single or up to 10 conidia |
Abbreviations: *Data from original description; **Data from Hawksworth & Pitt (1983); –: not observed.
All species except M. floridanus produced a species-specific series of extrolites, consistent with the phenotypic classification and the results obtained in the phylogenetic study. Monascus lunisporas, M. recifensis and M. flavipigmentosus are phylogenetically closely related. Their extrolite profiles are distinct. Monascus flavipigmentosus produces metabolites of biosynthetic family M and M. recifensis secalonic acid, asterric acid, sulochrin, questin and an anthraquinone with the same UV spectrum as physcion (physcion-like in Table 4). None of these extrolites were found in the closely related species M. lunisporas (CBS 142230T). An indole alkaloid (probably gypsetin) was produced by 6 of the 9 species (Table 4) and was the only metabolite found in section Floridani and Rubri. The metabolites mevinolins and xanthomonasin A were only detected in M. ruber-clade species. An important characteristic of Monascus ruber is its ability to produce citrinin, a compound with both antibiotic and toxic activity. According literature, this extrolite is also produced by M. purpureus, M. pallens, M. lunisporas and M. floridanus (Wang et al. 2005). In our study, citrinin was detected only in the type of M. purpureus. More strains in addition to other culture conditions stimulating citrinin production should be investigated to find out if other species besides M. purpureus can produce citrinin.
Table 4.
Extrolites detected in Monascus.
| Species | Extrolites |
|---|---|
| Monascus argentinensis | Anthraquinone Z, indole alkaloid (possibly gypsetin), rubratoxin-like |
| M.flavipigmentosus | Anthraquinone X (possibly atrochrysone), indole alkaloid (possibly gypsetin), unknown and unique metabolite biosynthetic family M and Y (Y1 and Y2) |
| M.floridanus | “ENDI”, orthosporin-like, “JOPS” |
| M.lunisporas | Citrinadin-like, indole alkaloid (possibly gypsetin), metabolite N series, metabolite O series, shamixanthone-like |
| M.mellicola | Indole alkaloid (possibly gypsetin), “GULLA” |
| M.pallens | Curvularin, dehydrocurvularin, indole alkaloid (possibly gypsetin) |
| M.purpureus | Citrinin, mevinolins, monascin, PP-V, PP-R, rubropunctamine, rubropunctatin, xanthomonasin A |
| M.recifensis | Anthraquinone X (= atrochrysone?), asterric acid, (−)-bisdechlorogeodin, “GULLA”, orthosporin-like, anthraquinone W (physcion-like), questin, red anthraquinone pigments, secalonic acid D, sulochrin |
| M.ruber | Indole alkaloid (possibly gypsetin), mevinolins, monascin, PP-V, PP-R, xanthomonasin A, rubropunctamine, rubropunctatin, rubratoxin-like |
Based on the results of our study combined with data from previous studies, we accept nine species in Monascus: M. argentinensis, M. floridanus, M. lunisporas, M. mellicola, M. pallens, M. purpureus, M. ruber, M. recifensis and M. flavipigmentosus (Hawksworth and Pitt, 1983, Park et al., 2004). Monascus pilosus, M. sanguineus are also often mentioned in literature as accepted species in Monascus. Phenotypically, M. pilosus is very similar to M. ruber and according literature, Hawksworth & Pitt (1983) indicated that they can differentiated by the size of ascomata (25–55 vs 30–50 (–60) μm), ascospores (5–7 (–8.5) × 3–3.5 (–4) vs 5–6.5 (–7.5) × (3.5–) 4–4.5 μm) and the presence of a brownish pigment in the cleistothecial walls and conidia. These sizes and colours are overlapping and during the study of the M. ruber isolates associated with bees, we also found considerable variation in pigmentation among the studied strains. Previous studies showed that M. pilosus shares ITS and partial LSU and β-tubulin sequences with M. ruber (Park and Jong, 2003, Park et al., 2004), suggesting that these are conspecific. Monascus pilosus clusters together with M. ruber in all of our single gene phylogenies, confirming these results. Additionally, M. ruber and M. pilosus are similar also in their metabolite profiles and share the production of mevinolins, rubropunctamine and xanthomonascin. Subsequently, there is no basis to accept M. pilosus as a separate species. Based on sequence data, M. sanguineus is treated here as a synonym of M. purpureus. Analysis of partial β-tubulin sequences (another part of the gene than used in this study) showed that M. sanguineus and M. purpureus are phylogenetically closely related and distinct from M. ruber (Park et al. 2004). These results are confirmed in our BenA, CaM, ITS and LSU phylograms, though statistical support was only found in the ITS phylogram. Based on the GCPSR concept, these species are treated as separate species. Phenotypically, M. sanguineus is differentiated from M. purpureus by its inability to grow on G25N and colour of ascomata and conidia; however, these characters might not be stable among a larger set of isolates, and this needs further investigation.
Many other species are described in Monascus: M. albidulus (= M. albidus nom. inval.), M. araneosus, M. aurantiacus, M. fumeus (= M. fuliginosus nom. inval.), M. kaoliang, M. major, M. paxii, M. pilosus nom. inval., M. pubigerus, M. rubiginosus, M. rutilus (= M. anka nom. inval.), M. rubropunctatus, M. serorubescens, M. vitreus. All these species belong to M. ruber-clade (Hawksworth and Pitt, 1983, Park and Jong, 2003). A detailed study is needed to determine the species diversity within the M. ruber-clade and to resolve the placement of the M. ruber/M. purpureus synonyms. Six Basipetospora species (B. chlamydospora, B. denticola, B. halophila, B. rubra, B. variabilis, B. vesicarum) are described and those might compete with the new species that are described here, especially those that lack a sexual state. However, Basipetospora rubra was described as the asexual state of M. ruber and is in the single name nomenclature system regarded as a synonym of this species. Basipetospora halophilica phylogenetically belongs to Aspergillus and was recently transferred to this genus (Samson et al., 2014, Kocsubé et al., 2016). Basipetospora chlamydospora, B. variabilis and B. denticola represented by CBS 228.84 (16S rRNA, AB024045), CBS 995.87 (16S rRNA, AF437892) and CBS 132.78 (ITS, LN850801), respectively, belong to Microascales. The first two species might represent a novel genus in this order (J. Woudenberg, pers. comm.) and the latter is a synonym of Scopulariopsis candida (Jagielski et al. 2016). Basipetospora vesicarum can be considered a synonym of M. ruber. This species was introduced based on examination of the type specimen of Sporotrichum vesicarum and analysis of this specimen revealed the presence of the Basipetospora anamorph of M. ruber (Stalpers 1984).
When Monascus eremophilus was described, Hocking & Pitt (1988) noted the unique features of this species. Based on colony colour and the mode of ascospore production they decided that the species could best be classified in Monascus. After its description, Monascus eremophilum was included in various phylogenetic studies; however, results concerning its placement inferred from different DNA regions were inconclusive. Park & Jong (2003) evaluated the use of D1/D2 sequences of the LSU rRNA for species differentiation in Monascus, and simultaneously performed a phylogenetic analysis. In their study, M. eremophilus was found in the clade containing the type of M. ruber; however, the bootstrap support of that clade was low (61 %). In 2004, Park et al. studied the genus Monascus by using ITS and partial beta-tubulin gene sequences. The position of M. eremophilus was unresolved in their ITS phylogram, and the species grouped together with M. lunisporas and M. pallens with less than 50 % bootstrap support. Moreover, when the beta-tubulin sequences were used, M. eremophilus was placed outside the ingroup. The authors commented that such an inconclusive placement of M. eremophilus might indicate: ‘… a unique and unpredictable genetic combination for this species. It might reflect enormous and extreme environmental stress and subsequent drastic genetic changes to adapt to extremely dry conditions’ (Park et al. 2004). More recently, based on D1/D2 sequence data, Vinnere-Pettersson et al. (2011) showed that M. eremophilus does not belong to Monascus, and appears to be related to Penicillium. In order to clarify the difference placements of M. eremophilus in literature, we re-analysed the LSU data set of Park & Jong (2003) and Vinnere-Pettersson et al. (2011) together with the data set generated in this study (data not shown). These results show that the sequence (AF365023) used in the study of Park et al. (2004) does not match with the other sequences generated from M. eremophilus, explaining the various phylogenetic placements of this species. Based on a 4-gene phylogeny, Houbraken et al. (2014) confirmed its placement in Penicillium. They confidently place the species on a branch together with members of section Charlesia (P. charlesii CBS 304.48T, P. fellutanum CBS 229.81), though there is sufficient genetic distance that would warrant placement of this species in a new section. Based on this literature review and additional (sequence) data generated in this study, we propose to transfer M. eremophilus in Penicillium. The placement of this species in Penicillium is unexpected. Penicillium eremophilum is, unlike any other Penicillium (and Monascus) species, an obligate xerophile. The species is not known to produce an asexual state and there were until now no strictly sexually reproducing species within Penicillium, though conidiophores can sometimes be sparsely produced in sexually reproducing Penicillium species. The formation of two-spored asci is also not shared with other Penicillium species. This feature, together with its xerophily, is shared with the phylogenetically distant species Xeromyces bisporus.
The ITS region is the official DNA barcode for fungi, and is good practice to include ITS sequences whenever new species are described (Schoch et al. 2012). However, not all species can be identified using this marker because certain species share identical ITS sequences (e.g. Houbraken et al., 2014, Chen et al., 2016). All Monascus species can be recognized on their ITS sequence only, even though the interspecific differences are low, especially between M. ruber and M. purpureus. Whether these barcode gaps remain present when a larger set of isolates is investigated remains unknown. A larger sequence variation was observed in the BenA gene. This gene is used as secondary barcode for the related genera Penicillium and Talaromyces and we propose the same for Monascus (Visagie et al., 2014, Yilmaz et al., 2014). The BenA gene is easy to amplify in Monascus and can distinguish all species. LSU has limited resolving power and RPB2 is more difficult to amplify and is therefore only recommended in phylogenetic studies.
Stingless beekeeping, or meliponiculture, is an ancient activity and many species of stingless bees are managed in the Americas, Africa, Asia and Australia; however, it remains a largely under-exploited business and technical knowledge is scarce. Much practical and academic work is being done about the best ways of keeping these bees, multiplying their colonies, and exploring the honey they produce (Cortopassi-Laurino et al., 2006, Villanueva-Gutiérrez et al., 2013, Jaffé et al., 2015). Melipona scutellaris is most known in the Northeast of Brazil. Furthermore, these bees are important pollinators in agricultural and natural ecosystems. Recently, a fungus cultivation mutualism in a social bee (Scaptotrigona postica) was reported for the first time (Menezes et al. 2015). The larvae of S. postica have a higher survival rate when they were fed with food grown with Monascus mycelium. The symbiotic relationships between microorganisms and stingless bees have been poorly explored, and during our investigation of fungi associated with Melipona scutellaris bees, we frequently isolated M. ruber from the inside of nests. This indicates that also other bee species, like Melipona scutellaris, might also have an (obligatory) relationship with M. ruber. Besides M. ruber, also M. mellicola was frequently isolated from honey, pollen and the inside of nests, followed M. recifensis and M. flavipigmentosus. This association with bees might be a novel unexplored ecological niche of Monascus species and can be the subject of future studies. The antibiotic and antifungal activity of some Monascus strains might play a role in the protection of the larvae food from microbial contaminations (Jůzlova et al., 1996, Menezes et al., 2015) and our discovery of many Monascus-unique extrolites in these species (metabolite families M, N, O, and Y) invites structure elucidation and bioactivity testing of those compounds. Stchigel & Guarro (2007) studied several cleistothecial ascomycetes and they concluded that the criterion of the production of closed ascomata without a predefined opening and with an irregular arrangement of asci at the centre is of little systematic value. A recent study about fungi living with association with solitary bees collected in Denmark suggest the convergent evolution of reduced fruiting bodies in Pezizomycotina is adaptive for spore dispersal to the bee habitat (Wynns 2015). Interesting to note in this context is that Monascus forms smaller cleistothecia than those produced in the related genera Aspergillus and Penicillium.
In the past, taxonomic studies on Monascus were solely based on phenotypic characters, or when sequence data was used, these were mostly applied for identification purposes. With the transfer of M. eremophilus to Penicillium, monophyly in Monascus is restored. The presented 5-gene phylogeny is a good robust starting point for future taxonomic studies in Monascus. Furthermore, a list of accepted species is provided, including information on (ex-)type strains and molecular markers (see Taxonomy section).
Taxonomy
Phylogenetically, two well-supported clades (M. floridanus-clade and M. ruber-clade) are present in Monascus and these groups can also be differentiated on phenotypic characters. Two sectional names are introduced for these clades and information on this taxonomic decision can be found in the Discussion. Our polyphasic approach revealed the presence of three new species and these are described below. Furthermore, a new combination for Monascus eremophilus is proposed.
Section Floridani R.N. Barbosa & Houbraken sect. nov. MycoBank MB820076.
Typus: Monascus floridanus P.F. Cannon & E.L. Barnard, Mycologia 79: 480. 1987. MycoBank MB132123.
Diagnosis: Colony diameter on MEA, PDA, CYA, CMA, OA, YES generally below 20 mm, no or restricted growth (<10 mm) on CREA and CYAS, and colony diameter less than 30 mm on MEA incubated at 30 and 37 °C. Colonies in shades of brown; conidia brown pigmented; mycelium white or in shades of brown.
Section Rubri R.N. Barbosa & Houbraken sect. nov. MycoBank MB820077.
Typus: Monascus ruber Tiegh., Bulletin de la Société Botanique de France 31: 227. 1884. MycoBank MB234876.
Diagnosis: Colony diameter on MEA, PDA, CYA, CMA, OA, YES generally above 15 mm, no or restricted growth on CREA and CYAS, good growth (>30 mm) on MEA incubated at 30 and 37 °C. Colonies in shades of brown to red; conidia brown pigmented; mycelium white or in shades of red or orange.
Fig. 6.
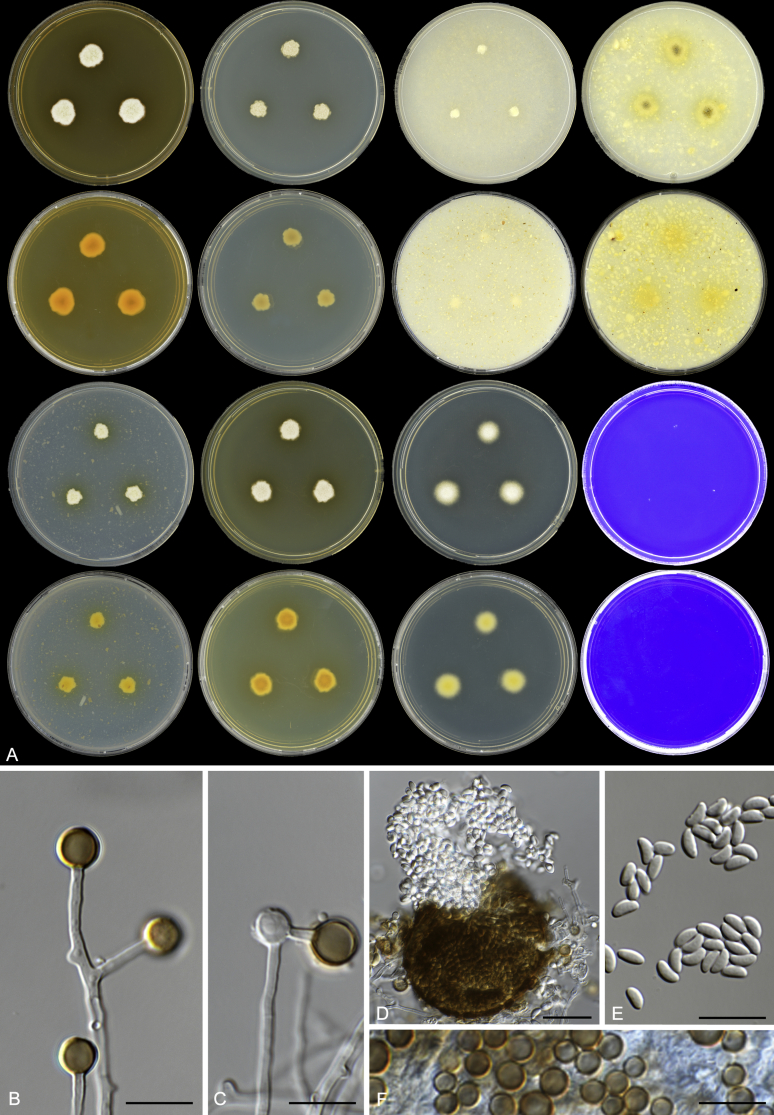
Monascus flavipigmentosus, URM 7536. A. Colonies from left to right (first row) MEA, CYA, OA, CMA; (second row) MEA reverse, CYA reverse, OA reverse, CMA reverse; (third row) PDA, YES, DG18, CREA; (forth row) PDA reverse, YES reverse, DG18 reverse, CREA reverse. B–C. Conidiophores. D. Ascoma. E. Ascospores. F. Conidia. Scale bars = 10 μm.
Etymology: flavipigmentosus is referring to yellow pigment produced on CMA and PDA.
Diagnosis: Monascus flavipigmentosus is phylogenetically distinct by BenA, CaM and ITS sequencing and characterized by the absence of growth on CREA 25 °C, and MEA and CYA incubated at 37 °C. Yellow soluble pigments present on CMA and PDA (and old cultures on DG18).
In: Monascus section Floridani
Typus: Brazil, Recife, isolate inside nests of Melipona scutellaris Jun 2014, isolated by R.N. Barbosa (holotype URM 90064; culture ex-type URM 7536 = CBS 142366 = DTO 353-A2).
Barcodes: ITS barcode: KY511751 (alternative markers: BenA = KY709168; CaM = KY611929; RPB2 = KY611968).
Colony diam, 7 d (mm): MEA 10–12; CYA 7–10; CMA 10–12; PDA 6–8; YES 10–11; OA 4–5; DG18 8–10; CYAS No growth; CREA No growth; CYA 30 °C 7–9; CYA 37 °C 0–2; MEA 30 °C 10–12; MEA 37 °C 0–3.
Description: Colonies characters after 7 d. MEA, 25 °C: colony texture velvety to floccose, pulvinate, mycelium white; sporulation absent; exudates absent; soluble pigments absent; reverse yellow. CYA, 25 °C: colony texture floccose low, mycelium white; sporulation absent; exudates absent; soluble pigments absent; reverse white to cream. CMA, 25 °C: colony texture lanose, low, mycelium inconspicuously white at the margin; sporulation weak at centre, conidia en masse dull brown; exudate absent; soluble pigments present, yellow; reverse yellow; ascomata abundantly produced, brown. PDA, 25 °C: colony texture floccose to lanose, low, mycelium white; sporulation absent; exudates absent; soluble pigments present, light yellow; colony reverse yellow. YES, 25 °C: colony texture floccose, low, mycelium white; sporulation absent; exudates absent; soluble absent; colony reverse yellow to brownish. OA, 25 °C: colony texture not determinate, mycelium white; sporulation absent, exudates absent; soluble pigments absent; colony reverse white to cream. DG18, 25 °C: colony texture velvety to floccose, low, mycelium white; sporulation absent; exudates absent; soluble pigments absent; reverse white to light yellow. CYAS, 25 °C: no growth. CREA, 25 °C: no growth. MEA, 30 °C: colony texture velvety, umbonate, mycelium white, sporulation absent, exudates absent; soluble pigments absent; reverse light brownish. CYA, 30 °C: mycelium brownish, sporulation weak, conidia en masse brownish; ascomata sparsely produced, brown; exudates absent; soluble pigments absent; reverse brownish. MEA, 37 °C: no growth. CYA, 37 °C: no growth.
Mycelium abundant, hyphae irregularly branched, hyaline to pale brown when old, smooth-walled, 1.8–3 μm wide. Conidiophores variable in length, smooth, 3–30 × 1.5–2.5 μm. Conidia single or formed in short basipetal chains, usually terminal, rarely intercalary, 5.5–7.5 × 5.5–7.5 μm diam, at first hyaline and pale brown to brown with age. Ascomata, stalked when young, non-ostiolate, globose to subglobose, 40–60 μm diam, initially light brown and dark brown in the age; peridium brown, developing irregularly polygonal plates, surrounded by short hyaline areas, in time filled with a compact mass of ascospores. Asci evanescent or no observed. Ascospores hyaline, 1-celled, reniform or allantoid, 4–5 × 1.7–2.5 μm, smooth-walled.
Notes: This species shares morphological features with M. lunisporas, but can be distinguished by the production of yellow soluble pigments on CMA and PDA, shorter conidiophores (3–28.5 × 1.5–2.5 μm vs 5–500 × 3–5 μm), smaller conidia (5.5–7.5 × 5.5–7.5 μm vs 6–11 μm) and ascospores (4–5 × 1.7–2.5 μm vs 6–7 × 2–2.5 μm).
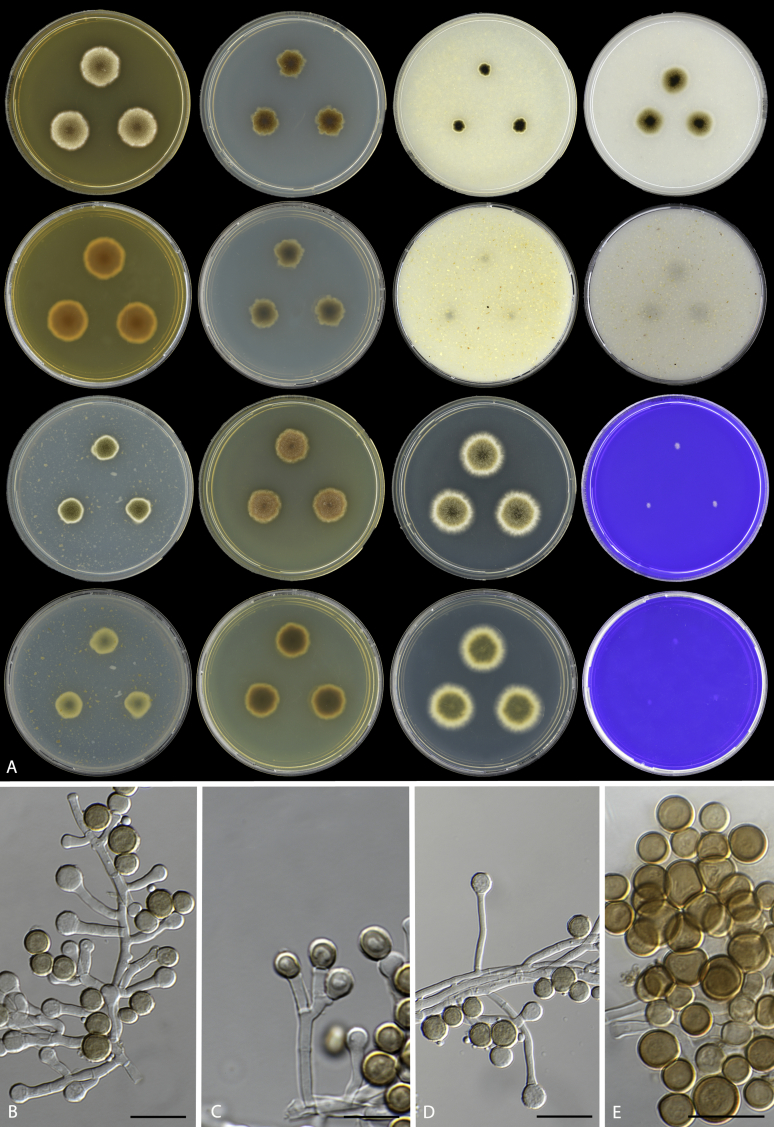
Fig. 7.
Monascus mellicola, URM 7510. A. Colonies from left to right (first row) MEA, CYA, OA, CMA; (second row) MEA reverse, CYA reverse, OA reverse, CMA reverse; (third row) PDA, YES, DG18, CREA; (forth row) PDA reverse, YES reverse, DG18 reverse, CREA reverse. B–E. Conidiophores with conidia chain. D. Conidia. Scale bars = 10 μm.
Etymology: mellicola refers to honey, the substrate from which the type species was isolated.
Diagnosis: Monascus mellicola is phylogenetically distinct by BenA, CaM and ITS sequencing, a sexual state is not observed in culture, and the species grows restricted on CREA incubated at 25 °C. No exudates and soluble pigments are produced on the agar media used in this study.
In: Monascus section Floridani
Typus: Brazil, Recife, honey from Melipona scutellaris Jun 2014, isolated by R.N. Barbosa (holotype URM 90065, culture ex-type URM 7510 = CBS 142364 = DTO 350-E6).
Barcodes: ITS barcode: KY511726 (alternative markers: BenA = KY709143; CaM = KY611904; RPB2 = KY611943).
Colony diam, 7 d (mm): MEA 11–12; CYA 8–10; CMA 9–10; PDA 9–10; YES 10–11; OA 9–10; DG18 7–10; CYAS No growth; CREA 5–7; CYA 30 °C 10–11; CYA 37 °C 6–8; MEA 30 °C 14–15; MEA 37 °C 5–6.
Description: Colonies characters after 7 d. MEA, 25 °C: colony texture floccose, raised in centre; mycelium white; sporulation strong, conidia en masse brown; exudates absent; soluble pigments absent, reverse brown. CYA, 25 °C: colony texture velvety, low; mycelium white, sometimes inconspicuously brown; sporulation weak, conidia en masse brown; exudates absent; soluble pigments absent, reverse dark brown at centre to brownish at margins. CMA, 25 °C: colony texture velvety, low; mycelium white sometimes inconspicuously greyish olive, sporulation moderate, conidia en masse brown; exudates absent; soluble pigments absent; reverse dark brown. PDA, 25 °C: colony texture velvety, low; mycelium white sometimes inconspicuously brown; sporulation strong, conidia en masse brown; exudates absent; soluble pigments absent; reverse brownish. YES, 25 °C: colony texture velvety to floccose, low; mycelium white; sporulation strong, conidia en masse brownish; exudates absent; soluble pigments absent; reverse dark brown. OA, 25 °C: colony texture velvety, low; mycelium white, sporulation weak, conidia en masse brown; exudates absent; soluble pigments absent, reverse brown. DG18, 25 °C: colony texture velvety to floccose, low, mycelium white; sporulation absent; exudates absent; soluble pigments absent; reverse white in the margins and dark brown at centre. CYAS, 25 °C: no growth. CREA, 25 °C: mycelium white, sporulation absent; no acid production. CYA, 30 °C: colony texture velvety to floccose, low; mycelium brown, sporulation weak, conidia en masse brownish; exudates absent; soluble pigments absent; reverse brown. CYA, 37 °C: mycelium white, sporulation absent; exudates absent; soluble pigments absent, reverse brown. MEA 30 °C: mycelium white, sporulation moderate to strong, conidia en masse in shades of brown; exudates absent; soluble pigments absent; reverse brownish. MEA, 37 °C: mycelium white, sporulation in centre, weak, conidia en masse in shades of brown; exudates absent; soluble pigments absent; reverse yellow-brownish.
Mycelium abundant, hyphae irregularly branched, hyaline to pale brown when old, smooth-walled, 2.5–3 μm wide. Conidiophores basipetospora-type, variable in length, smooth, 16–32 × 1.5–2.0 μm. Conidia formed basipetally, in long chains, up to 17 conidia, globose to subglobose, smooth-walled, 2.5–5.0 × 3.5–5.0 μm diam, hyaline when young, becoming pale brown to brown with age. Agglomeration of conidia with variable size observed, 45–65 × 55–65 μm diam. Sexual morph not observed.
Fig. 8.
Monascus recifensis, URM 7524. A. Colonies from left to right (first row) MEA, CYA, OA, CMA; (second row) MEA reverse, CYA reverse, OA reverse, CMA reverse; (third row) PDA, YES, DG18, CREA; (forth row) PDA reverse, YES reverse, DG18 reverse, CREA reverse. B–D. Conidiophores. E. Conidia. Scale bars = 10 μm.
Etymology: recifensis refers to the Brazilian city Recife, the location of the type strain of this species.
Diagnosis: Monascus recifensis is phylogenetically distinct by BenA, CaM and ITS sequencing. The species is characterized by restricted growth on agar media, a sexual state is not observed, and the species doesn't produce exudates and soluble pigments on the agar media used in this study.
In: Monascus section Floridani
Typus: Brazil, Recife, isolated from pollen inside nests of Melipona scutellaris Jun 2014, isolated by R.N. Barbosa, (holotype URM 90066; culture ex-type URM 7524 = CBS 142365 = DTO 350-G6).
Barcodes: ITS barcode: KY511740 (alternative markers: BenA = KY709157; CaM = KY611918; RPB2 = KY611957).
Colony diam, 7 d (mm): MEA 16–18; CYA 12–14; CMA 10–12; PDA 10–11; YES 14–15; OA 3–5; DG18 20–21; CYAS not growth; CREA 1–2; CYA 30 °C 13–15; CYA 37 °C 7–8; MEA 30 °C 17–20; MEA 37 °C 3–4.
Description: Colonies characters after 7 d. MEA, 25 °C: colony texture floccose to lanose, pulvinate, mycelium white, sporulation strong, conidia en masse brown; exudates absent; soluble pigments absent; reverse brownish. CYA, 25 °C: colony texture lanose, pulvinate; mycelium white sometimes inconspicuously brown; sporulation weak to moderate, conidia en masse brownish; exudates absent; soluble pigments absent; reverse dark brown to light brown close at margins. CMA, 25 °C: colony texture velvety, low; mycelium brown; sporulation moderate to strong at centre, conidia en masse dark brown; exudates absent; soluble pigments absent; reverse black. PDA, 25 °C: colony texture velvety to floccose; mycelium white; sporulation strong, en masse brownish; exudates absent; soluble pigments absent; reverse white to cream close at margins, dark brown at centre. YES, 25 °C: colony texture velvety, mycelium white sometimes inconspicuously brownish; sporulation strong, conidia en masse in shades of brown; exudates absent; soluble pigments present after 10 d. incubation, in shades of brown; reverse dark brown to light brown close at margins. OA, 25 °C: colony texture velvety; mycelium white; sporulation weak, conidia en masse dark brown; exudates absent; soluble pigments absent; reverse dark brown. DG18, 25 °C: colony floccose, mycelium white; sporulation weak to moderate, conidia en masse brownish; exudates absent; soluble pigments absent; reverse white close to margins and dark brownish at centre. CYAS, 25 °C: no growth. CREA, 25 °C: growth very poor. MEA, 30 °C: colony texture velvety; mycelium white; sporulation moderate to strong, conidia en masse brown; exudates absent; soluble pigments absent; reverse brownish. CYA, 30 °C: colony texture velvety, mycelium brownish; sporulation moderate, conidia en masse brown; exudates absent; soluble pigments absent; reverse dark brown, white at margins. MEA, 37 °C: colony texture velvety; mycelium white; sporulation absent; exudates absent; soluble pigments absent; reverse cream. CYA, 37 °C: colony texture velvety to floccose; mycelium brownish; sporulation moderate, conidia en masse in shades of brown; exudates absent; soluble pigments absent; reverse dark brown.
Mycelium abundant, hyphae irregularly branched, hyaline to pale brown when old, smooth-walled, 1.8–2.5 μm wide. Conidiophores variable in length, smooth, 4.5–21.0 × 1.8–2.5 μm, sometimes with additional branch, Conidia single, globose, 4.0–7.0 × 4.0–7.0 μm diam, at first hyaline, pale brown to brown with age. Sexual morph not observed after 60 d incubation.
Notes: Monascus lunisporas and M. flavipigmentosus are phylogenetically closely related to M. recifensis and the latter species doesn't produce ascomata, exudates and soluble pigments. These species can also be differentiated by their unique extrolite profiles (Table 4).
Penicillium eremophilum (A.D. Hocking & Pitt) Houbraken, Leong & Vinnere-Pettersson comb. nov. MycoBank MB820075.
Basionym: Monascus eremophilus A.D. Hocking & Pitt, Mycologia 80: 84. 1988. MycoBank MB132383.
Typus: Australia, New South Wales, Sydney, isolated from mouldy prunes, isolated by A.D. Hocking, 1986 (Herb.: FRR 3338; Ex-type: IMI 313774 = CBS 123361 = ATCC 62925).
Barcodes: ITS barcode: GU733347 (alternative markers: BenA = KY709170; CaM = KY611931; RPB2 = KY611970).
Notes: The colony morphology was identical to that described by Hocking and Pitt in 1998. Monascus eremophilus is indeed an obligate xerophile. No growth was observed on either MEA or MA20S at any temperature after incubation of one year. Monascus eremophilus grew well on MY50G within the range 10–25 °C. Good growth at 30 °C and absence of growth at 37 °C has been previously reported (Leong et al. 2011). Upon microscopy, ascomatal initials were observed after approximately a month of cultivation. However, these cleistothecia never matured and thus no ascospores were observed. No anamorph was observed during the time of cultivation or mentioned in the original description. The fact that we did not observe any fertile cleistothecia may indicate that the type strain (FRR 3338) is deteriorating. Molecular data shows that this species is related to Penicillium (Park et al., 2004, Vinnere-Pettersson et al., 2011, Houbraken et al., 2014) and is transferred to Penicillium (this study).
List of accepted species in Monascus
Monascus argentinensis Stchigel & Guarro, Stud. Mycol. 50: 301. 2004. [MB500076]. — Herb.: FMR 6778. Ex-type: CBS 109402 = FMR 6778. Section Floridani. ITS barcode: JF922046 (Alternative markers: BenA = KY709174; CaM = KY611935; RPB2 = JN121423).
Monascus flavipigmentosus R.N. Barbosa, Souza-Motta, N.T. Oliveira & Houbraken (this study). [MB820072]. — Herb.: URM 90064. Ex-type: URM 7536 = CBS 142366 = DTO 353-A2. Section Floridani. ITS barcode: KY511751 (Alternative markers: BenA = KY709168; CaM = KY611929; RPB2 = KY611968).
Monascus floridanus P.F. Cannon & E.L. Barnard, Mycologia 79: 480. 1987. [MB132123]. — Herb.: IMI 282587. Ex-type: FLAS F54662 = CBS 142228 = CGMCC 3.5843 = BCRC 33310 = UAMH 4180. Section Floridani. ITS barcode: KY635848 (Alternative markers: BenA = KY709172; CaM = KY611933; RPB2 = KY611972).
Monascus lunisporas Udagawa & H. Baba, Cryptogamie Mycol 19: 270. 1998. [MB446999]. — Herb.: SUM 3116. Ex-type: CBS: 142230 = CGMCC 3.7951 = ATCC 204397 = NBRC 33241 = BCRC 33640. Section Floridani. ITS barcode: KY635847 (Alternative markers: BenA = KY709171; CaM = KY611932; RPB2 = KY611971).
Monascus mellicola R.N. Barbosa, Souza-Motta, N.T. Oliveira & Houbraken (this study). [MB820073]. — Herb.: URM 90065. Ex-type: URM 7510 = CBS 142364 = DTO 350-E6. Section Floridani. ITS barcode: KY511726 (Alternative markers: BenA = KY709143; CaM = KY611904; RPB2 = KY611943).
Monascus pallens P.F. Cannon, Abdullah & B.A. Abbas, Mycol. Res. 99: 659. 1995. [MB413476]. — Herb.: IMI 356820. Ex-type: BSRA 10266 = CBS 142229 = CGMCC 3.5844 = ATCC 200612 = BCRC 33641. Section Floridani. ITS barcode: KY635849 (Alternative markers: BenA = KY709173; CaM = KY611934; RPB2 = KY611973).
Monascus purpureus Went, Ann. Sci. Nat., Bot. Ser. 8, 1, 1–18. 1895. [MB235390]. — Herb.: IMI 210765. Ex-type: CBS 109.07 = IF0 45 13 = ATCC 16426 = NRRL 1596 = FRR 1596. Section Rubri. ITS barcode: KY635851 (Alternative markers: BenA = KY709176; CaM = KY611937; RPB2 = JN121422).
Monascus recifensis R.N. Barbosa, Souza-Motta, N.T. Oliveira & Houbraken (this study). [MB820074]. — Herb.: URM 90066. Ex-type: URM 7524 = CBS 142365 = DTO 350-G6. Section Floridani. ITS barcode: KY511740 (Alternative markers: BenA = KY709157; CaM = KY611918; RPB2 = KY611957).
Monascus ruber Tiegh, Bull. Soc. Bot. France. 31: 227. 1884. [MB234876]. — Herb.: IMI 81596. Ex-type: CBS 135.60 = IFO 8451 = ATCC 15670. Section Rubri. ITS barcode: KY635850 (Alternative markers: BenA = KY709175; CaM = KY611936; RPB2 = KY611974).
Overview and status of Basipetospora species
Basipetospora chlamydospora Matsush., Icones Microfungorum a Matsushima lectorum 13. 1975. [MB309463]. — Herb.: MFC 2307. Ex-type: CBS 228.84 = MFC 2409. 18S rDNA: AB024045. Note: BLAST analysis of the 18S rDNA sequences shows that this species belongs to Microascales.
Basipetospora denticola (C. Moreau) C. Moreau, Bull. Soc. Mycol. France 87: 43. 1971. nom. inval., (Art. 6.10, 41.1 & 41.5) [MB309464]. Basionym: Chrysosporium keratinophilum var. denticola C. Moreau [as ‘denticolum’], Mycopathologia et Mycologia Applicata. 37: 37. 1969. nom. inval., (Art. 39.1 & 40.1) [MB353354]. — Herb.: n/a. Representative culture: CBS 132.78. ITS barcode: LN850801. Note: Basipetospora denticola is based on the invalidly described species C. keratinophilum var. denticola. A representative culture of B. denticola (CBS 132.78) belongs to Microascales and is a synonym of Scopulariopsis candida (Jagielski et al. 2016).
Basipetospora halophila (J.F.H. Beyma) Pitt & A.D. Hocking, Mycotaxon 22: 198. 1985. [MB105087]. Basionym: Oospora halophila J.F.H. van Beyma Zentralblatt für Bakteriologie und Parasitenkunde Abteilung, Abt. II 88: 134. 1933. [MB266778]. — Herb.: n/a. Representative culture: CBS 232.32 = VKM F-204. Note: This species was formerly described as Oospora halophila by van Beyma (1933) and was recently transferred to Aspergillus under the new name A. baarnensis (Samson et al., 2014, Kocsubé et al., 2016).
Basipetospora rubra G.T. Cole & W.B. Kendr., Canadian Journal of Botany 46: 991. 1968. [MB326938]. — Herb.: ATCC 18199. Ex-type: FRR 2452. Note: The herbarium and ex-type culture of B. rubra and M. ruber differ. Basipetospora rubra was described as the asexual state of M. ruber and is in the single name nomenclature system regarded as a synonym of this species.
Basipetospora variabilis Matsush., Icones Microfungorum a Matsushima lectorum 13. 1975. [MB309465]. — Herb.: MFC 2428. Ex-type: CBS 995.87. 18S rDNA: AF437892. Note: Comparison of the publically available 18S rDNA sequence on GenBank shows that this species belongs to Microascales.
Basipetospora vesicarum (Link) Stalpers, Studies in Mycology 24: 91. 1984. [MB106627]. — Herb.: n/a. Ex-type: n/a. Note: This fungus was originally described as Sporotrichum vesicarum by Link (Sprengel et al. 1818). Stalpers (1984) examined a herbarium specimen from B and this specimen contained the anamorph of M. ruber, which he named B. vesicarum. This species is tentatively placed in synonymy with M. ruber.
Acknowledgements
We would like to thank National Council for Scientific and Technological Development (CNPq) (Process 201478/2015-3 – SWE) and Coordination for the Improvement of Higher Education Personnel (CAPES) for financial support and scholarship for R.N. Barbosa and Associação Pernambucana de Apicultores e Meliponicultores (APIME) is thanked for their help in collecting the honey/pollen samples. We would like to acknowledge Martin Meijer, Xuewei Wang and Jadson Bezerra for their support and Konstanze Bench for nomenclatural assistance.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.simyco.2017.04.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Arx J.A. von. A re-evaluation of the Eurotiales. Persoonia. 1987;13:273–300. [Google Scholar]
- Barnard E.L., Cannon P.F. A new species of Monascus from pine tissues in Florida. Mycologia. 1987;79:479–484. [Google Scholar]
- Benny G.L., Kimbrough J.W. Synopsis of the orders and families of Plectomycetes with keys to genera. Mycotaxon. 1980;12:1–91. [Google Scholar]
- Berbee M.L., Yoshimura A., Sugiyama J. Is Penicillium monophyletic? An evaluation of phylogeny in the family Trichocomaceae from 18S, 5.8S and ITS ribosomal DNA sequence data. Mycologia. 1995;87:210–222. [Google Scholar]
- Beyma F.H. van. Beschreibung einiger neuer Pilzarten aus dem Centraalbureau voor Schimmelcultures – Baarn (Holland) Zentralblatt für Bakteriologie und Parasitenkunde Abteilung. 1933;88:132–141. [Google Scholar]
- Blanc P.J., Loret M.O., Goma G. Production of citrinin by various species of Monascus. Biotechnology Letters. 1995;17:291–294. [Google Scholar]
- Cannon P.F., Abdullah S.K., Abbas B.A. Two new species of Monascus from Iraq, with a key to known species of the genus. Mycological Research. 1995;99:659–662. [Google Scholar]
- Chang J.M., Di Tommaso P., Lefort V. TCS: a web server for multiple sequence alignment evaluation and phylogenetic reconstruction. Nucleic Acids Research. 2015;43:W3–W6. doi: 10.1093/nar/gkv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.J., Sun B.D., Houbraken J. New Talaromyces species from indoor environments in China. Studies in Mycology. 2016;84:119–144. doi: 10.1016/j.simyco.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G.T., Samson R.A. Pitman; London: 1979. Patterns of development in conidial fungi. [Google Scholar]
- Cortopassi-Laurino M., Imperatriz-Fonseca V.L., Roubik D.W. Global meliponiculture: challenges and opportunities. Apidologie. 2006;37:275–292. [Google Scholar]
- Dietrich R., Usleber E., Martlbauer E. Detection of the nephrotoxic mycotoxin citrinin in foods and food colorants derived from Monascus spp. Archiv für Lebensmittelhygiene. 1999;50:17–21. [Google Scholar]
- Gams W., Christensen M., Onions A.H. Infrageneric taxa of Aspergillus. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus systematics. Plenum Press; New York: 1985. pp. 55–62. [Google Scholar]
- Hawksworth D.L., Pitt J.I. A new taxonomy for Monascus species based on cultural and microscopical characters. Australian Journal of Botany. 1983;31:51–61. [Google Scholar]
- Hocking A.D., Pitt J.I. Two new species of xerophilic fungi and a further record of Eurotium halophilicum. Mycologia. 1988;80:82–88. [Google Scholar]
- Houbraken J., de Vries R.P., Samson R.A. Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Advances in Applied Microbiology. 2014;86:199–249. doi: 10.1016/B978-0-12-800262-9.00004-4. [DOI] [PubMed] [Google Scholar]
- Houbraken J., Samson R.A. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology. 2011;70:1–51. doi: 10.3114/sim.2011.70.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J., Spierenburg H., Frisvad J.C. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie Van Leeuwenhoek. 2012;101:403–421. doi: 10.1007/s10482-011-9647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W.-H., Pan T.-M. Monascus purpureus-fermented products and oral cancer: a review. Applied Microbiology and Biotechnology. 2012;93:1831–1842. doi: 10.1007/s00253-012-3891-9. [DOI] [PubMed] [Google Scholar]
- Huang Z.-B., Li Y.-P., Wang Y.-H. Studies on citrinin and pigments in fermented rice of Monascus aurantiacus (As3.4384) and its mutant strains by high performance liquid chromatography. Chinese Journal of Analytical Chemistry. 2007;35:474–478. [Google Scholar]
- Iriart X., Fior A., Blanchet D. Monascus ruber: invasive gastric infection caused by dried and salted fish consumption. Journal of Clinical Microbiology. 2010;48:3800–3802. doi: 10.1128/JCM.01000-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffé R., Pope N., Carvalho A.T. Bees for development: Brazilian survey reveals how to optimize stingless beekeeping. PLoS ONE. 2015;10:e0121157. doi: 10.1371/journal.pone.0121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagielski T., Sandoval-Denis M., Yu J. Molecular taxonomy of scopulariopsis-like fungi with description of new clinical and environmental species. Fungal Biology. 2016;120:586–602. doi: 10.1016/j.funbio.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Jůzlova P., Martinkova L., Kren V. Secondary metabolites of the fungus Monascus: a review. Journal of Industrial Microbiology. 1996;16:163–170. [Google Scholar]
- Katoh K., Kuma K., Toh H. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Kim H.-J., Oh J.-H. Characteristics of Monascus sp. isolated from Monascus fermentation products. Food Science and Biotechnology. 2010;19:1151–1157. [Google Scholar]
- Klitgaard A., Iversen A., Andersen M.R. Aggressive dereplication using UHPLC-DAD-QTOF – screening extracts for up to 3000 fungal secondary metabolites. Analytical and Bioanalytical Chemistry. 2014;406:1933–1943. doi: 10.1007/s00216-013-7582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsubé S., Perrone G., Magistà D. Aspergillus is monophyletic: evidence from multiple gene phylogenies and extrolites profiles. Studies in Mycology. 2016;85:199–213. doi: 10.1016/j.simyco.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-L., Pan T.-M. Red mold fermented products and Alzheimer's disease: a review. Applied Microbiology and Biotechnology. 2011;91:461–469. doi: 10.1007/s00253-011-3413-1. [DOI] [PubMed] [Google Scholar]
- Lee C.-L., Pan T.-M. Benefit of Monascus-fermented products for hypertension prevention: a review. Applied Microbiology and Biotechnology. 2012;94:1151–1161. doi: 10.1007/s00253-012-4076-2. [DOI] [PubMed] [Google Scholar]
- Leong S.L., Vinnere-Pettersson O., Rice T. The extreme xerophilic mould Xeromyces bisporus – growth and competition at various water activities. International Journal of Food Microbiology. 2011;145:57–63. doi: 10.1016/j.ijfoodmicro.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhou Y.-C., Yang M.-H. Natural occurrence of citrinin in widely consumed traditional Chinese food red yeast rice, medicinal plants and their related products. Food Chemistry. 2012;132:1040–1045. [Google Scholar]
- Li Y.-P., Tang X.C., Wu W. The ctnG gene encodes carbonic anhydrase involved in mycotoxin citrinin biosynthesis from Monascus aurantiacus. Food Additives & Contaminants: Part A. 2015;32:577–583. doi: 10.1080/19440049.2014.990993. [DOI] [PubMed] [Google Scholar]
- Li Z.Q., Guo F. A further studies on the species of Monascus. Mycosystema. 2004;23:1–6. [Google Scholar]
- Maddison W.P., Maddison D.R. 2016. Mesquite: a modular system for evolutionary analysis.http://mesquiteproject.org Version 3.11. [Google Scholar]
- McNeill J., Barrie F.R., Buck W.R., editors. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum Vegetabile 154. Koeltz Scientific Books; Königstein: 2012. [Google Scholar]
- Menezes C., Vollet-Neto A., Marsaioli A.J. A Brazilian social bee must cultivate fungus to survive. Current Biology. 2015;25:2851–2855. doi: 10.1016/j.cub.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Moreau C. Presence du Monascus purpureus Went dans du mais ensile. Remarques sur la forme imperfecte Basipetospora Cole et Kendrick. Bulletin trimestriel de la Société mycologique de France. 1971;87:39–44. [Google Scholar]
- Negishi S., Huang Z.-C., Hasumi K. Productivity of monacolin K (mevinolin) in the genus Monascus. Hakko Kogaku Kaishi. 1986;64:584–590. (in Japanese) [Google Scholar]
- Nielsen K.F., Månsson M., Rank C. Dereplication of microbial natural products by LC-DAD-TOFMS. Journal of Natural Products. 2011;74:2338–2348. doi: 10.1021/np200254t. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Sugiyama J. Evolutionary relationships of the cleistothecial genera with Penicillium, Geosmithia, Merimbla and Sarophorum anamorphs as inferred from 18S rDNA sequence divergence. In: Samson R.A., Pitt J.I., editors. Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Plenum Press; New York: 2000. pp. 149–161. [Google Scholar]
- Ogawa H., Yoshimura A., Sugiyama J. Polyphyletic origins of species of the anamorphic genus Geosmithia and the relationships of the cleistothecial genera: evidence from 18S, 5S and 28S rDNA sequence analyses. Mycologia. 1997;89:756–771. [Google Scholar]
- Park H.G., Jong S.C. Molecular characterization of Monascus strains based on the D1/D2 regions of LSU rRNA genes. Mycoscience. 2003;44:25–32. [Google Scholar]
- Park H.G., Stamenova E.K., Jong S.C. Phylogenetic relationships of Monascus species inferred from the ITS and the partial beta-tubulin gene. Botanical Bulletin of Academia Sinica. 2004;45:325–330. [Google Scholar]
- Pattangul P., Pinthong R., Phianmongkhol Mevinolin, citrinin and pigments of adlay angkak fermented by Monsacus sp. International Journal of Food Microbiology. 2008;126:20–23. doi: 10.1016/j.ijfoodmicro.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Peterson S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia. 2008;100:205–226. doi: 10.3852/mycologia.100.2.205. [DOI] [PubMed] [Google Scholar]
- Pisareva E., Savov V., Kujumdzieva A. Pigments and citrinin biosynthesis of fungi belonging to genus Monascus. Zeitschrift für Naturforschung. 2005;60c:116–120. doi: 10.1515/znc-2005-1-221. [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Rambaut A. 2009. FigTree v. 1.3.1.http://tree.bio.ed.ac.uk/software/ Computer program and documentation distributed by the author at. [Google Scholar]
- Ronquist F., Teslenko M., van derMark P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman A.Y., Allen W.C., Braun U. Overlooked competing asexual and sexually typified generic names of Ascomycota with recommendations for their use or protection. IMA Fungus. 2016;7:289–308. doi: 10.5598/imafungus.2016.07.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R.A., Houbraken J., Thrane U. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2010. Food and indoor fungi. (CBS Laboratory manual series; No. 2). [Google Scholar]
- Samson R.A., Visagie C.M., Houbraken J. Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Seifert K.A., Huhndorf S. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Xu L., Chen F. Genetic diversity analysis of Monascus strains using SRAP and ISSR markers. Mycoscience. 2011;52:224–233. [Google Scholar]
- Shi Y.-C., Pan T.-M. Red mold, diabetes, and oxidative stress: a review. Applied Microbiology and Biotechnology. 2012;94:47–55. doi: 10.1007/s00253-012-3957-8. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kinoshita H., Ishihara S. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Applied and Environmental Microbiology. 2005;71:3453–3457. doi: 10.1128/AEM.71.7.3453-3457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsgaard J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. Journal of Chromatography A. 1997;760:264–270. doi: 10.1016/s0021-9673(96)00803-5. [DOI] [PubMed] [Google Scholar]
- Sprengel K., Schrader A.H., Link H.F. G.C. Nauck's Buchhandlung; Berlin und Leipzig, Germany: 1818. Jahrbücher der Gewächskunde 1. [Google Scholar]
- Stalpers J.A. A revision of the genus Sporotrichum. Studies in Mycology. 1984;24:1–105. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stchigel A.M., Cano J.F., Abdullah S.K. New and interesting species of Monascus from soil, with a key to the known species. Studies in Mycology. 2004;50:299–306. [Google Scholar]
- Stchigel A.M., Guarro J. A reassessment of cleistothecia as a taxonomic character. Mycological Research. 2007;111:1100–1115. doi: 10.1016/j.mycres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieghem P. van. Monascus, genre nouveau de l'ordre des Ascomycétes. Bulletin De La Société Botanique De France. 1884;31:226–231. [Google Scholar]
- Udagawa S., Baba H. Monascus lunisporas, a new species isolated from mouldy feeds. Cryptogamie Mycologie. 1998;19:269–276. [Google Scholar]
- Vellinga E.C., Kuyper T.W., Ammirati J. Six simple guidelines for introducing new genera of fungi. IMA Fungus. 2015;6:65–68. [Google Scholar]
- Vendruscolo F., Schmidell W., Ninow J.L. Antimicrobial activity of Monascus pigments produced in submerged fermentation. Journal of Food Processing and Preservation. 2014;38:1860–1865. [Google Scholar]
- Villanueva-Gutiérrez R., Roubik D.W., Colli-Ucán W. A critical view of colony losses in managed Mayan honey-making bees (Apidae: Meliponini) in the heart of Zona Maya. Journal of the Kansas Entomological Society. 2013;86:352–362. [Google Scholar]
- Vinnere-Pettersson O., Leong S.L., Lantz H. Phylogeny and intraspecific variation of the extreme xerophile, Xeromyces bisporus. Fungal Biology. 2011;115:1100–1111. doi: 10.1016/j.funbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Visagie C.M., Houbraken J., Frisvad J.C. Identification and nomenclature of the genus Penicillium. Studies in Mycology. 2014;78:343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.J., Lee C.L., Pan T.M. Improvement of monacolin K, γ-aminobutyric acid and citrinin production ratio as a function of environmental conditions of Monascus purpureus NTU 601. Journal of Industrial Microbiology and Biotechnology. 2003;30:669–676. doi: 10.1007/s10295-003-0097-2. [DOI] [PubMed] [Google Scholar]
- Wang Y.Z., Ju X.L., Zhou Y.G. The variability of citrinin production in Monascus type cultures. Food Microbiology. 2005;22:145–148. [Google Scholar]
- Wynns A.A. Convergent evolution of highly reduced fruiting bodies in Pezizomycotina suggests key adaptations to the bee habitat. BMC Evolutionary Biology. 2015;15:145. doi: 10.1186/s12862-015-0401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz N., Visagie C.M., Houbraken J. Polyphasic taxonomy of the genus Talaromyces. Studies in Mycology. 2014;78:175–341. doi: 10.1016/j.simyco.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.