Abstract
Objective:
Osteoporosis is the well-known major complication in chronic hepatitis B virus (HBV) infection. Fewer reports are available of the relationship between bone loss and chronic HBV infection. We investigated the bone mineral density (BMD) and prevalence of osteoporosis in chronic HBV patients in comparison with healthy subjects.
Methods:
We assessed 148 chronic HBV patients and 148 age- and gender-matched healthy controls by dual energy X-ray absorptiometry (DEXA) for determination of BMD. T-score was used to define bone status according to the World Health Organization’s classification.
Results:
The BMD values were significantly lower in HBV patients in all scan of specific regions compared with the controls (P < 0.05). The prevalence of osteoporosis in either of lumbar spine (LS), total hip (TH) or the femoral neck (FN) was significantly higher in the HBV patients group compared with the healthy controls. The rate of osteopenia and osteoporosis for HBV patients aged 45–54 years was significantly higher than that of the healthy controls.
Conclusions:
Chronic HBV infection was associated with low BMD and increased the risk of developing subsequent osteoporosis.
Keywords: BMD, Chronic HBV infection, Chronic liver disease, Osteoporosis, Osteopenia
INTRODUCTION
Osteoporosis is an escalating disease with high morbidity and mortality rates and large socioeconomic expenses. The term “hepatic osteodystrophy” is a common complication among chronic liver disease (CLD) patients.1 It is a metabolic bone disease secondary to increased resorption and reduced formation of bone.2 Osteoporosis is the well-known major complication in this chronic hepatitis.3 The prevalence of osteoporosis in CLD varies widely, ranges from 20 to 50% depending on patient selection and diagnostic criteria.4 Many researches have reported significant osteoporosis in patients with cirrhosis, especially secondary to hepatitis B.1,2
Hepatitis B is a global health problem caused by infection with the hepatitis B virus (HBV); it is estimated that as many as 350 million people are affected with HBV worldwide, and about 75% of them are from Asia.5 In addition, many extrahepatic manifestations are associated with chronic HBV infection.6 However, fewer reports are available of the relationship between bone loss and chronic HBV infection.
To determine whether chronic HBV infection is at increased risk of developing low bone mass, we investigated the prevalence of osteoporosis in chronic HBV patients in comparison with age- and sex-matched healthy subjects.
METHODS
A total of 148 patients with chronic HBV patients were assessed, they were recruited at Department of Infectious Diseases, Union Hospital, Tongji Medical College from January 2014 to December 2015 and were defined by clinical, biochemical, serological, immunological and histopathological investigations with duration up to 6 months. Also, we selected 148 healthy controls with age- and gender-matched from the Medical Examination Center, Union Hospital, Tongji Medical College. The study protocol was approved by the Ethics Committee of Union Hospital, Tongji Medical College, according to the ethical and moral principles stated in the Helsinki 1966 Declaration on Human Rights.
Demographics, lifestyle, and menopause status of study participants were collected. Exclusion criteria were: any treatments that could affect bone mass (calcium, bisphosphonates, estrogens, vitamin D supplements, corticosteroids, and active intravenous drugs.), history of hip or lumbar spine fracture, history of malignancy, severe heart disease, chronic kidney disease, human immunodeficiency virus (HIV) coinfection, malnutrition, and other chronic liver disease, such as chronic hepatitis C, primary biliary cirrhosis, alcoholic liver disease, autoimmune liver disease, bone disorders.
Bone mineral density measurements
Bone mineral density of lumbar spine (LS, L1–L4), total hip (TH), femoral neck (FN), trochanter (Tro) and ward’s triangle (WT) were measured by dual-energy X-ray absorptiometry (DXA) (QDR-4500A; Hologic, Waltham, MA). All measurements were realized by two technicians in all patients and controls. According to the World Health Organization criteria,7 osteoporosis was defined as a T score below -2.5 SD, osteopenia was defined as a T score between -1 and -2.5 SD and low BMD was defined as Z score below -2.
Statistical Analysis
All data were expressed as mean ± SD. The Chi square test was used to analyze differences in non-continuous variables and the Student’s t test in continuous variables. P < 0.05 was considered as a significant difference. All statistical analyses were performed by SPSS version 13 (SPSS, Inc., Chicago, IL).
RESULTS
The demographic and clinical characteristics of the HBV patients and the healthy controls are presented in Table-I. The mean age of HBV patients was 43.7 ± 11.9 years (range 15–72 years). There were 26 (17.8%) females and 122 (82.4%) males. The mean BMI was 22.7 ± 1.9 kg/m2 (range 17.7–27.7). A total of 42.3% (11/26) of the females in HBV patients were postmenopausal.
Table-I.
The demographic and clinical characteristics of the patients and the controls.
| HBV Patients | Healthy Controls | Pvalue | |
|---|---|---|---|
| N | 148 | 148 | |
| Sex (M, %) | 122(82.4) | 122(82.4) | |
| Age (years, Mean ± SD) | 43.7(11.9) | 43.6(11.9) | 0.581 |
| BMI (kg/m2, Mean ± SD) | 22.7(1.9) | 23.0(1.8) | 0.925 |
| Smoke (n, %) | 91(61.5) | 87(58.8) | 0.225 |
| Menopause (n, %) | 11(42.3) | 11(42.3) |
M = male, BMI = body mass index.
BMD values (g/cm2), Z scores and T scores of the lumbar spine, femoral neck, total hip, trochanter and ward’s triangle in HBV patients and healthy controls are shown in Table-II. The BMD values were significantly lower in HBV patients in all scan of specific regions compared with the controls (P < 0.05). In addition, the T scores and Z scores showed significantly lower than the controls at the lumbar spine, femoral neck and total hip sites except Z scores in women.
Table-II.
Comparison of BMD, T and Z-scores between HBV patients and healthy controls.
| All | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HBV patients (Mean ± SD) | Healthy Controls (Mean ± SD) | P value | HBV patients (Mean ± SD) | Healthy Controls (Mean ± SD) | P value | HBV patients (Mean ± SD) | Healthy Controls (Mean ± SD) | P value | |
| N | 148 | 148 | 122 | 122 | 26 | 26 | |||
| BMD, g/cm2 | |||||||||
| TH | 0.874 ± 0.058 | 0.945 ± 0.060 | 0.01 | 0.871 ± 0.047 | 0.952 ± 0.047 | 0.01 | 0.860 ± 0.093 | 0.910 ± 0.093 | 0.01 |
| FN | 0.829 ± 0.060 | 0.915 ± 0.061 | 0.01 | 0.825 ± 0.052 | 0.921 ± 0.050 | 0.01 | 0.823 ± 0.093 | 0.889 ± 0.094 | 0.01 |
| Tro | 0.732 ± 0.067 | 0.797 ± 0.070 | 0.01 | 0.748 ± 0.045 | 0.819 ± 0.043 | 0.01 | 0.642 ± 0.078 | 0.694 ± 0.078 | 0.01 |
| WT | 0.756 ± 0.075 | 0.823 ± 0.076 | 0.01 | 0.771 ± 0.059 | 0.844 ± 0.055 | 0.01 | 0.658 ± 0.082 | 0.724 ± 0.081 | 0.01 |
| T-score | |||||||||
| LS | -1.05 ± 0.87 | -0.49± 0.76 | 0.01 | -1.15 ± 0.87 | -0.32 ± 0.64 | 0.01 | -1.23 ± 0.85 | -0.56 ± 0.74 | 0.080 |
| TH | -1.05 ± 0.88 | -0.52 ± 0.62 | 0.01 | -1.16 ± 0.85 | -0.47 ± 0.57 | 0.01 | -0.65 ± 0.84 | -0.56 ± 0.81 | 0.648 |
| FN | -1.12 ± 0.91 | -0.50 ± 0.60 | 0.01 | -1.26 ± 0.88 | -0.49 ± 0.52 | 0.01 | -0.55 ± 0.90 | -0.51 ± 0.74 | 0.851 |
| Z-score | |||||||||
| LS | -0.42 ± 0.56 | -0.04 ± 0.43 | 0.01 | -0.39 ± 0.56 | 0.03 ± 0.38 | 0.01 | -0.56 ± 0.54 | -0.42 ± 0.46 | 0.361 |
| TH | -0.38 ± 0.57 | 0.16 ± 0.46 | 0.01 | -0.47 ± 0.53 | 0.20 ± 0.39 | 0.01 | -0.04 ± 0.55 | 0.04 ± 0.66 | 0.613 |
| FN | -0.44 ± 0.62 | -0.10 ± 0.40 | 0.01 | -0.55 ± 0.56 | -0.14 ± 0.37 | 0.01 | 0.07 ± 0.64 | 0.04 ± 0.50 | 0.850 |
BMD = bone mineral density, LS = lumbar spine, TH = total hip, FN = femoral neck, Tro = trochanter, WT = ward’s triangle.
The prevalence of osteopenia was showed in Table-III. The prevalence of osteopenia in HBV patients at LS, TH and FN were 23.6%, 24.3%, 23%, respectively. In healthy control, 10.1%, 9.5%, 9.5% participants were osteopenic at LS, TH and FN, respectively. The prevalence of osteopenia was significantly higher in male and female HBV patients regardless of their menopausal status than their controls. The prevalence of osteoporosis is shown in Table-IV. The prevalence of osteoporosis in either of LS, TH or the FN was significantly higher in the HBV patients group (19/148, 12.8%; 17/148, 11.5%; 18/148, 12.2%) compared with the healthy control (7/148, 4.7%; 6/148, 4.1%; 7/148, 4.7%) (P = 0.022; P = 0.028; P = 0.035). When the prevalence of osteopenia and osteoporosis were evaluated according to ranges of age, all categories showed higher rates of osteopenia and osteoporosis in HBV patients than the healthy controls with a statistically significant difference for HBV patients between 45 and 54 years (P = 0.008, P = 0.048) (Fig.1).
Table-III.
Prevalence of osteopenia in chronic HBV patients and healthy controls.
| LS | TH | FN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HBV Patients | Healthy Controls | Pvalue | HBV Patients | Healthy Controls | P value | HBV Patients | Healthy Controls | P value | |
| All | 35/148(23.6%) | 15/148(10.1%) | 0.003 | 36/148(24.3%) | 14/148(9.5%) | 0.001 | 34/148(23%) | 14/148(9.5%) | 0.002 |
| Men | 22/122(18%) | 10/122(8.2%) | 0.036 | 22/122(18%) | 9/122(7.4%) | 0.02 | 21/122(17.2%) | 9/122(7.4%) | 0.031 |
| Women | 13/26(50%) | 5/26(19.2%) | 0.04 | 14/26(53.8%) | 5/26(19.2%) | 0.02 | 13/26(50%) | 5/26(19.2%) | 0.04 |
| Menopausal women | 5/12(41.7%) | 3/12(25%) | 0.667 | 6/12(50%) | 3/12(25%) | 0.4 | 5/12(41.7%) | 3/12(25%) | 0.667 |
| Non-menopausal women | 8/14(57.1%) | 2/14(14.3%) | 0.046 | 8/14(61.5%) | 2/14(14.3%) | 0.046 | 8/14(57.1%) | 2/14(14.3%) | 0.046 |
LS = lumbar spine, TH = total hip, FN = femoral neck.
Table-IV.
Prevalence of osteoporosis in chronic HBV patients and healthy controls.
| LS | TH | FN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HBV Patients | Healthy Controls | P value | HBV Patients | Healthy Controls | P value | HBV Patients | Healthy Controls | P value | |
| All | 19/148(12.8%) | 7/148(4.7%) | 0.022 | 17/148(11.5%) | 6/148(4.1%) | 0.028 | 18/148(12.2%) | 7/148(4.7%) | 0.035 |
| Men | 11/122(9%) | 4/122(3.3%) | 0.107 | 10/122(8.2%) | 3/122(2.5%) | 0.084 | 10/122(8.2%) | 4/122(3.3%) | 0.167 |
| Women | 8/26(30.7%) | 3/26(11.5%) | 0.173 | 7/26(26.9%) | 3/26(11.5%) | 0.291 | 8/26(30.7%) | 3/26(11.5%) | 0.173 |
| Menopausal women | 4/12(33.3%) | 2/12(16.7%) | 0.64 | 3/12(25%) | 2/12(16.7%) | 0.615 | 4/12(33.3%) | 2/12(16.7%) | 0.64 |
| Non-menopausal women | 4/14(28.5%) | 1/14(7.1%) | 0.326 | 4/14(28.5%) | 1/14(7.1%) | 0.326 | 4/14(28.5%) | 1/14(7.1%) | 0.326 |
LS = lumbar spine, TH = total hip, FN = femoral neck.
Fig.1.
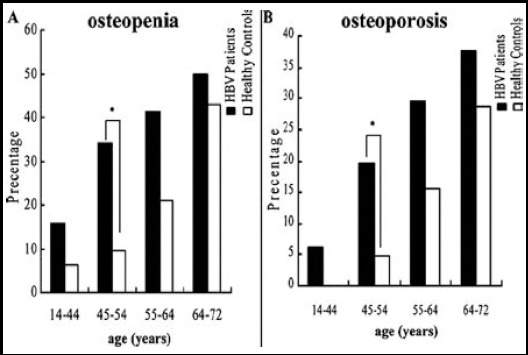
Comparison of the prevalence of osteopenia (A), osteoporosis (B) between chronic HBV patients and health controls. *P < 0.05.
DISSCUSSION
The present study reveals a higher prevalence of osteoporosis and osteopenia in HBV patients than healthy controls. However, the overall prevalence of osteoporosis in previous studies with chronic liver diseases patients was ranging from 12% to 55% and that prevalence in viral hepatitis patients was 20% to 55%.2 The total prevalence of osteoporosis in the present study was 12.8% in HBV patients.
Our study showed that the rate of osteoporosis at the femoral neck in HBV patients was lower than that at the lumbar spine. It might partly be explained by the reason that the turnover of cortical bone was lower than that of trabecular bone and the lumbar spine in chronic liver disease patients was mostly affected by cirrhosis.8 However, it was still controversial. Guggenbuhl P et al.9 reported lower BMD in the femoral neck compared the lumbar spine. While Valenti L et al.10 suggested an increased prevalence of osteoporosis at lumbar spine compared to femoral neck.
As we know, senile osteoporosis and postmenopausal osteoporosis are the main etiologies of primary osteoporosis. Our study showed that the osteoporosis incidence increased with age in HBV patients. Moreover, women was found with higher prevalence of osteoporosis than men in HBV patients and that prevalence increased significantly in the postmenopausal women. Our findings are consistent with previous study.11 Some studies reported that lower BMI showed lower BMD in chronic liver diseases.12 However, the association between BMI and BMD in HBV patients was not observed in our study.
The mechanisms for the association between HBV and osteoporosis are not clear. Chronic inflammation and decompensated liver or cirrhosis induced by HBV may be the potential mechanism. First, inflammatory cytokines (e.g., interleukin-1 (IL-1), and interleukin-6 (IL-6), tumor necrosis factor-alpha (TNFα)) associated with chronic HBV infection can increase receptor activator of nuclear factor kappa-B ligand (RANKL) to stimulate osteoclastogenesis and bone resorption.13 Moreover, TNFα was reported to inhibit the osteoblast differentiation and promote osteoblast apoptosis.13 The combined effects of these inflammatory cytokines can result in bone formation decreasing and bone resorption increasing, leading to reduced bone mineral density and then causing osteoporosis. Second, chronic HBV infection associated liver decompensation and cirrhosis can impair insulin-like growth factor 1(IGF-1) produced by liver, which inhibit osteoblast differentiation and proliferation promoted by IGF-1. In addition, previous study showed that hypogonadism observed in decompensated liver could increase osteoclast activity with reducing of estrogen and testosterone in blood levels.14 Third, metabolic acidosis in decompensated cirrhosis can also reduce bone mineral density through inducing calcium efflux from bone.15 Finally, decreasing of hydroxylation of vitamin D3 to D25 and blood levels of osteocalcin in advanced liver disease can accelerate bone loss and decrease bone formation.16
The measurement of BMD has been widely recommended in patients with chronic liver disease. Patients with cirrhosis were recommended with BMD assessment according to the societies of American and British gastroenterology.17,18 A recent report showed that chronic hepatitis B infection was associated with risk of hip fracture.19 Moreover, it was estimated that fracture risk would be increased twofold in cirrhosis regardless of aetiology2 and cumulatively by two fold or threefold for each decrease of one SD in BMD. In clinical practice, fracture risk could be prevented through the implementation of therapeutic measures by the systematic determination of BMD to limit the morbidity of patients with cirrhosis.20
Limitations of the study
First, bone health associated with virus content was not determined in the present study. Second, lifestyle factors except smoking of osteoporosis were not fully ascertained in this study. Third, our analyses accounted only for baseline of BMD measurement but not use during follow-up.
CONCLUSION
The present study demonstrated that chronic HBV infection was associated with low BMD and increased the risk of developing subsequent osteoporosis. Future studies are needed to confirm these findings, to determine the potential mechanisms and to evaluate the changes of bone mass during follow-up in these patients.
Authors’ Contribution
XZL conceived, designed and did statistical analysis & editing of manuscript.
ZFH, HW & CC did data collection and manuscript writing.
JW & SHY did review and final approval of manuscript.
Footnotes
Grant Support: This work was supported by the National Natural Science Foundation of China (Grants: 81201413, 81302344, 81371973 and 81401573).
Declaration of interest: None
REFERENCES
- 1.Hay JE, Guichelaar MM. Evaluation and management of osteoporosis in liver disease. Clin Liver Dis. 2005;9(4):747–766, viii. doi: 10.1016/j.cld.2005.07.003. doi:10.1016/j.cld.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Collier J. Bone disorders in chronic liver disease. Hepatology. 2007;46(4):1271–1278. doi: 10.1002/hep.21852. doi:10.1002/hep.21852. [DOI] [PubMed] [Google Scholar]
- 3.Wariaghli G, Mounach A, Achemlal L, Benbaghdadi I, Aouragh A, Bezza A, et al. Osteoporosis in chronic liver disease:a case-control study. Rheumatol Int. 2010;30(7):893–899. doi: 10.1007/s00296-009-1071-8. doi:10.1007/s00296-009-1071-8. [DOI] [PubMed] [Google Scholar]
- 4.Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33(1):301–307. doi: 10.1053/jhep.2001.20533. doi:10.1053/jhep.2001.20533. [DOI] [PubMed] [Google Scholar]
- 5.Jha V, Prasad N. CKD and Infectious Diseases in Asia Pacific:Challenges and Opportunities. Am J Kidney Dis. 2016;68(1):148–160. doi: 10.1053/j.ajkd.2016.01.017. doi:10.1053/j.ajkd.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Cacoub P, Saadoun D, Bourliere M, Khiri H, Martineau A, Benhamou Y, et al. Hepatitis B virus genotypes and extrahepatic manifestations. J Hepatol. 2005;43(5):764–770. doi: 10.1016/j.jhep.2005.05.029. doi:10.1016/j.jhep.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis:synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–381. doi: 10.1007/BF01622200. doi :10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 8.Chen CC, Wang SS, Jeng FS, Lee SD. Metabolic bone disease of liver cirrhosis:is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol. 1996;11(5):417–421. doi: 10.1111/j.1440-1746.1996.tb00284.x. doi:10.1111/j.1440-1746.1996.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 9.Guggenbuhl P, Deugnier Y, Boisdet JF, Rolland Y, Perdriger A, Pawlotsky Y, et al. Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int. 2005;16(12):1809–1814. doi: 10.1007/s00198-005-1934-0. doi:10.1007/s00198-005-1934-0. [DOI] [PubMed] [Google Scholar]
- 10.Valenti L, Varenna M, Fracanzani AL, Rossi V, Fargion S, Sinigaglia L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int. 2009;20(4):549–555. doi: 10.1007/s00198-008-0701-4. doi:10.1007/s00198-008-0701-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Lin CL, Kao CH. Association between chronic hepatitis b virus infection and risk of osteoporosis:A Nationwide Population-Based Study. Medicine (Baltimore) 2015;94(50):e2276. doi: 10.1097/MD.0000000000002276. doi:10.1097/MD.0000000000002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbu EC, Chitu-Tisu CE, Lazar M, Olariu CM, Olteanu D, Bojinca M, et al. Body Composition Changes in Patients with Chronic Hepatitis C. J Gastrointestin Liver Dis. 2016;25(3):323–329. doi: 10.15403/jgld.2014.1121.253.hpc. doi:10.15403/jgld.2014.1121.253.hpc. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277(4):2695–2701. doi: 10.1074/jbc.M106339200. doi:10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 14.Pignata S, Daniele B, Galati MG, Esposito G, Vallone P, Fiore F, et al. Oestradiol and testosterone blood levels in patients with viral cirrhosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1997;9(3):283–286. doi: 10.1097/00042737-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Krieger NS, Frick KK, Bushinsky DA. Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens. 2004;13(4):423–436. doi: 10.1097/01.mnh.0000133975.32559.6b. doi:10.1097/01.mnh.0000133975.32559.6b. [DOI] [PubMed] [Google Scholar]
- 16.Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest. 1995;95(6):2581–2586. doi: 10.1172/JCI117959. doi:10.1172/JCI117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Gastroenterological Association medical position statement:osteoporosis in hepatic disorders. Gastroenterology. 2003;125(3):937–940. doi: 10.1016/s0016-5085(03)01060-6. doi:10.1016/S0016-5085(03)01060-6. [DOI] [PubMed] [Google Scholar]
- 18.Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50(Suppl 1):i1–i9. doi: 10.1136/gut.50.suppl_1.i1. doi:10.1136/gut.50.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne DD, Newcomb CW, Carbonari DM, Nezamzadeh MS, Leidl KB, Herlim M, et al. Risk of hip fracture associated with untreated and treated chronic hepatitis B virus infection. J Hepatol. 2014;61(2):210–218. doi: 10.1016/j.jhep.2014.04.001. doi:10.1016/j.jhep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoudi A, Sellier N, Reboul-Marty J, Chales G, Lalatonne Y, Bourcier V, et al. Bone mineral density assessed by dual-energy X-ray absorptiometry in patients with viral or alcoholic compensated cirrhosis. A prospective study. Clin Res Hepatol Gastroenterol. 2011;35(11):731–737. doi: 10.1016/j.clinre.2011.07.009. doi:10.1016/j.clinre.2011.07.009. [DOI] [PubMed] [Google Scholar]


