Abstract
OBJECTIVE: We investigated the efficacy of early lung cancer screening with low-dose spiral computed tomography(LDCT) in both smokers and nonsmokers based on the current situation of community health service, with integration of superior resources of medical institutions at all levels in Shanghai. METHODS: From August 2013 to August 2014, we screened 11,332 (male 7144; female 4188) high-risk individuals in selected communities of Minhang, Shanghai City, for early diagnosis of lung cancer with LDCT combined with multidisciplinary comprehensive treatment pattern including minimally invasive surgery, exploring the medical service network covering prevention, diagnosis, treatment, rehabilitation, and follow-up. RESULTS: Screening resulted in a diagnosis of cancer in 29 participants. Of these participants, 27 had primary lung cancer, 1 had lung metastatic cancer, and 1 had breast cancer. The detection rate of primary lung cancer was 238.26 cases per 100,000 person-years among all the participants. Specifically, the incidence of primary lung cancer was 336.97 cases per 100,000 person-years among the nonsmoking participants, as compared with 159.06 cases per 100,000 person-years among the smoking participants (P = .054). Among the 27 primary lung cancers, 22 (81.48%) had stage 0 to I lung cancer. CONCLUSION: Based on community health service, screening with LDCT could improve the early diagnosis rate of lung cancer in both smokers and nonsmokers with feasibility and validity, which could be applicable in qualified eligible medical centers and communities in China. It is not reasonable to exclude nonsmokers from screening with LDCT.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with more than 1.6 million new cases and 1.4 million deaths each year [1]. China is the largest consumer of tobacco in the world. The 2015 Chinese Adults Tobacco Survey Report revealed that, in 2015, about 27.7% of Chinese adults (defined as age ≥ 15 years; 52.1% of men and 2.7% of women), corresponding to more than 316 million people, were current smokers, and average daily consumption was 15.2 cigarettes [2]. In 2010, the Global Adult Tobacco Survey reported similar results. Furthermore, they showed that among those who had smoked at some time, only 16.9% had quit smoking and were not smoking currently, whereas 33.1% had quit smoking in the past but were currently smoking [3]. If the high smoking prevalence among Chinese adults persists, deaths in China caused by tobacco will rise from about 1 million in 2010 to 2 million in 2030 and 3 million in 2050 [4]. With the huge smoking population, the morbidity and mortality of lung cancer are expected to remain high in the coming decades in China. Early diagnosis and rational treatment are key methods to improve the prognosis of lung cancer.
There are still many problems to be explored in early-stage lung cancer screening in China's current health service status. In this collaborative study, we carried out an early lung cancer screening project among high-risk population in pilot communities based on current community medical service in Shanghai and integration of medical institutions at different levels, aiming to explore a comprehensive work pattern for screening of this lethal disease.
Methods
From August 2013 to August 2014, we conducted an early lung cancer screening program in selected communities including Wujing, Gumei, Jiangchuan, Pujiang, Xinzhuang, Zhuanqiao, and Maqiao in Minhang District of Shanghai. Participants were inhabitants of these communities who were a high-risk population of lung cancer. This program was based on a previously founded database of high-risk population of lung cancer and combined medical resources from university hospitals, disease control institutions, and community health service centers. We explored a community-based lung cancer comprehensive prevention and control network using low-dose spiral computed tomography (LDCT) in a high-risk population of lung cancer.
We recruited participants according to the following eligibility criteria: age 50 to 80 years; asymptomatic; no history of malignant disease in the recent 5 years; adequate performance status (assessed on the basis of the patient's eligibility to undergo thoracic surgery and no lethal disease); and 1) current or former smokers (quit smoking for less than 5 years) with a minimum of 20 pack-years smoking history, 2) passive smokers, or 3) never smokers with other risk factors of lung cancer including lung cancer family history, history of kitchen fume, or dust exposure. All participants gave written informed consent, and the ethics committee of Fudan University Shanghai Cancer Center approved the study.
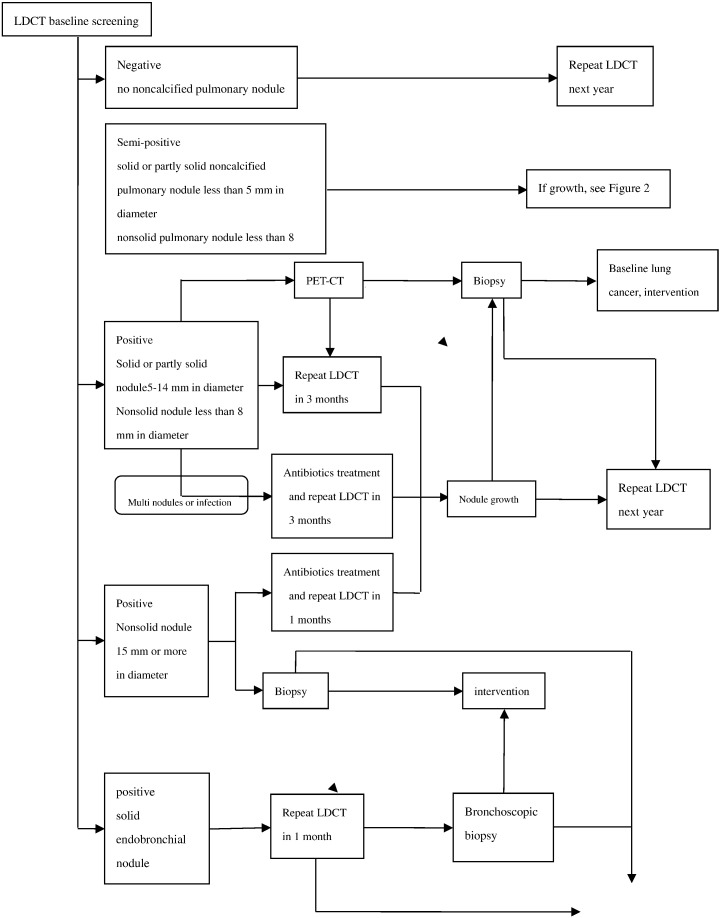
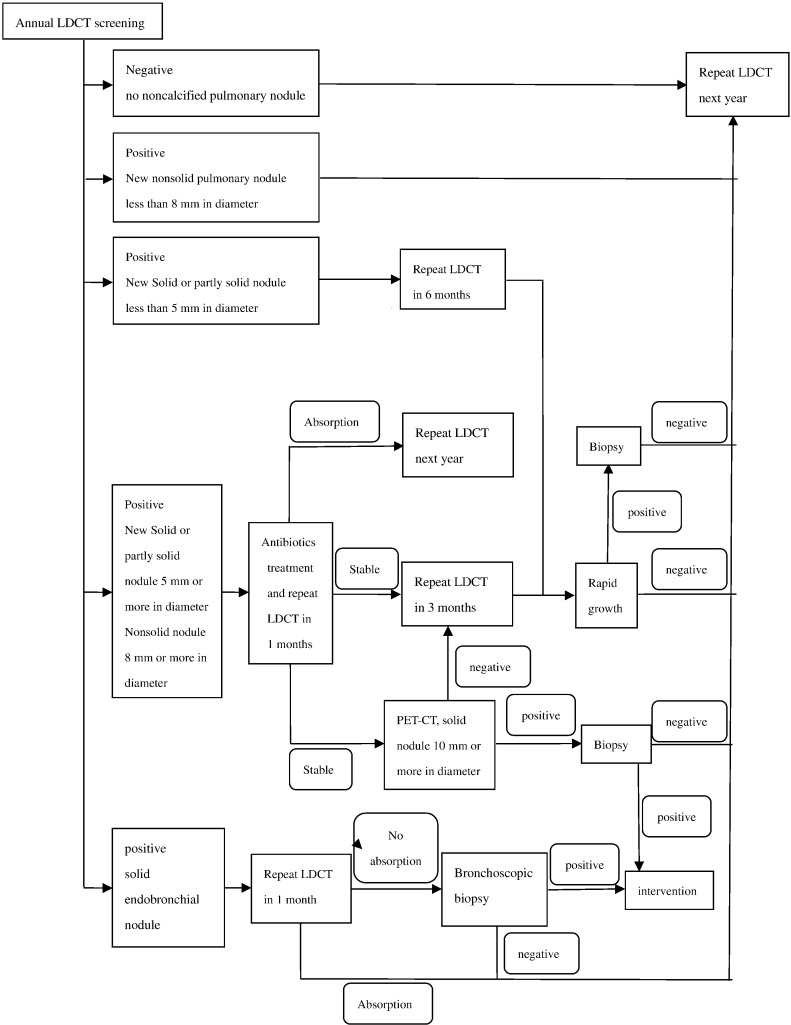
In this multicenter collaborative study, we used LDCT and computer-aided detection for screening. The protocol of baseline screening was shown in Figure 1, and protocol of annually repeat screening was shown in Figure 2.
Figure 1.
Protocol of baseline screening.
Figure 2.
Protocol of annually repeat screening.
The imaging criteria were the same for baseline and annual LDCT screening. Unenhanced CT scans of the chest were made with Siemens Somatom 64-multidetector spiral CT scanners with a low-dose protocol: 120 kVp, 40 mA, and 1.5-mm collimation. Participants were asked to hold breath while being scanned from the superior aperture of thorax to the adrenal gland. Any further diagnostic workup was required to follow the same low-dose protocol.
The image data were presented with standard pulmonary (window width 1500 Hounsfield units [HU], window level 650 HU) and mediastinum windows (window width 350 HU, window level 25 HU). Examinations were independently reported by two experienced radiologists on a Siemens workstation. In the event of a disagreement, a third radiologist was consulted. The site, dimension, density (solid, partly solid, or nonsolid), calcification, speculated sign, and other radiological features of each nodule were defined and recorded at baseline and repeat CT.
For baseline screening, a positive result on the initial LDCT scan was defined as the identification of at least one solid or partly solid noncalcified pulmonary nodule 5 mm or more in diameter, at least one nonsolid noncalcified pulmonary nodule 8 mm or more in diameter, or a solid endobronchial nodule. If noncalcified nodules identified were too small to meet the study criteria for a positive result, the result was regarded as semipositive, and LDCT was repeated 12 months later. If the result was positive, the type of workup depended on the diameter of the largest nodule. For nodules 5 to14 mm in diameter, the preferred option was to perform another LDCT at 3 months; if the images showed growth of the nodule (growth was defined as a change in volume of at least 25% between two scans), then biopsy was to be performed, whereas if there was no growth, the workup was stopped. For nodules 15 mm in diameter or larger (whether solid, partly solid, or nonsolid), immediate biopsy was an option in addition to the options already specified for smaller nodules. When infection was suspected, antibiotics followed 1 month later by LDCT was preferred. For solid endobronchial nodules, another LDCT was to be repeated 1 month later. If the nodule showed no resolution or growth, bronchoscope examination was recommended.
Nodules were divided into four types according to location: 1) subpleural nodules were those clung to the pleural; 2) peripheral nodules were the ones located close to the pleural within 2 cm but not connected to the pleural; 3) hilum nodules were located close to the hilum of lung within 2 cm; and 4) central nodules were located between the peripheral nodules and hilum nodules. Subpleural and peripheral nodules were defined as outfield pulmonary nodules, whereas hilum and central nodules were defined as infield pulmonary nodules.
Knowledge propaganda and education, screening mobilization, and clinical data collection of lung cancer patients at high risk of lung cancer were done by Fudan University Shanghai Cancer Center, Minhang District Health and Family Planning Commission, Fudan University Zhongshan Hospital, the Sixth People's Hospital Affiliated to Shanghai Jiaotong University, Xinhua Hospital Affiliated to Shanghai Jiaotong University, Minhang Cancer Center, Wujing Hospital, Gumei, Jiangchuan, Wujing, Pujiang, Xinzhuang, Zhuanqiao, and Maqiao Community Health Service Center.
LDCT screening was performed by Fudan University Shanghai Cancer Center, Fudan University Zhongshan Hospital, the Sixth People's Hospital Affiliated to Shanghai Jiaotong University, Xinhua Hospital Affiliated to Shanghai Jiaotong University, and Wujing Hospital.
Radiodiagnosis were performed by Fudan University Shanghai Cancer Center, Fudan University Zhongshan Hospital, the Sixth People's Hospital Affiliated to Shanghai Jiaotong University, and Xinhua Hospital Affiliated to Shanghai Jiaotong University.
Minimally invasive surgery of positive patients was performed by Fudan University Shanghai Cancer Center. The specimens obtained from participants who underwent surgical resection were independently examined by two experienced pathologists.
Rehabilitation and follow-up after treatment were done by Fudan University Shanghai Cancer Center, the Sixth People's Hospital Affiliated to Shanghai Jiaotong University, Xinhua Hospital Affiliated to Shanghai Jiaotong University, Minhang Cancer Center, Wujing hospital, Gumei, Jiangchuan, Wujing, Pujiang, Xinzhuang, Zhuanqiao, and Maqiao Community Health Service Center.
Data analysis was done by Fudan University Shanghai Cancer Center and Minhang District Health and Family Planning Commission.
Results
Thirteen thousand three hundred and twenty-two persons were enrolled from August 2013 to August 2014. Average age was 63.46 ± 6.79 years; 7144 (53.6%) were men. Table 1 shows the clinical characteristics of participants. At baseline examination, we detected 253 pulmonary nodules in 195 participants. The detailed information of these nodules is listed in Table 2. Primary lung cancer was pathologically diagnosed in 27 individuals, including 24 adenocarcinomas, 2 squamous cell carcinomas, and 1 malignant pleural effusion (Table 3). In addition, one metastatic lung cancer and one breast cancer were diagnosed. Fourteen participants underwent surgery for benign disease because of false-positive results, and the distribution according to the type of diseases is shown in Table 4. The incidence of the primary lung cancer in baseline screening was 238.26/100,000. Twenty-two cases were diagnosed as stage 0 to I lung cancer, accounting for 81.48% of primary lung cancer. Specifically, the incidence of primary lung cancer was 336.97 cases per 100,000 person-years among the nonsmoking participants as compared with 159.06 cases per 100,000 person-years among the smoking participants (P = .054, Table 5).
Table 1.
Clinical Characteristics of Participants
| Characteristics | Male (n, %) | Female (n, %) |
|---|---|---|
| Age | ||
| 50-60 years | 2506 (35.08%) | 1507 (35.98%) |
| 60-70 years | 3568 (49.94%) | 1585 (37.85%) |
| 70-80 years | 1070 (14.98%) | 1096 (26.17%) |
| Smoking history | ||
| Never smoker | 522 (7.31%) | 1380 (32.95%) |
| Smoker | ||
| Smoking dose ≤ 10 pack-years | 608 (8.51%) | 25 (0.60%) |
| Smoking dose 10-20 pack-years | 683 (9.56%) | 11 (0.26%) |
| Smoking dose 20-40 pack-years | 4358 (61.01%) | 7 (0.17%) |
| smoking dose >40 pack-years | 592 (8.29%) | 3 (0.07%) |
| Passive smoker | 381 (5.33%) | 2762 (65.95%) |
| Kitchen fume contact history | ||
| No | 5355 (74.96%) | 586 (13.99%) |
| Yes | 1789 (25.04%) | 3602 (86.01%) |
Table 2.
Morphology and Size of Nodules
| Morphology and Size | No. of Individuals | No. of Nodules |
|---|---|---|
| Size | ||
| Largest diameter < 5 mm | 133 | 179 |
| Largest diameter 5-10 mm | 27 | 33 |
| Largest diameter 10-20 mm | 23 | 29 |
| Largest diameter 20-30 mm | 5 | 5 |
| Largest diameter ≥ 30 mm | 7 | 7 |
| Density | ||
| Pure GGO | 88 | 106 |
| Partly GGO | 15 | 17 |
| Solid nodule | 20 | 23 |
| Calcified nodule | 72 | 107 |
Table 3.
Primary Lung Cancer
| Pathology and TNM stage | No. | Note |
|---|---|---|
| Adenocarcinoma | ||
| AAH | 1 | |
| AIS | 2 | |
| MIA | 4 | |
| T1aN0M0 | 8 | |
| T1bN0m0 | 5 | |
| T2aN0M0 | 2 | |
| T3NxM0 | 1 | Lung biopsy |
| IV | 1 | Supraclavicular lymph node biopsy |
| Squamous cell carcinoma | ||
| T2bN0M0 | 1 | |
| T3NxM0 | 1 | Lung biopsy |
| Malignant pleural effusion | 1 | Pleural fluid cytology |
AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma.
Table 4.
Benign Lesion
| Category | No. |
|---|---|
| Proliferation of fibrous tissue and inflammatory cell infiltration | 6 |
| Granulomatous lesion | 3 |
| Hamartoma | 2 |
| Thymic cyst | 1 |
| Neurilemmoma | 1 |
| B1 thymoma | 1 |
Table 5.
Detection Rate of Primary Lung Cancer in Smokers and Nonsmokers
| Detection Rate | P | |
|---|---|---|
| Smokers | 159.06/100,000 | .054 |
| Nonsmokers | 336.97/100,000 |
Discussion
Lung carcinoma is the most fatal cancer worldwide, with a 5-year survival rate of only 16% [5]. Most early lung cancer patients have no clinical symptoms. Cough, chest pain, sputum with blood, and other clinical symptoms are often presented in advanced-stage patients. However, the cost for advanced lung cancer is high, and the prognosis is poor. Results from the International Early Lung Cancer Action Program demonstrated that the estimated 10-year survival rate of clinical stage I lung cancer was 88% (95% CI, 84-91), and among those with clinical stage I lung cancer who underwent surgical resection within 1 month after the diagnosis, the rate was 92% (95% CI, 88-95) [6]. To explore a practical and feasible method for early lung cancer screening, a series of clinical studies has been carried out. Early detection trials from Mayo Clinic with conventional sputum cytology and chest radiography proved unable to decrease lung cancer mortality [7]. The United States Prostate, Lung, Colorectal, and Ovarian Cancer screening trial illustrated that annual routine chest radiography screening could not improve the mortality of lung cancer [8].
The National Lung Screening Trial is a prospective randomized controlled clinical study which showed that LDCT had enormous advantage in early lung cancer screening. The mortality rate of LDCT group decreased by 20% compared with conventional chest radiography screening among a high-risk population in the trial [9].
Lung cancer screening should clearly point to high-risk population rather than all. The current international recommendations on the target population of lung cancer screening mainly referred to the eligibility criteria of the National Lung Screening Trial. There are far more nonsmoking female lung adenocarcinoma patients in East Asia than in Europe and the United States. This subgroup of patients harbors more targetable EGFR gene mutations and results in favorable outcome if comprehensively treated [10]. If these nonsmoking women should also be included in the scope of screening, they would benefit from early detection. However, with regard to the delineation of high-risk lung adenocarcinoma population, there is still no consensus.
In a lung cancer screening study in the United Kingdom in 2012, the risk model covered age, sex, smoking history, and history of occupation factors [11]. A concise and effective prediction model of high-risk lung cancer population will be more helpful to delineate the characteristics of high-risk population in the future [12], [13].
The exclusion criteria for screening are also important. Patients suffering from other severe health threats (such as acute myocardial infarction, etc.) should not be included in lung cancer screening to be prevented from radiation exposure.
Air pollution is suspected to be one of the etiologies of lung cancer [14]. Relationship between kitchen fume or inspirable fine particles and lung cancer carcinogenesis has not been clearly demonstrated. How to define the exposure dosage of kitchen fume and inspirable fine particles and include these risk factors into lung cancer screening is also a public health problem.
The radiation dose of LDCT is much less than that of ordinary CT [15]. A new generation of dual-source CT has a lower radiation dose. It is suggested that the interaction between radiation and smoking could increase the risk of lung cancer [16].
In making decisions about instituting cancer screening in communities, a number of factors need to be taken into consideration, including the sensitivity and specificity of the examination, cost-effectiveness, and even the customs and habits. Cancer screening in the community population should be acceptable for inhabitants, families, and society [9], [13]. At present, it is generally believed that lung cancer patients detected by LDCT screening are mainly early-stage ones. Surgery for early-stage lung cancer is less than half the cost of late-stage treatment and results in better outcome [17]. Furthermore, a recent study suggested that lung cancer screening combined with smoking cessation guidance could reduce tobacco consumption [18].
In conclusion, it has become a consensus that LDCT screening can lead to early detection and intervention of lung cancer and improve the survival of lung cancer patients. More clinical data and community experience are needed for guiding the practice of LDCT screening in both smoker and nonsmoker populations of lung cancer in China. Shanghai community-based practice of early lung cancer screening with LDCT has improved the early diagnosis rate of lung cancer. We found LDCT screening for lung cancer among smoking and nonsmoking individuals to be feasible and could be applicable in qualified eligible medical centers and communities in China.
Footnotes
This study was supported by a grant from Shanghai Hospital Development Center (no. SHDC12012308), grant from Health and Family Planning Commission of Shanghai Municipality (no. 2013ZYJB0301), and grant from Science and Technology Commission of Shanghai Municipality (no. 14495810800).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Ma C, Xi B. Tobacco control in China: still a long way to go. Lancet. 2016;387(10026):1375–1376. doi: 10.1016/S0140-6736(16)30080-0. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Hsia J, Yang G. Prevalence of smoking in China in 2010. N Engl J Med. 2011;364(25):2469–2470. doi: 10.1056/NEJMc1102459. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Peto R, Zhou M, Iona A, Smith M, Yang L, Guo Y, Chen Y, Bian Z, Lancaster G. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 2015;386(10002):1447–1456. doi: 10.1016/S0140-6736(15)00340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63(3):151–166. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 6.International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 7.Strauss GM, Gleason RE, Sugarbaker DJ. Screening for lung cancer reexamined. A reinterpretation of the Mayo Lung Project randomized trial on lung cancer screening. Chest. 1993;103(4):337–341. [PubMed] [Google Scholar]
- 8.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, Crawford ED, Fouad MN, Isaacs C, Reding DJ. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306(17):1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 9.National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Ren Y, Fang Z, Li C, Fang R, Gao B, Han X, Tian W, Pao W, Chen H. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28(30):4616–4620. doi: 10.1200/JCO.2010.29.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raji OY, Duffy SW, Agbaje OF, Baker SG, Christiani DC, Cassidy A, Field JK. Predictive accuracy of the Liverpool lung project risk model for stratifying patients for computed tomography screening for lung cancer. Ann Intern Med. 2012;157(4):242–250. doi: 10.7326/0003-4819-157-4-201208210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tammemägi MC, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, Riley TL, Commins J, Oken MM, Berg CD. Lung cancer risk prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst. 2011;103(13):1058–1068. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 15.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology. 2004;231(2):440–445. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 17.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States:2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. doi: 10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]