Abstract
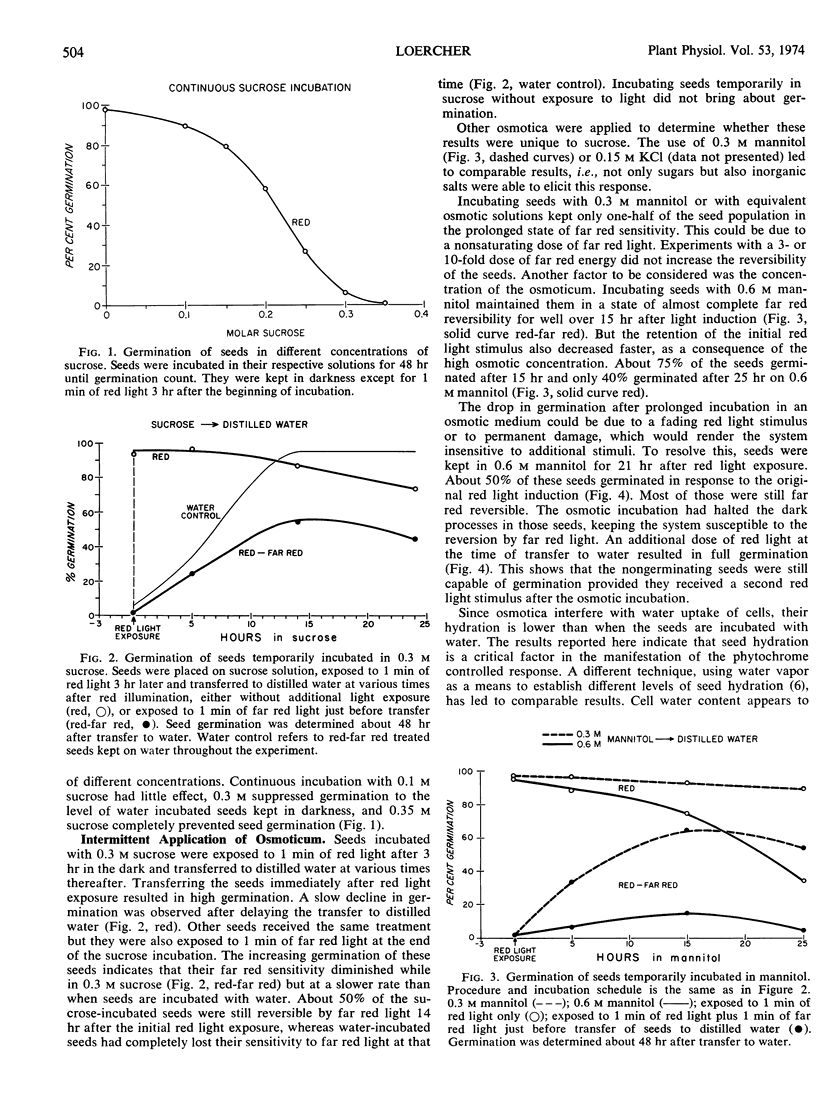
Incubation of lettuce seeds (Lactuca sativa L. cv. Grand Rapids) in 0.3 m mannitol allows sufficient water uptake to make seeds fully sensitive to red light. But germination is possible only after lowering the osmotic potential of the incubation medium. The red light induction of these incompletely hydrated seeds can be reversed by far red light. Their reversibility declines with time at a slower rate than seeds incubated in water. About half the seeds in 0.3 m mannitol respond to far red light when all seeds in water have escaped control by far red light. Close to 100% of the seeds remain sensitive to far red exposure if 0.6 m mannitol is used as osmoticum. The retention of the original red light stimulus is inversely related to the concentration of the incubation medium.
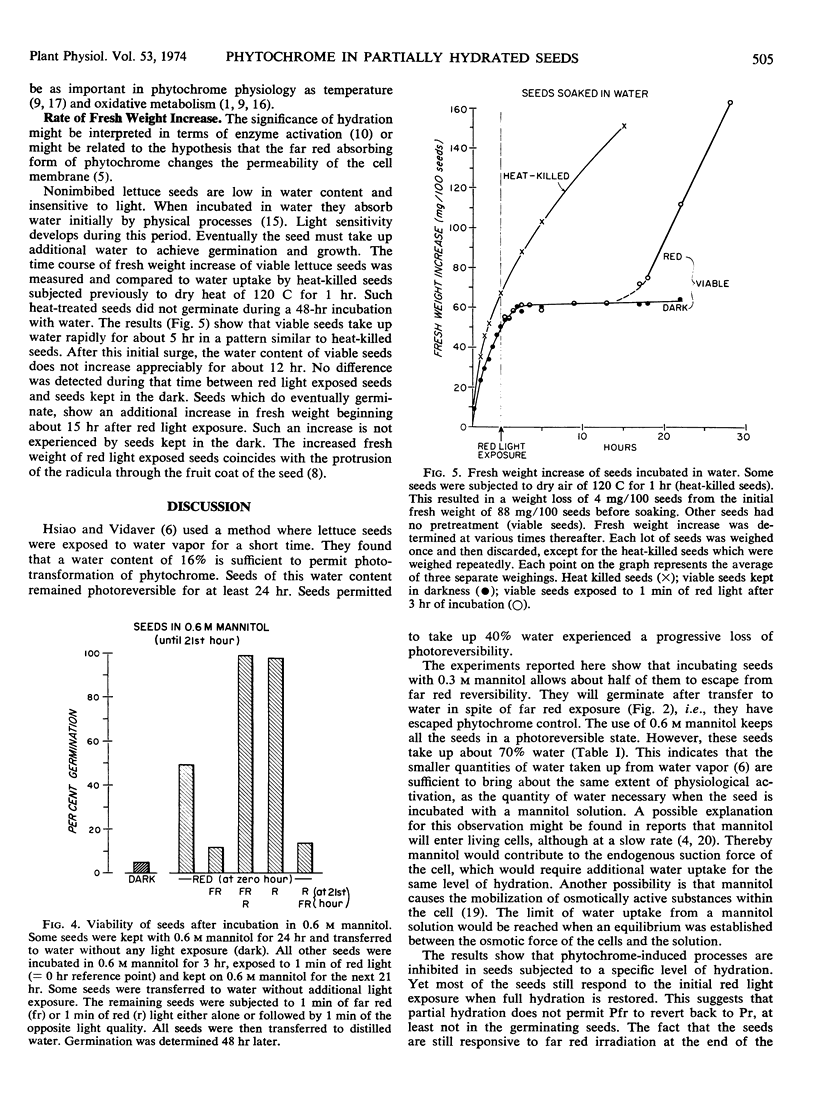
The fresh weight of viable seeds incubated with water or with an osmoticum increases rapidly during the first 5 hours, then remains stationary for about 12 hours. After that only germinating seeds experience a second increase in fresh weight. Heat-killed seeds do not show such a discontinuity in water uptake.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Norris K. H., Siegelman H. W., Hendricks S. B. DETECTION, ASSAY, AND PRELIMINARY PURIFICATION OF THE PIGMENT CONTROLLING PHOTORESPONSIVE DEVELOPMENT OF PLANTS. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1703–1708. doi: 10.1073/pnas.45.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner W. R., Nieman R. H. Lower Limit of Water Availability to Plants. Science. 1964 Mar 27;143(3613):1460–1462. doi: 10.1126/science.143.3613.1460. [DOI] [PubMed] [Google Scholar]
- Greenway H. Effects of slowly permeating osmotica on metabolism of vacuolated and nonvacuolated tissues. Plant Physiol. 1970 Aug;46(2):254–258. doi: 10.1104/pp.46.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks S. B., Borthwick H. A. The function of phytochrome in regulation of plant growth. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2125–2130. doi: 10.1073/pnas.58.5.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao A. I., Vidaver W. Water Content and Phytochrome-induced Potential Germination Responses in Lettuce Seeds. Plant Physiol. 1971 Feb;47(2):186–188. doi: 10.1104/pp.47.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Armstrong J. E. Evidence for osmotic regulation of hydrolytic enzyme production in germinating barley seeds. Plant Physiol. 1971 Aug;48(2):137–142. doi: 10.1104/pp.48.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick R. E., Hillman W. S. Dark Reversion of Phytochrome in Sinapis alba L. Plant Physiol. 1970 Oct;46(4):596–598. doi: 10.1104/pp.46.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. O. Persistent photoreversibility of leaf development. Plant Physiol. 1969 Jun;44(6):897–902. doi: 10.1104/pp.44.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur J. A., Briggs W. R. In vivo phytochrome reversion in immature tissue of the alaska pea seedling. Plant Physiol. 1971 Jul;48(1):46–49. doi: 10.1104/pp.48.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylorson R. B., Hendricks S. B. Action of Phytochrome During Prechilling of Amaranthus retroflexus L. Seeds. Plant Physiol. 1969 Jun;44(6):821–825. doi: 10.1104/pp.44.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylorson R. B., Hendricks S. B. Rehydration of Phytochrome in Imbibing Seeds of Amaranthus retroflexus L. Plant Physiol. 1972 Apr;49(4):663–665. doi: 10.1104/pp.49.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Loos G. M., Samuel E. W. Penetration of Mannitol into Potato Discs. Plant Physiol. 1960 Nov;35(6):848–853. doi: 10.1104/pp.35.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]


