Introduction
Psoriasis is a common chronic disease and may be the most prevalent autoimmune disease. The estimated prevalence is 2% to 4% of the global population.1 Fortunately, providers have a plethora of medications to choose from, but even so, some cases can be very difficult to treat. Herein, we present a challenging case of palmoplantar pustulosis primarily affecting the soles of the feet. Successful treatment requires use of a novel combination of biologic therapy. To our knowledge, this is the first published case highlighting adalimumab and ustekinumab in combination for the treatment of severe palmoplantar pustulosis.
Case report
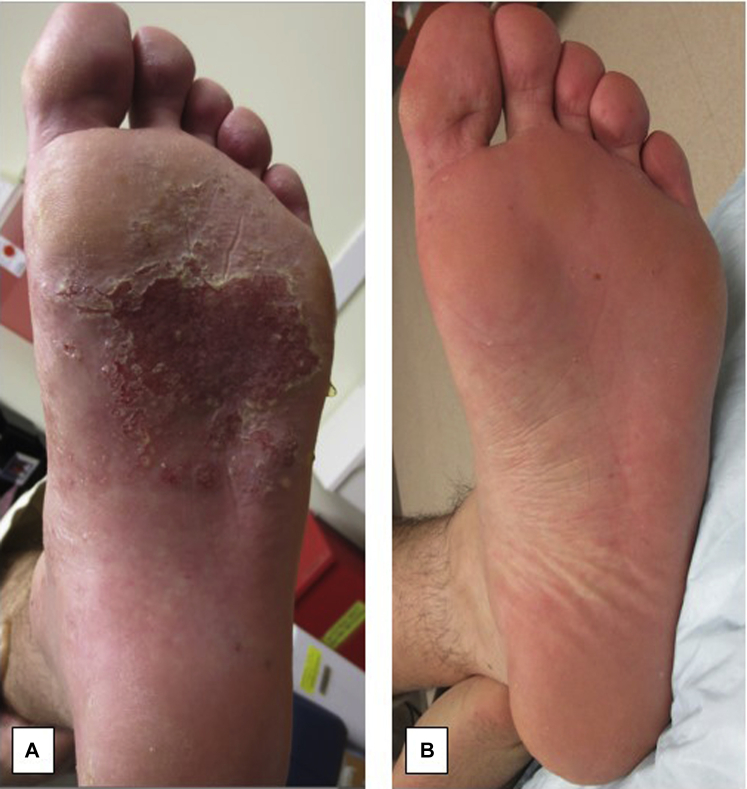
A 33-year-old, 170-pound fit, white male auto mechanic presented with severe palmoplantar inflammation characterized by deep-seated pustules, discrete vesicles, and weeping, eroded plaques within a background of erythema and scale (Fig 1, A). The feet were significantly more involved than the hands. Aside from left great toe dactylitis, no other skin or nail findings were present. At the time of presentation, the differential diagnosis favored palmoplantar pustular psoriasis but included dyshidrotic eczema, allergic contact dermatitis, and dermatitis secondary to hyperhidrosis. Initial treatment interventions included topical steroids, narrow-band ultraviolet B therapy, and oral prednisone (maximum of 60 mg/d), but all failed to provide improvement. Botox injections, to treat underlying hyperhidrosis, were attempted but could not be tolerated. Subsequently, cyclosporine, 150 mg twice daily (∼4 mg/kg/d), and methotrexate, 10 mg weekly, were initiated, resulting in 85% to 90% disease clearance after 4 weeks. Despite his initial response, we were unable to reduce the dosage of cyclosporine. Addition of mycophenolate (1 g twice daily) was also inadequate to taper the cyclosporine dose. Consequently, we opted for a trial of adalimumab, 40 mg, initially taken subcutaneously every other week. Methotrexate was discontinued on initiation of adalimumab; however, mycophenolate was continued. After 4 months, the adalimumab was increased to 40 mg weekly, and the patient was on this dose, along with mycophenolate, for 6 months. Although this treatment helped, it did not clear his condition nor did it facilitate tapering of cyclosporine. Finally, we opted to add the higher dosing regimen of ustekinumab, 90 mg, to his treatment plan (day 1, day 28, then every 3 months) given the severity of disease. The addition of ustekinumab facilitated the removal of all other immunosuppressants by the third dose with the exception of adalimumab. After 3 doses of ustekinumab in combination with adalimumab (40 mg weekly), the patient obtained 95% clearance and has remained clear for more than 4 months (Fig 1, B). Importantly, no adverse effects occurred.
Fig 1.
Severe palmoplantar pustulosis before and after combination treatment. A, Before: Severe palmoplantar inflammation with vesicles and eroded plaque on the left sole B, After: Clear left sole after treatment with 3 doses of ustekinumab in combination with adalimumab.
Discussion
Palmoplantar pustulosis, felt by many to be a variant of psoriasis, is a complex disorder that is often recalcitrant to single-agent therapy. It is likely that the pathophysiologies of psoriasis and palmoplantar pustulosis overlap and result from a combination of genetic, epigenetic, and environmental factors leading to unregulated hyperproliferation of keratinocytes.2 Traditional therapies including corticosteroids, systemic immunosuppressants, and phototherapy can be effective in treating palmoplantar pustulosis, including palmoplantar psoriasis and many of the inflammatory conditions in our patient's differential diagnosis. In our case, however, topical therapies and phototherapy were ineffective. Systemic agents, including methotrexate, mycophenolate, and cyclosporine (noteworthy in combination) were effective but insufficient in facilitating tapering of cyclosporine, which over time can lead to hypertension and renal toxicity.
Unlike the above therapies, biologic therapies target individual cytokines and pro-inflammatory proteins. Given the inconclusiveness of the diagnosis in our case, biologic therapies were only considered after multiple more broadly effective therapies failed. Even among the biologics, options abound, and our choice to start with adalimumab was not done without reason. Tumor necrosis factor-α has a role as a regulator of the interleukin (IL)-23/T-helper-17 axis in that it promotes dendritic cell production of proinflammatory IL-23, and thus has a broader inflammatory target compared with ustekinumab, which only targets cytokines primarily derived from dendritic cells, namely IL-12 and IL-23.1, 3
Although adalimumab kept our patient's condition stable, it did not provide full relief or adequately manage his condition, as he was still cyclosporine dependent. It was at this point that ustekinumab was added as a therapy. Despite limited literature on the safety of combination biologic therapy,4 the concept of adding a biologic in another class is logical, as targeting multiple components of the inflammatory cascade might be therapeutically superior. Indeed, it was this combination therapy that afforded our patient near complete clearance and a sustained response.
When deciding whether to initiate any combination therapy, in particular, combinations of biologic agents, both safety and cost are important factors to consider. In addition to the potential for risks associated with individual agents, such as malignancy and infection, other yet unknown adverse effects could occur. Thus, close monitoring, including more frequent follow-up visits and additional laboratory tests, may be warranted. In our case, after starting the therapy, we regularly communicated with the patient to assess his tolerability to the medication including any adverse effects, and none were reported.
Cost is obviously also a significant hurdle when deciding on combination therapies. All of the newer biologic therapies are expensive.5 It is important to note, however, that patients with severe inflammatory skin disease accumulate costs related to loss of productivity and missed workdays because of the daily impact disease has on their lives. Costly yet effective drug treatments also may have the potential to reduce long-term overall cost through reduction in other aspects such as laboratory monitoring and office visits.6
A limitation of our case is the lack of a skin biopsy. Because the patient was on multiple therapies, he preferred not to suspend treatment solely for diagnostic confirmation. Additionally, the patient's insurance afforded us the ability to use multiple biologics, a luxury likely not applicable to many patients at this time.
As the pathophysiology of psoriasis becomes more fully understood, new therapeutic agents and combinations of existing therapies are likely to become options for refractory cases. In this case, we found successful treatment with combination therapy using adalimumab and ustekinumab. Although long-term safety and cost are strong considerations, this approach may be an option for other patients with similar or more extensive palmoplantar disease resistant to standard treatments.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Payette has been an adviser to Amgen, Abbvie, and Eli Lilly. Ms Torre declares no conflicts of interest.
References
- 1.Nestle F.O., Kaplan D.H., Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Deng Y., Chang C., Lu Q. The inflammatory response in psoriasis: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:377–389. doi: 10.1007/s12016-016-8535-x. [DOI] [PubMed] [Google Scholar]
- 3.Zaba L.C., Cardinale I., Gilleaudeau P. Amelioration of epidermal hyperplasia by TNF inhibition Is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babalola O., Lakdawala N., Strober B.E. Combined biologic therapy for the treatment of psoriasis and psoriatic arthritis: A case report. JAAD Case Rep. 2015;1:3–4. doi: 10.1016/j.jdcr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J., Feldman S.R. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212–266. doi: 10.7573/dic.212266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Souza L.S., Payette M.J. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2014;72:589–598. doi: 10.1016/j.jaad.2014.11.028. [DOI] [PubMed] [Google Scholar]