ABSTRACT
Decay of the HIV reservoir is slowed over time in part by expansion of the pool of HIV-infected cells. This expansion reflects homeostatic proliferation of infected cells by interleukin-7 (IL-7) or antigenic stimulation, as well as new rounds of infection of susceptible target cells. As novel therapies are being developed to accelerate the decay of the latent HIV reservoir, it will be important to identify interventions that prevent expansion and/or repopulation of the latent HIV reservoir. Our previous studies showed that HIV protease cleaves the host protein procaspase 8 to generate Casp8p41, which can bind and activate Bak to induce apoptosis of infected cells. In circumstances where expression of the anti-apoptotic protein BCL2 is high, Casp8p41 instead binds BCL2, and cell death does not occur. This effect can be overcome by treating cells with the clinically approved BCL2 antagonist venetoclax, which prevents Casp8p41 from binding BCL2, thereby allowing Casp8p41 to bind Bak and kill the infected cell. Here we assess whether the events that maintain the HIV reservoir are also antagonized by venetoclax. Using the J-Lat 10.6 model of persistent infection, we demonstrate that proliferation and HIV expression are countered by the use of venetoclax, which causes preferential killing of the HIV-expressing cells. Similarly, during new rounds of infection of primary CD4 T cells, venetoclax causes selective killing of HIV-infected cells, resulting in decreased numbers of HIV DNA-containing cells.
IMPORTANCE Cure of HIV infection requires an intervention that reduces the HIV reservoir size. A variety of approaches are being tested for their ability to impact HIV reservoir size. Even if successful, however, these approaches will need to be combined with additional complementary approaches that prevent replenishment or repopulation of the HIV reservoir. Our previous studies have shown that the FDA-approved BCL2 antagonist venetoclax has a beneficial effect on the HIV reservoir size following HIV reactivation. Here we demonstrate that venetoclax also has a beneficial effect on HIV reservoir size in a model of homeostatic proliferation of HIV as well as in acute spreading infection of HIV in primary CD4 T cells. These results suggest that venetoclax, either alone or in combination with other approaches to reducing HIV reservoir size, is a compound worthy of further study for its effects on HIV reservoir size.
KEYWORDS: Bcl-2 family, HIV, antiapoptosis, reservoir
INTRODUCTION
The latent HIV reservoir, comprised of CD4 T cells with an integrated, replication-competent, transcriptionally silent HIV provirus, is established early in HIV infection (1, 2), has a half-life estimated at 3.6 years (3), and is responsible for the invariant rebound in viral replication that occurs upon interruption of suppressive antiretroviral therapy (ART). The HIV reservoir exists in the peripheral blood, lymphoid organs, and possibly other sanctuary sites (4–6) and is maintained in at least two ways—by homeostatic proliferation and, probably to a lesser extent, by new rounds of infection of uninfected CD4 T cells, some of which then enter latency. Proliferation of latently infected cells can be induced by the presence of either antigenic stimulation or homeostatic cytokine signals (7, 8), resulting in daughter cells that contain integrated HIV. Ongoing viral replication, even in the setting of apparently suppressive ART, also contributes to maintaining the latent reservoir (9, 10), possibly because of poor penetration of ART into lymph nodes (11) and other privileged anatomic sites.
Diverse approaches have been proposed to reduce HIV reservoir size, with some showing early signs of success. Examples include bone marrow transplantation, which reduces HIV reservoir size (12, 13), albeit not to levels associated with cure in all patients; gene editing with clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 to excise HIV provirus, which has shown promise ex vivo and in animal models of HIV (14, 15); and the use of designer chimeric antigen receptor (CAR) T cells, which reduce HIV reservoir size in vitro by selectively killing infected cells (16–18). In addition, noncellular, anti-HIV immunomodulatory therapies, for instance, those using anti-programed death-ligand 1 (PD-L1) (19) or interleukin-15 (IL-15) (20, 21), may be combined with other strategies for HIV cure. However, if any approach is successful at reducing HIV reservoir size, it will be necessary to also prevent repopulation of the HIV reservoir in order to cure patients of HIV infection.
Many of these treatments act, at least in part, by inducing death in cells that harbor latent HIV. One of the important mechanisms of cell death, particularly in lymphocytes, is apoptosis. The mitochondrial pathway of apoptosis is regulated in large part by BCL2, an oncoprotein that protects cells against death induced by a variety of death stimuli, such as growth factor withdrawal, certain chemotherapeutics (22), and, of pertinence to HIV biology, Casp8p41 (23). BCL2 is localized to the mitochondrial outer membrane, where it interacts with other BCL2 family proteins to determine cell fate (24). Once activated, the proapoptotic family members Bax and Bak promote mitochondrial permeabilization and release of cytochrome c, triggering an apoptotic cascade (24). Bax and Bak are activated by BH3-only members of the BCL2 protein family (25) and inhibited by anti-apoptotic proteins (e.g., BCL2, MCL1, and BCLXL), which bind and neutralize the BH3-only proteins as well as Bax and Bak.
Central memory CD4 T cells are a dominant cell type in which HIV provirus is found (26). In previous studies, we have shown that central memory CD4 T cells resist the cytotoxic effects of HIV replication as well as death induced by multiple other stimuli (23).This death resistance is associated with decreased expression of proapoptotic proteins such as procaspase 8 and with increased expression of anti-apoptotic proteins, including BCL2 (23). Moreover, antagonizing BCL2 in primary patient cells ex vivo, in concert with reactivating HIV from latency, results in selective death of HIV-infected but not uninfected cells and decreased total cell-associated HIV DNA levels as a surrogate marker of reservoir size (23). This selective killing of infected cells occurs, at least in part, because HIV protease, which is present only in HIV-infected cells, cleaves the host cell cytoplasmic protein procaspase 8 to generate Casp8p41 (27, 28), which binds and activates Bak (29, 30) but can be neutralized by BCL2 (23). The idea of this HIV protease → Casp8p41 → Bak pathway is supported by a variety of observations, including the ability of procaspase 8 downregulation to protect cells from HIV protease-induced death (31), the demonstration that expression of Casp8p41 alone is sufficient to activate Bak and kill cells (30), and the identification of Bak knockdown as a potent suppressor of HIV protease-induced cell death in a lentiviral short hairpin RNA (shRNA) screen (30).
More recently, we reported on the role of BCL2 in determining the outcome of HIV reactivation. In particular, when HIV-infected cells were reactivated and produced Casp8p41 under conditions where BCL2 levels were high, BCL2 bound Casp8p41 and cell death was averted (23). However, when HIV-infected cells were reactivated in the presence of a compound (venetoclax) that selectively occupies the BCL2 BH3 binding groove, HIV protease-generated Casp8p41 could not bind BCL2 and instead activated Bak, causing death of HIV-infected cells (23).
Based on this emerging understanding of the role of BCL2 in HIV protease-mediated cell death, we hypothesized that occupation of the BH3 groove of BCL2 by venetoclax might promote HIV-induced cell death during acute infection and possibly during proliferation of cells that constitute the HIV reservoir. To test these hypotheses, we evaluated the effects of this FDA-approved BCL2 antagonist on HIV dynamics in in vitro models of chronic infection and proliferation and of acute CD4 T cell infection with HIV.
RESULTS
BCL2 inhibition during proliferation of chronically HIV-infected cells.
The latent HIV reservoir is composed primarily of memory CD4 T cells containing transcriptionally silent integrated HIV. The size of the HIV reservoir can be maintained over time by de novo spreading of infection of CD4 T cells, resulting in more cells containing HIV, or by proliferation of preexisting infected cells as a consequence of antigenic stimulation or stimulation by interleukin-7 (IL-7) (7). Thus, proliferation of cells containing HIV provirus is critical to the stability and, possibly, the expansion of the HIV reservoir over time.
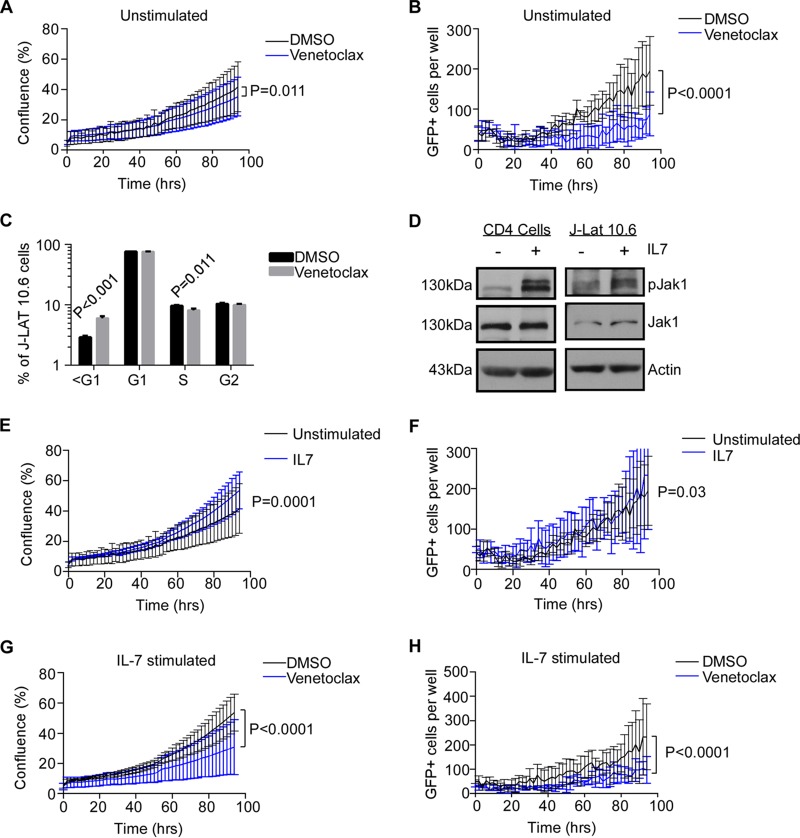
J-Lat 10.6 cells contain one integrated HIV provirus per cell (with the gene encoding green fluorescent protein [GFP] inserted into the Nef gene) (32). In the absence of stimulation, cells of this cell line proliferate slowly (Fig. 1A) and are typically <5% GFP positive (Fig. 1B), consistent with chronically HIV-infected cells, with the majority of provirus transcriptionally silent. We assessed whether antagonizing BCL2 in the J-Lat 10.6 cells with venetoclax (33) impacted either the viral or cellular dynamics of these cells undergoing proliferation. Venetoclax treatment decreased cell confluence in culture over 96 h compared to treatment with a dimethyl sulfoxide (DMSO) control (P = 0.011, Fig. 1A) and decreased the number of GFP-expressing cells compared to the DMSO control (P < 0.0001, Fig. 1B). We next questioned whether reduced proliferation of J-Lat 10.6 cells was due to cell cycle arrest induced by venetoclax. In fact, venetoclax did not significantly alter G1 or G2/M cell cycle phases in J-Lat 10.6 cells (Fig. 1C) but instead increased the percentage of cells containing hypodiploid DNA (P < 0.001, Fig. 1C), consistent with inducing apoptosis in these cells. As we have previously published that 1 μM venetoclax does not significantly impact either cell viability or proliferation of uninfected primary CD4 T cells (23), the present results in J-Lat 10.6 cells suggest that venetoclax may have a specific effect on the subset of infected cells that actively transcribe HIV.
FIG 1.
BCL-2 antagonism impairs proliferation of chronically HIV-infected cells. (A) J-Lat 10.6 cells were cultured in the presence or absence of venetoclax (1 μM) or a DMSO control and monitored for proliferation over time by live-cell phase contrast imaging. (B) GFP expression was monitored over time in the same cultures as those described for panel A using live-cell fluorescence detection. (C) J-Lat 10.6 cells treated with venetoclax or a DMSO control for 72 h were analyzed for cell cycle results by DNA content analysis. (D) Uninfected primary CD4 T cells or J-Lat 10.6 cells were treated with IL-7 and assessed for Jak1 phosphorylation. (E and F) J-Lat 10.6 cells were treated with a control or IL-7 (25 ng/ml) and were monitored for confluence (E) and GFP expression (F) over time. (G and H) J-Lat 10.6 cells were treated with a DMSO control or venetoclax (1 μM) plus IL-7 stimulation and monitored for confluence (G) and GFP expression (H) over time. Data in panels E to H represent means ± standard errors of the means of results from triplicate samples within each experiment. Data are representative of results of 4 independent experiments.
We questioned whether venetoclax would impact either cell proliferation or viral production under conditions of cytokine stimulation. It has been previously reported that the parental line of J-Lat 10.6 cells expresses IL-7 receptor alpha (34). Consistent with this, treatment of J-Lat 10.6 cells with IL-7 resulted in phosphorylation of Jak1 at Tyr1022/1023 (Fig. 1D), indicating that there is activation of the downstream signaling pathways. Treatment of J-Lat 10.6 cells with IL-7 also induced downstream events following IL-7 receptor engagement and Jak1 phosphorylation, including both proliferation (P = 0.0001, Fig. 1E) and GFP expression (P = 0.03, Fig. 1F), compared to the results seen with unstimulated cells. The addition of venetoclax significantly decreased proliferation and GFP expression in IL-7-stimulated J-Lat 10.6 cells compared to DMSO treatment (P < 0.0001, Fig. 1G and H, respectively).
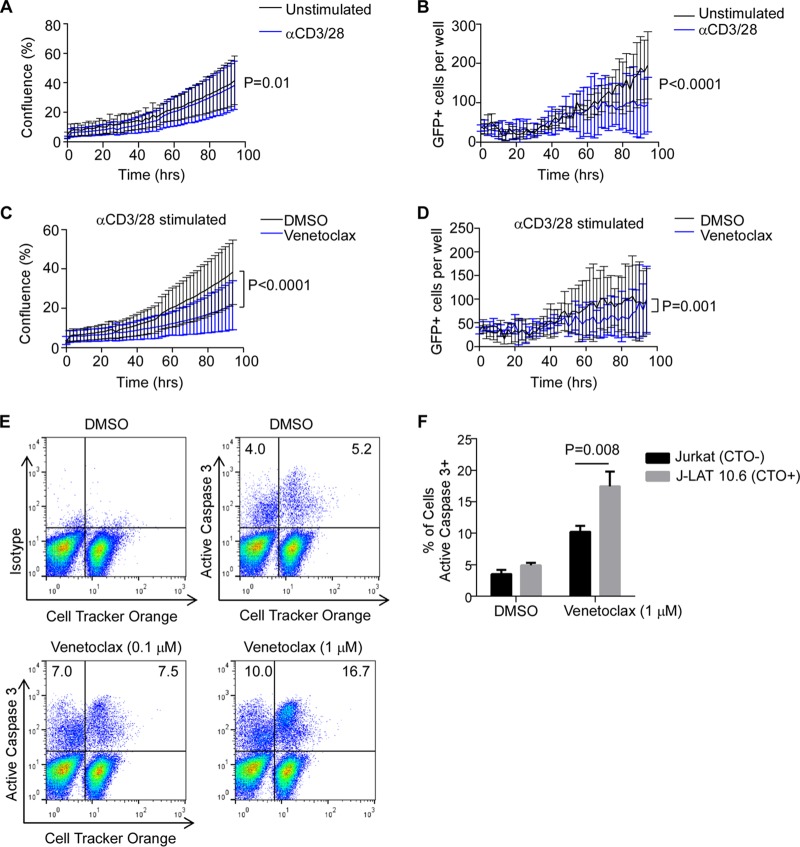
We next questioned whether venetoclax would similarly alter cell proliferation or viral protein production under conditions of T cell receptor stimulation. Treatment with αCD3/CD28 has been previously used as a positive control for HIV reactivation (35). Cell proliferation of J-Lat 10.6 cells was modestly reduced by T cell receptor stimulation alone (Fig. 2A), whereas HIV expression was significantly reduced at later time points (P < 0.0001, Fig. 2B), consistent with previous reports of HIV reactivation mediated by αCD3/CD28 stimulation inducing viral cytopathic effects in HIV-infected cells (36). Notably, the addition of venetoclax to T cell receptor stimulation further reduced cell proliferation (P < 0.0001, Fig. 2C) and HIV expression (P = 0.001, Fig. 2D).
FIG 2.
BCL2 antagonism promotes preferential apoptosis in HIV-infected cells. (A and B) J-Lat 10.6 cells were treated with αCD3/28 or a control and monitored for confluence (A) and GFP expression (B) over time. (C and D) J-Lat 10.6 cells were treated with a DMSO control or venetoclax (1 μM) plus αCD3/CD28 stimulation and monitored for confluence (C) and GFP expression (D) over time. (E) Unlabeled, uninfected Jurkat T cells were cocultured 1:1 with cell tracker orange-labeled J-Lat 10.6 cells, stimulated with αCD3/28 in the presence of antiretrovirals with or without venetoclax, and assessed for active caspase 3 expression by flow cytometry. (F) Data represent means (standard deviations [SD]) of results from three independent experiments performed as described for panel E.
Taken together, these data suggest that by impairing proliferation of chronically infected cells and expression of HIV in response to reactivating stimuli, venetoclax may antagonize the persistence and/or replenishment of the HIV reservoir.
BCL2 inhibition increases death rates of HIV-containing cells.
We next assessed whether the reduced GFP expression in J-Lat 10.6 cells associated with venetoclax treatment is due to death of reactivated cells. We have previously demonstrated that relying on detection of intracellular expression of viral protein (i.e., p24 or GFP) or nucleic acids to define infected cells in the context of apoptosis is unreliable due to the activation of endogenous proteases and nucleases during the programmed cell death process (23). In fact, this is consistent with the late decrease in GFP expression in J-Lat 10.6 cells treated with αCD3/CD28 (Fig. 2D) described above. Therefore, inducing cell death impairs the ability to track intracellular protein markers of infection, such as GFP and, therefore, likely HIV p24 as well.
To overcome this methodological limitation, we instead cocultured unlabeled, uninfected parental Jurkat cells with J-Lat 10.6 cells that had been previously labeled with the lipophilic tracking dye Cell Tracker Orange (CTO), the signal of which is not degraded during apoptosis. Unlabeled Jurkat T cells were mixed in a 1:1 ratio with CTO-labeled J Lat 10.6 cells and were treated with venetoclax or a diluent control followed by αCD3/CD28 to mimic antigenic stimulation. Treatment with venetoclax preferentially increased apoptosis to a greater degree in the HIV-infected J-Lat 10.6 cells (CTO+) than in the unlabeled and uninfected Jurkat cells, as measured by active caspase 3 expression (P = 0.008, Fig. 2E and F). The small increase in the death rate noted in uninfected Jurkat cells in response to venetoclax is consistent with clinical trial data which suggest an infrequent incidence of neutropenia upon treatment (37). These data indicate that venetoclax treatment of proliferating T cells results in the killing of more HIV-infected cells than uninfected cells.
BCL2 inhibition during acute HIV infection reduces HIV reservoir size.
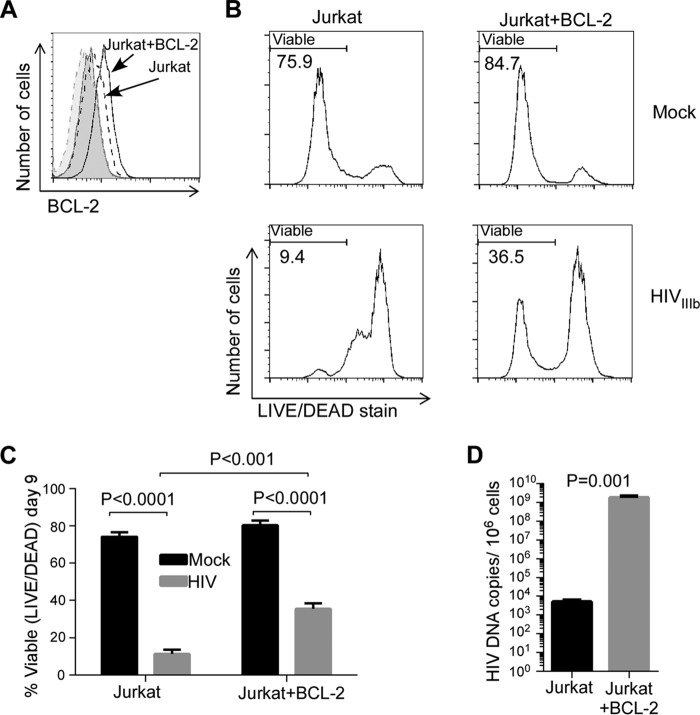
The HIV reservoir may also be repopulated by new rounds of CD4 T cell infection. HIV replicates preferentially in activated CD4 T cells, and HIV replication causes the death of some of these activated CD4 T cells but not before the HIV replicative machinery has produced a sufficient number of progeny virions to permit viral dissemination. Because BCL2 supports the survival of HIV-infected cells that reactivate HIV from latency (23), we sought to determine whether BCL2 alters the outcome of acute HIV infection in vitro using Jurkat T cells and Jurkat T cells stably overexpressing BCL2 (Jurkat+BCL2). Following HIVIIIb infection, we analyzed cultures for viability, HIV p24 production, and cell-associated HIV DNA content to determine whether these parameters were altered by BCL2. BCL2 overexpression in Jurkat+BCL2 cells compared to parental cells was confirmed by intracellular flow cytometry (Fig. 3A). The Jurkat cells that overexpressed BCL2 had increased cell survival following HIV infection (mean, 36% versus 11% viability at day 9 postinfection) (P < 0.001, Fig. 3B and C), increased cell-associated HIV DNA levels (5.5 log increase) (P = 0.001, Fig. 3D), and increased HIV p24 production in culture supernatants (2.5 log increase) (P = 0.023, data not shown) compared to parental cells. These striking differences between Jurkat and Jurkat+BCL2 cells demonstrate that BCL2 inhibits HIV-induced cell death in acute productive infection and increases the number of HIV-infected cells (reflected by increased HIV DNA), thereby allowing more cells to produce progeny virions (reflected by increased p24); moreover, it suggests that antagonizing BCL2 might have desirable reciprocal effects.
FIG 3.
BCL2 promotes survival of acutely HIV-infected T cells. (A) Parental Jurkat T cells or Jurkat T cells stably overexpressing BCL2 (Jurkat-BCL2) were stained for intracellular BCL2. Dashed lines represent Jurkat cells; solid lines represent Jurkat-BCL2 cells. Tinted areas represent isotype antibody staining. (B) Jurkat and Jurkat-BCL2 cells were infected with HIVIIIb or and were assessed for viability by LIVE/DEAD staining at day 9 postinfection. Representative flow data from three independent experiments are shown. (C) Mean (SD) of results from 3 independent experiments performed as described for panel B. (D) Cell-associated HIV DNA content was measured at day 9 postinfection.
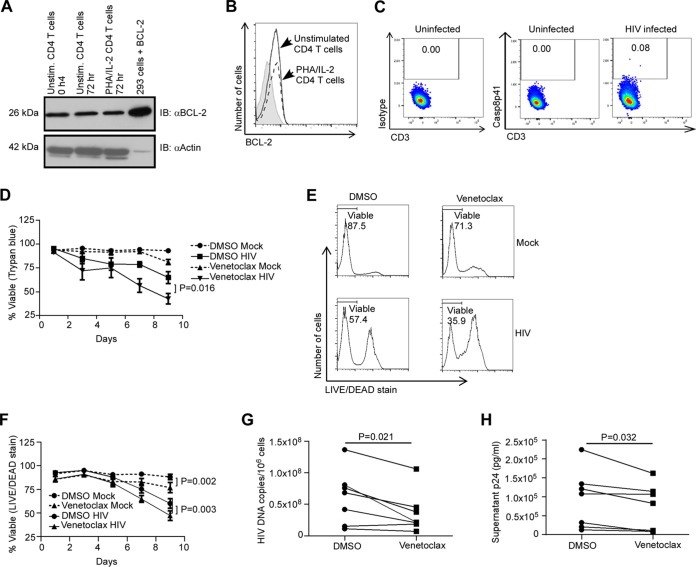
We next examined the impact of pharmacologic inhibition of BCL2 function by venetoclax on primary uninfected CD4 T cells and primary CD4 T cells acutely infected with HIV. First, we confirmed that BCL2 is expressed in both resting and activated CD4 T cells by Western blotting (Fig. 4A) and flow cytometry (Fig. 4B). Consistent with our previous results (28), activated CD4 T cells infected with HIV contained Casp8p41, whereas uninfected cells did not (Fig. 4C). Importantly, the toxicity of venetoclax in mock-infected, activated primary CD4 T cells was minimal and did not reach significance (P = 0.054, Fig. 4D) at achievable in vivo plasma concentrations (1 μM), consistent with the favorable safety profile of venetoclax in clinical trials (38). In contrast, when primary activated CD4 T cells from 7 donors were infected with HIV and analyzed for survival over time, venetoclax treatment reduced survival, as measured by trypan blue exclusion, of the infected cells compared to vehicle control treatment (P = 0.016, Fig. 4D). Similar results were observed by flow cytometry (Fig. 4E and F) using vital staining (39). These observations indicate that, similarly to proliferating HIV-infected cells (Fig. 1 and 2), acutely HIV-infected cells die preferentially in the presence of venetoclax. Consistent with this suggestion, HIV-infected cultures treated with venetoclax had decreased cell-associated HIV-1 DNA levels (mean difference, −35%) (P = 0.021, Fig. 4G) and reduced p24 production levels compared to vehicle control treatment (mean, 24.4% reduction) (P = 0.032, Fig. 4H).
FIG 4.
BCL2 antagonism decreases survival and HIV replication in acutely infected T cells. (A) BCL2 expression was assessed in resting (Unstim.) or PHA/IL-2-stimulated primary CD4 T cells by Western blotting. IB, immunoblot. (B) BCL2 expression was assessed in resting or stimulated CD4 T cells by flow cytometry. The shaded area represents an isotype control. (C) Activated primary CD4 T cells were infected with HIVIIIb or not infected and were assessed for Casp8p41 expression by flow cytometry. (D to H) Primary CD4 T cells from seven uninfected donors were infected with HIVIIIb or mock infected, treated with venetoclax (1 μM) or diluent, and assayed for cell viability by trypan blue exclusion (D) or flow cytometry (E and F). (G and H) At day 9 postinfection, levels of cell-associated HIV DNA (G) and HIV p24 concentrations in culture supernatant (H) were measured.
DISCUSSION
BCL2 is a critical regulatory molecule in lymphoid tissue homeostasis, as forced overexpression results in B cell hyperplasia (40), mutations in BCL2 are overrepresented in lymphoid malignancies (41), absence of BCL2 impairs memory cell generation and function (42), and inhibition of BCL2 is an FDA-approved approach to therapy for treatment of chronic lymphocytic leukemia (33). It is hardly surprising, then, that BCL2 also impacts HIV homeostasis in infected lymphoid cells. Additional evidence supporting the idea of the importance of BCL2 in HIV persistence is derived from an artificial model of HIV latency wherein primary CD4 T cells infected with HIV and transduced with BCL2 in vitro recapitulate many aspects of latently HIV-infected cells in vivo (43). Mechanistically, we now know that BCL2 directly binds Casp8p41, preventing the latter from binding and activating Bak to induce apoptosis (23, 30). Consequently, in the setting of HIV reactivation from latency, the BCL2 antagonist venetoclax increases Casp8p41-mediated apoptosis to promote selective infected-cell death (23). In the current report, we extend the understanding of the role of BCL2 in HIV infection by demonstrating that BCL2 is necessary for maintaining HIV in the settings of homeostatic proliferation of infected cell lines and acute spreading infection of primary CD4 T cells.
During HIV infection, inhibition of BCL2 therefore has the dual effects of (i) preventing replenishment of the HIV reservoir and (ii) directly facilitating the death of reactivating cells. Both of these effects depend on enhancing the cytotoxicity of Casp8p41, an HIV protease-specific cleavage product of cellular procaspase 8 (27) that is present exclusively in HIV-infected cells (28). Casp8p41 is expressed both in vitro and in vivo (28) and both during acute infection and during reactivation of latent HIV infection (23, 31). Once formed by cleavage of procaspase-8, Casp8p41 translocates to mitochondria (29, 44), where it binds to and activates Bak, inducing mitochondrial-dependent apoptosis (29). Casp8p41 expression in memory CD4 T cells in HIV-positive patients is inversely correlated with absolute CD4 T cell count, and changes in Casp8p41 expression predict changes in CD4 T cell count over time at the beginning of and during stable antiretroviral therapy (45, 46).
Expression of HIV proteins, which occurs de facto in the context of HIV replication, is known to modulate BCL2 expression and promote death resistance of HIV target cells. For instance, overexpressing HIV Tat, or treatment with exogenous recombinant Tat, increases BCL2 expression in multiple cell types and decreases the susceptibility of treated cells to cell death induced by multiple diverse stimuli compared to control cells (47–50). Similarly, endogenous overexpression of HIV Vpr increases BCL2 expression and decreases Bax expression, resulting in decreased susceptibility of cells to apoptosis induced by the presence of cycloheximide, tumor necrosis alpha (TNF-α), or Fas or by serum starvation treatment (51). Both HIV Tat and Vpr are proteins expressed early in the viral life cycle when the virus necessarily depends on cell survival to replicate. Conversely, later in the viral life cycle, cell death and caspase 8 activation synergistically enhance NFKB activation (52), which in turn drives HIV replication (53). HIV protease is generated late in the HIV life cycle coincident with induction of cell death. Expression of HIV protease alone leads to apoptosis, and it cleaves BCL2 between phenylalanine 112 and alanine 113 (54), along with other cellular targets (55–57); however, only cleavage of procaspase 8 is required for protease-mediated cell death (31). Casp8p41, which is generated as a consequence, also activates NF-κB and drives HIV long terminal repeat (LTR) transcription (58). Therefore, it is logical that HIV-induced increased BCL2 expression early in the viral life cycle contributes to maintenance of the HIV reservoir by promoting survival of infected cells in the setting of ongoing viral replication.
In addition to ongoing HIV replication leading to the presence of new latently infected cells, the HIV reservoir is maintained by the long-term survival and proliferation of already-infected cells. We and others have previously reported that the endogenous expression of BCL2 in central memory CD4 T cells is associated with intrinsic cell death resistance (23). Moreover, proliferation of latently infected memory CD4 T cells can be triggered by antigenic stimulation or homeostatic cytokine signals (7). Our observation that the selective BCL2 antagonist venetoclax impairs proliferation of latently infected cells induced by αCD3/CD28 and IL-7 signaling (Fig. 1 and 2) suggested that BCL2 expression and function are necessary for the survival of proliferating latently infected cells. Consistent with this conclusion, venetoclax preferentially increased cell death in latently infected cells while preferentially sparing uninfected cells that had been stimulated to proliferate in mixed cultures in our clonotypic assay (Fig. 2). Since our present study utilized a chronically HIV-infected cell line (J-Lat 10.6 cells), it would be of interest to confirm these findings using in vitro primary cell models of HIV latency in future studies.
An additional mechanism that has been proposed to contribute to clonal expansion of latently HIV-infected cells involves integration of HIV provirus into proto-oncogenes, including MKL2 and BACH2 (59). As BCL2 is also a proto-oncogene and as BCL2 inhibition favors killing of HIV-infected cells, it may also be of interest to see if target-specific inhibitors inhibit proliferation of latently infected cells with HIV provirus integrated into aberrantly activated oncogenes.
The ability of venetoclax to preferentially kill HIV-infected cells is consistent with several previous reports stating that inhibition of apoptosis in the context of productive HIV infection results in increased viral replication. Initial experiments with Jurkat T cells overexpressing the anti-apoptotic adenovirus E1B 19K protein, and infected with HIV, demonstrated decreased cell death rates and increased viral replication compared to parental cell results (60). Similarly, BCL2 overexpression in T cell clones enhanced viral replication and inhibited syncytial apoptosis compared to control cell results (61). Likewise, U937 monocytoid cells overexpressing BCL2 had decreased HIV-induced cell death and increased viral replication levels (62). Of perhaps more physiologic relevance, IL-2 treatment of peripheral blood mononuclear cells (PBMCs) from HIV-infected patients upregulated endogenous BCL2 expression and inhibited spontaneous apoptosis in vitro compared to the results seen with control treated cells (63). In addition, pharmacologic inhibition of apoptosis using the cellular pan-caspase inhibitor z-VAD-fmk increased viral replication during HIV infection in vitro and stimulated viral replication in PBMCs from HIV-infected patients ex vivo (64).
Collectively, our findings suggest that BCL2 antagonism occurring in vivo under conditions of clinically suppressive antiretroviral therapy may decrease the replenishment of the latent HIV reservoir and promote the death of latently HIV-infected cells undergoing HIV reactivation (23). These results support our “prime, shock, and kill” paradigm (65) wherein sensitization of cells toward an apoptosis-prone phenotype before HIV reactivation, before homeostatic proliferation, or before the spread of HIV infection facilitates the selective killing of HIV-infected cells. These observations suggest that further investigation of venetoclax treatment in animal models of HIV and pilot studies in HIV-positive patients might be warranted.
MATERIALS AND METHODS
Cell culture.
Primary CD4 T cells from anonymous, HIV-negative apheresis donors were obtained from leukoreduction system chambers (66). Bulk CD4 T cells were isolated by negative selection using RosetteSep Human CD4+ T Cell Enrichment Cocktail (Stemcell Technologies) according to the manufacturer's protocol and maintained in RPMI 1640 (Mediatech Inc.) supplemented with 10% fetal bovine serum (Atlanta Biologicals), 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco) at 37°C and 5% CO2. All human samples were obtained according to a Mayo Clinic Institutional Review Board-approved protocol (Mayo IRB 1039-03) in compliance with all pertinent federal regulations.
Homeostatic proliferation model.
J-Lat full-length cells (clone no. 10.6), which contain one replication-incompetent, integrated HIV provirus with a GFP gene insertion in place of the Nef gene per cell, were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, from Eric Verdin. J-Lat 10.6 cells were cultured at 10,000 cells per well in 96-well plates and treated with interleukin-7 (25 ng/ml) or plate-bound αCD3 (clone OKT3) and soluble αCD28 (1 μg/ml) or with venetoclax (1 μM) or diluent control (DMSO). Cell proliferation, as defined by culture confluence (67), and GFP expression, as defined by the number of GFP-positive cells per well, were measured over time using an IncuCyte Zoom live-cell analysis system (Essen BioScience).
Immunoblotting.
Nonactivated primary CD4 T cells and J-Lat-10.6 cells were treated with IL-7 (R&D Systems, Inc.) (25 ng/ml) for 0 or 15 min. Cells were lysed and processed for Western blot analysis. Cell lysate (30 μg) was run on 8% polyacrylamide gels and then transferred onto polyvinylidene difluoride (PVDF) membranes for 2 h at 1,200 mA in transfer buffer (24 mM Tris, 192 mM glycine). The membranes were then blocked in Tris-buffered saline–Tween (TBST) (20 mM Tris, 150 mM NaCl, 0.05% Tween 20) with 5% bovine serum albumin (BSA) (Sigma, St. Louis, MO) for 1 h at room temperature. Membranes were blotted with Phospho JAK1 (Tyr1022/1023) or total JAK1 (Cell Signaling, Danvers, MA) or anti-actin (Sigma, St. Louis, MO) primary antibody. The blot was washed, exposed to horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h, and, finally, examined by chemiluminescence using a SuperSignal West Pico chemiluminescent substrate kit (Thermo Fisher Scientific, Waltham, MA).
Acute HIV infections.
Jurkat and Jurkat-BCL2 cells were infected overnight, while primary phytohemagglutinin (PHA)-activated CD4 T cells were infected for 6 h with HIV-1 IIIb (NIH AIDS Reagent Program). Aliquots of the same pooled infectious supernatant were used for all experiments. Cells were then washed 3 times and incubated in fresh complete medium. HIV-1 p24 levels in the cell culture supernatant were measured by the use of RETROtek enzyme-linked immunosorbent assay (ELISA) kits (Zeptometrix Corporation) according to the manufacturer's protocol. Cell-associated HIV-1 DNA levels were measured by quantitative PCR (qPCR) using a validated assay as previously described (23). The lower limit of detection of HIV-1 DNA using the qPCR assay was 50 copies/106 cells; levels in all mock-infected cultures were below this limit.
Cell mixing experiment.
J-Lat-10.6 cells were labeled with Cell Tracker Orange (C2927; Molecular Probes, Invitrogen, CA) according to the manufacturer's instructions. The Cell Tracker Orange-labeled J-Lat-10.6 cells were washed and mixed with unlabeled Jurkat cells at a ratio of 1:1 using RPMI 1640 containing antiretroviral agents. Coculture cells were treated with or without venetoclax (0.1 μM or 1 μM) for 3 days. At the end of experimental period, cells were collected, washed, and fixed with 2% paraformaldehyde for 24 h. Coculture cells were processed for intracellular staining of active caspase 3 (BD Biosciences) and then analyzed by flow cytometry using an LSR Fortessa X-20 cell analyzer (BD Biosciences). CellTracker orange-positive (J-LAT10.6) and -negative (Jurkat) cells were analyzed and gated using FlowJo software (FlowJo LLC, Ashland, OR).
Flow cytometry.
Intracellular BCL2 levels were measured using anti-human BCL2-fluorescein isothiocyanate (BCL2-FITC) (clone BCL2/100; eBioscience, San Diego, CA). Briefly, fixed cells were permeabilized in 0.1% NP-40–phosphate-buffered saline (PBS)–5% bovine serum albumin (BSA) on ice for 30 min, stained with 0.4 μg/ml anti-BCL2-FITC or an isotype control on ice for 60 min, washed, and fixed. Cell death was measured using LIVE/DEAD fixable aqua dead-cell stain (Invitrogen) according to the manufacturer's protocol. Intracellular detection of Casp8p41 was performed as previously described (23). Cell cycle analysis using DNA content determinations was performed as previously described (68). Fluorescence-activated cell sorter (FACS) analysis was performed using either a FACScan analyzer or LSRII flow cytometer (BD Biosciences) based on multiparameter needs. FACS data were analyzed using FlowJo software (Tree Star Inc.).
Statistics.
Pooled data are generally expressed as means and standard deviations, except where noted. Paired or unpaired t tests, or nonparametric equivalents, depending on the experimental design, were used to compare differences between means. Repeated measures were analyzed by linear regression. P values of <0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc.).
ACKNOWLEDGMENTS
We thank the study participants that provided samples.
This publication was made possible by CTSA grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), as well as by NIH grants R01 AI110173 and R01 AI120698.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. 1998. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavez L, Calvanese V, Verdin E. 2015. HIV latency is established directly and early in both resting and activated primary CD4 T cells. PLoS Pathog 11:e1004955. doi: 10.1371/journal.ppat.1004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siliciano JM, Siliciano RF. 2015. The remarkable stability of the latent reservoir for HIV-1 in resting memory CD4+ T cells. J Infect Dis 212:1345–1347. doi: 10.1093/infdis/jiv219. [DOI] [PubMed] [Google Scholar]
- 4.von Stockenstrom S, Odevall L, Lee E, Sinclair E, Bacchetti P, Killian M, Epling L, Shao W, Hoh R, Ho T, Faria NR, Lemey P, Albert J, Hunt P, Loeb L, Pilcher C, Poole L, Hatano H, Somsouk M, Douek D, Boritz E, Deeks SG, Hecht FM, Palmer S. 2015. Longitudinal genetic characterization reveals that cell proliferation maintains a persistent HIV type 1 DNA pool during effective HIV therapy. J Infect Dis 212:596–607. doi: 10.1093/infdis/jiv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney MF, Wiegand A, Shao W, Coffin JM, Mellors JW, Lederman M, Gandhi RT, Keele BF, Li JZ. 2015. Origin of rebound plasma HIV includes cells with identical proviruses that are transcriptionally active before stopping of antiretroviral therapy. J Virol 90:1369–1376. doi: 10.1128/JVI.02139-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonetti FR, Sobolewski MD, Fyne E, Shao W, Spindler J, Hattori J, Anderson EM, Watters SA, Hill S, Wu X, Wells D, Su L, Luke BT, Halvas EK, Besson G, Penrose KJ, Yang Z, Kwan RW, Van Waes C, Uldrick T, Citrin DE, Kovacs J, Polis MA, Rehm CA, Gorelick R, Piatak M, Keele BF, Kearney MF, Coffin JM, Hughes SH, Mellors JW, Maldarelli F. 2016. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci U S A 113:1883–1888. doi: 10.1073/pnas.1522675113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandergeeten C, Fromentin R, DaFonseca S, Lawani MB, Sereti I, Lederman MM, Ramgopal M, Routy JP, Sekaly RP, Chomont N. 2013. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 121:4321–4329. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Günthard HF, Frost SD, Leigh-Brown AJ, Ignacio CC, Kee K, Perelson AS, Spina CA, Havlir DV, Hezareh M, Looney DJ, Richman DD, Wong JK. 1999. Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J Virol 73:9404–9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frenkel LM, Wang Y, Learn GH, McKernan JL, Ellis GM, Mohan KM, Holte SE, De Vange SM, Pawluk DM, Melvin AJ, Lewis PF, Heath LM, Beck IA, Mahalanabis M, Naugler WE, Tobin NH, Mullins JI. 2003. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J Virol 77:5721–5730. doi: 10.1128/JVI.77.10.5721-5730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Kosakovsky Pond SL, Chung YS, Penugonda S, Chipman JG, Fletcher CV, Schacker TW, Malim MH, Rambaut A, Haase AT, McLean AR, Wolinsky SM. 2016. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 530:51–56. doi: 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, Hill AL, Busch MP, Armand P, Soiffer RJ, Altfeld M, Kuritzkes DR. 2014. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavigner M, Watkins B, Lawson B, Lee ST, Chahroudi A, Kean L, Silvestri G. 2014. Persistence of virus reservoirs in ART-treated SHIV-infected rhesus macaques after autologous hematopoietic stem cell transplant. PLoS Pathog 10:e1004406. doi: 10.1371/journal.ppat.1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaminski R, Bella R, Yin C, Otte J, Ferrante P, Gendelman HE, Li H, Booze R, Gordon J, Hu W, Khalili K. 2016. Excision of HIV-1 DNA by gene editing: a proof-of-concept in vivo study. Gene Ther 23:690–695. doi: 10.1038/gt.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminski R, Chen Y, Salkind J, Bella R, Young WB, Ferrante P, Karn J, Malcolm T, Hu W, Khalili K. 2016. Negative feedback regulation of HIV-1 by gene editing strategy. Sci Rep 6:31527. doi: 10.1038/srep31527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH. 2012. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahu GK, Sango K, Selliah N, Ma Q, Skowron G, Junghans RP. 2013. Anti-HIV designer T cells progressively eradicate a latently infected cell line by sequentially inducing HIV reactivation then killing the newly gp120-positive cells. Virology 446:268–275. doi: 10.1016/j.virol.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B, Zou F, Lu L, Chen C, He D, Zhang X, Tang X, Liu C, Li L, Zhang H. 2016. Chimeric antigen receptor T cells guided by the single-chain Fv of a broadly neutralizing antibody specifically and effectively eradicate virus reactivated from latency in CD4+ T lymphocytes isolated from HIV-1-infected individuals receiving suppressive combined antiretroviral therapy. J Virol 90:9712–9724. doi: 10.1128/JVI.00852-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill AL, Green SA, Abdullah S, Le Saout C, Pittaluga S, Chen H, Turnier R, Lifson J, Godin S, Qin J, Sneller MC, Cuillerot JM, Sabzevari H, Lane HC, Catalfamo M. 2016. Programed death-1/programed death-ligand 1 expression in lymph nodes of HIV infected patients: results of a pilot safety study in rhesus macaques using anti-programed death-ligand 1 (Avelumab). AIDS 30:2487–2493. doi: 10.1097/QAD.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lum JJ, Schnepple DJ, Nie Z, Sanchez-Dardon J, Mbisa GL, Mihowich J, Hawley N, Narayan S, Kim JE, Lynch DH, Badley AD. 2004. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. J Virol 78:6033–6042. doi: 10.1128/JVI.78.11.6033-6042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seay K, Church C, Zheng JH, Deneroff K, Ochsenbauer C, Kappes JC, Liu B, Jeng EK, Wong HC, Goldstein H. 2015. In vivo activation of human NK cells by treatment with an interleukin-15 superagonist potently inhibits acute in vivo HIV-1 infection in humanized mice. J Virol 89:6264–6274. doi: 10.1128/JVI.00563-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correia C, Lee SH, Meng XW, Vincelette ND, Knorr KL, Ding H, Nowakowski GS, Dai H, Kaufmann SH. 2015. Emerging understanding of Bcl-2 biology: implications for neoplastic progression and treatment. Biochim Biophys Acta 1853:1658–1671. doi: 10.1016/j.bbamcr.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummins NW, Sainski AM, Dai H, Natesampillai S, Pang YP, Bren GD, de Araujo Correia MC, Sampath R, Rizza SA, O'Brien D, Yao JD, Kaufmann SH, Badley AD. 2016. Prime, shock, and kill: priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J Virol 90:4032–4048. doi: 10.1128/JVI.03179-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luna-Vargas MP, Chipuk JE. 2016. The deadly landscape of pro-apoptotic BCL-2 proteins in the outer mitochondrial membrane. FEBS J 283:2676–2689. doi: 10.1111/febs.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doerflinger M, Glab JA, Puthalakath H. 2015. BH3-only proteins: a 20-year stock-take. FEBS J 282:1006–1016. doi: 10.1111/febs.13190. [DOI] [PubMed] [Google Scholar]
- 26.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol 78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nie Z, Phenix BN, Lum JJ, Alam A, Lynch DH, Beckett B, Krammer PH, Sekaly RP, Badley AD. 2002. HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome c, caspase cleavage and nuclear fragmentation. Cell Death Differ 9:1172–1184. doi: 10.1038/sj.cdd.4401094. [DOI] [PubMed] [Google Scholar]
- 28.Nie Z, Bren GD, Vlahakis SR, Schimnich AA, Brenchley JM, Trushin SA, Warren S, Schnepple DJ, Kovacs CM, Loutfy MR, Douek DC, Badley AD. 2007. Human immunodeficiency virus type 1 protease cleaves procaspase 8 in vivo. J Virol 81:6947–6956. doi: 10.1128/JVI.02798-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sainski AM, Natesampillai S, Cummins NW, Bren GD, Taylor J, Saenz DT, Poeschla EM, Badley AD. 2011. The HIV-1-specific protein Casp8p41 induces death of infected cells through Bax/Bak. J Virol 85:7965–7975. doi: 10.1128/JVI.02515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sainski AM, Dai H, Natesampillai S, Pang YP, Bren GD, Cummins NW, Correia C, Meng XW, Tarara JE, Ramirez-Alvarado M, Katzmann DJ, Ochsenbauer C, Kappes JC, Kaufmann SH, Badley AD. 2014. Casp8p41 generated by HIV protease kills CD4 T cells through direct Bak activation. J Cell Biol 206:867–876. doi: 10.1083/jcb.201405051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie Z, Bren GD, Rizza SA, Badley AD. 2008. HIV protease cleavage of procaspase 8 is necessary for death of HIV-infected cells. Open Virol J 2:1–7. doi: 10.2174/1874357900802010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan A, Bisgrove D, Verdin E. 2003. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts AW, Huang D. 26 November 2016. Targeting BCL2 with BH3 mimetics: basic science and clinical application of venetoclax in chronic lymphocytic leukemia and related B cell malignancies. Clin Pharmacol Ther doi: 10.1002/cpt.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HR, Hwang KA, Kim KC, Kang I. 2007. Down-regulation of IL-7Ralpha expression in human T cells via DNA methylation. J Immunol 178:5473–5479. doi: 10.4049/jimmunol.178.9.5473. [DOI] [PubMed] [Google Scholar]
- 35.Venkatachari NJ, Zerbato JM, Jain S, Mancini AE, Chattopadhyay A, Sluis-Cremer N, Bar-Joseph Z, Ayyavoo V. 2015. Temporal transcriptional response to latency reversing agents identifies specific factors regulating HIV-1 viral transcriptional switch. Retrovirology 12:85. doi: 10.1186/s12977-015-0211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. 2012. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, Puvvada S, Kipps TJ, Anderson MA, Salem AH, Dunbar M, Zhu M, Peale F, Ross JA, Gressick L, Desai M, Kim SY, Verdugo M, Humerickhouse RA, Gordon GB, Gerecitano JF. 2017. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 35:826–833. doi: 10.1200/JCO.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF. 2016. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummins NW, Sainski AM, Natesampillai S, Bren GD, Badley AD. 2014. Choice of antiretroviral therapy differentially impacts survival of HIV-infected CD4 T cells. Mol Cell Ther 2:1. doi: 10.1186/2052-8426-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, Korsmeyer SJ. 1989. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 41.Pegoraro L, Palumbo A, Erikson J, Falda M, Giovanazzo B, Emanuel BS, Rovera G, Nowell PC, Croce CM. 1984. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci U S A 81:7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nuñez G, Hockenbery D, McDonnell TJ, Sorensen CM, Korsmeyer SJ. 1991. Bcl-2 maintains B cell memory. Nature 353:71–73. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- 43.Yang HC, Xing S, Shan L, O'Connell K, Dinoso J, Shen A, Zhou Y, Shrum CK, Han Y, Liu JO, Zhang H, Margolick JB, Siliciano RF. 2009. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest 119:3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Algeciras-Schimnich A, Belzacq-Casagrande AS, Bren GD, Nie Z, Taylor JA, Rizza SA, Brenner C, Badley AD. 2007. Analysis of HIV protease killing through caspase 8 reveals a novel interaction between caspase 8 and mitochondria. Open Virol J 1:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cummins NW, Jiang W, McGinty J, Bren GD, Bosch RJ, Landay A, Deeks SG, Martin JN, Douek D, Lederman MM, Brenchley J, Badley AD. 2010. Intracellular Casp8p41 content is inversely associated with CD4 T cell count. J Infect Dis 202:386–391. doi: 10.1086/653705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummins NW, Neuhaus J, Sainski AM, Strausbauch MA, Wettstein PJ, Lewin SR, Plana M, Rizza SA, Temesgen Z, Touloumi G, Freiberg M, Neaton J, Badley AD. 2014. Short communication: CD4 T cell declines occurring during suppressive antiretroviral therapy reflect continued production of Casp8p41. AIDS Res Hum Retroviruses 30:476–479. doi: 10.1089/aid.2013.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zauli G, Gibellini D, Caputo A, Bassini A, Negrini M, Monne M, Mazzoni M, Capitani S. 1995. The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood 86:3823–3834. [PubMed] [Google Scholar]
- 48.Wang Z, Morris GF, Reed JC, Kelly GD, Morris CB. 1999. Activation of Bcl-2 promoter-directed gene expression by the human immunodeficiency virus type-1 Tat protein. Virology 257:502–510. doi: 10.1006/viro.1999.9688. [DOI] [PubMed] [Google Scholar]
- 49.Zhang M, Li X, Pang X, Ding L, Wood O, Clouse KA, Hewlett I, Dayton AI. 2002. Bcl-2 upregulation by HIV-1 Tat during infection of primary human macrophages in culture. J Biomed Sci 9:133–139. doi: 10.1007/BF02256024. [DOI] [PubMed] [Google Scholar]
- 50.Zheng L, Yang Y, Guocai L, Pauza CD, Salvato MS. 2007. HIV Tat protein increases Bcl-2 expression in monocytes which inhibits monocyte apoptosis induced by tumor necrosis factor-alpha-related apoptosis-induced ligand. Intervirology 50:224–228. doi: 10.1159/000100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conti L, Rainaldi G, Matarrese P, Varano B, Rivabene R, Columba S, Sato A, Belardelli F, Malorni W, Gessani S. 1998. The HIV-1 Vpr protein acts as a negative regulator of apoptosis in a human lymphoblastoid T cell line: possible implications for the pathogenesis of AIDS. J Exp Med 187:403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaudhary PM, Eby MT, Jasmin A, Kumar A, Liu L, Hood L. 2000. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene 19:4451–4460. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 53.Kretzschmar M, Meisterernst M, Scheidereit C, Li G, Roeder RG. 1992. Transcriptional regulation of the HIV-1 promoter by NF-kappa B in vitro. Genes Dev 6:761–774. doi: 10.1101/gad.6.5.761. [DOI] [PubMed] [Google Scholar]
- 54.Strack PR, Frey MW, Rizzo CJ, Cordova B, George HJ, Meade R, Ho SP, Corman J, Tritch R, Korant BD. 1996. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc Natl Acad Sci U S A 93:9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoeman RL, Sachse C, Honer B, Mothes E, Kaufmann M, Traub P. 1993. Cleavage of human and mouse cytoskeletal and sarcomeric proteins by human immunodeficiency virus type 1 protease. Actin, desmin, myosin, and tropomyosin. Am J Pathol 142:221–230. [PMC free article] [PubMed] [Google Scholar]
- 56.Shoeman RL, Huttermann C, Hartig R, Traub P. 2001. Amino-terminal polypeptides of vimentin are responsible for the changes in nuclear architecture associated with human immunodeficiency virus type 1 protease activity in tissue culture cells. Mol Biol Cell 12:143–154. doi: 10.1091/mbc.12.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rumlová M, Křížová I, Keprová A, Hadravová R, Doležal M, Strohalmová K, Pichová I, Hájek M, Ruml T. 2014. HIV-1 protease-induced apoptosis. Retrovirology 11:37. doi: 10.1186/1742-4690-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bren GD, Whitman J, Cummins N, Shepard B, Rizza SA, Trushin SA, Badley AD. 2008. Infected cell killing by HIV-1 protease promotes NF-kappaB dependent HIV-1 replication. PLoS One 3:e2112. doi: 10.1371/journal.pone.0002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH. 2014. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345:179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antoni BA, Sabbatini P, Rabson AB, White E. 1995. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J Virol 69:2384–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandstrom PA, Pardi D, Goldsmith CS, Chengying D, Diamond AM, Folks TM. 1996. bc1-2 expression facilitates human immunodeficiency virus type-1 mediated cytopathic effects during acute spreading infections. J Virol 70:4617–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marshall WL, Datta R, Hanify K, Teng E, Finberg RW. 1999. U937 cells overexpressing bcl-xl are resistant to human immunodeficiency virus-1-induced apoptosis and human immunodeficiency virus-1 replication. Virology 256:1–7. doi: 10.1006/viro.1999.9599. [DOI] [PubMed] [Google Scholar]
- 63.Adachi Y, Oyaizu N, Than S, McCloskey TW, Pahwa S. 1996. IL-2 rescues in vitro lymphocyte apoptosis in patients with HIV infection: correlation with its ability to block culture-induced down-modulation of Bcl-2. J Immunol 157:4184–4193. [PubMed] [Google Scholar]
- 64.Chinnaiyan AM, Woffendin C, Dixit VM, Nabel GJ. 1997. The inhibition of pro-apoptotic ICE-like proteases enhances HIV replication. Nat Med 3:333–337. doi: 10.1038/nm0397-333. [DOI] [PubMed] [Google Scholar]
- 65.Badley AD, Sainski A, Wightman F, Lewin SR. 2013. Altering cell death pathways as an approach to cure HIV infection. Cell Death Dis 4:e718. doi: 10.1038/cddis.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dietz AB, Bulur PA, Emery RL, Winters JL, Epps DE, Zubair AC, Vuk-Pavlovic S. 2006. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion 46:2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 67.Single A, Beetham H, Telford BJ, Guilford P, Chen A. 2015. A comparison of real-time and endpoint cell viability assays for improved synthetic lethal drug validation. J Biomol Screen 20:1286–1293. doi: 10.1177/1087057115605765. [DOI] [PubMed] [Google Scholar]
- 68.Cummins NW, Klicpera A, Sainski AM, Bren GD, Khosla S, Westendorf JJ, Badley AD. 2011. Human immunodeficiency virus envelope protein Gp120 induces proliferation but not apoptosis in osteoblasts at physiologic concentrations. PLoS One 6:e24876. doi: 10.1371/journal.pone.0024876. [DOI] [PMC free article] [PubMed] [Google Scholar]