ABSTRACT
In 2007, we reported a patient with an atypical form of Creutzfeldt-Jakob disease (CJD) heterozygous for methionine-valine (MV) at codon 129 who showed a novel pathological prion protein (PrPTSE) conformation with an atypical glycoform (AG) profile and intraneuronal PrP deposition. In the present study, we further characterize the conformational properties of this pathological prion protein (PrPTSE MVAG), showing that PrPTSE MVAG is composed of multiple conformers with biochemical properties distinct from those of PrPTSE type 1 and type 2 of MV sporadic CJD (sCJD). Experimental transmission of CJD-MVAG to bank voles and gene-targeted transgenic mice carrying the human prion protein gene (TgHu mice) showed unique transmission rates, survival times, neuropathological changes, PrPTSE deposition patterns, and PrPTSE glycotypes that are distinct from those of sCJD-MV1 and sCJD-MV2. These biochemical and experimental data suggest the presence of a novel prion strain in CJD-MVAG.
IMPORTANCE Sporadic Creutzfeldt-Jakob disease is caused by the misfolding of the cellular prion protein, which assumes two different major conformations (type 1 and type 2) and, together with the methionine/valine polymorphic codon 129 of the prion protein gene, contribute to the occurrence of distinct clinical-pathological phenotypes. Inoculation in laboratory rodents of brain tissues from the six possible combinations of pathological prion protein types with codon 129 genotypes results in the identification of 3 or 4 strains of prions. We report on the identification of a novel strain of Creutzfeldt-Jakob disease isolated from a patient who carried an abnormally glycosylated pathological prion protein. This novel strain has unique biochemical characteristics, does not transmit to humanized transgenic mice, and shows exclusive transmission properties in bank voles. The identification of a novel human prion strain improves our understanding of the pathogenesis of the disease and of possible mechanisms of prion transmission.
KEYWORDS: Creutzfeldt-Jakob disease, humanized mice, prion strain, prions
INTRODUCTION
The pathogenic event common to all transmissible spongiform encephalopathy (TSE) or prion diseases is the conformational conversion of the cellular prion protein (PrPC) into an abnormal, protease-resistant form (PrPTSE). Because PrPC is a glycosylphosphatidylinositol (GPI)-anchored glycoprotein with two variably occupied N-linked glycan sites, PrPC and PrPTSE appear on Western blots as di-, mono-, and unglycosylated isoforms (1).
Sporadic Creutzfeldt-Jakob disease (sCJD) is the most common form of TSE disease in humans and is clinically characterized by progressive dementia associated with a wide spectrum of neurological signs and a mean disease duration of about 7 months (2). The different clinical and pathological phenotypes of sCJD are grouped into molecular subtypes according to the methionine (M)/valine (V) polymorphic prion protein gene (PRNP) at codon 129 and the PrPTSE glycotype (type 1 or 2) (3, 4). PrPTSE type 1 and type 2 are distinguishable based on the distinct electrophoretic mobilities of proteinase K (PK)-resistant PrP fragments at 19 kDa for type 1 and 17.5 kDa for type 2 (5–7). PrPTSE type 1 has a main N-terminal PK cleavage site at residue G82 and type 2 at residue S97 (8). The combination of M and V with type 1 or 2 of PrPTSE gives six basic combinations (MM1, MV1, VV1, MM2, VV2, and MV2), but there are two distinct MM2 subtypes: MM2C, where PrPTSE type 2 accumulates mostly in the brain cortex, and MM2T, where it is mostly located in the thalamus (6, 8). Finally, in several cases, both PrPTSE types have occurred in the brain of the same patient (9). Despite the fact that molecular subtyping recognizes 7 different possible forms of sCJD, experimental transmission in bank voles (10) or gene-targeted transgenic mice expressing human PrP (TgHu) (11) revealed 4 different strains, based on survival times and central nervous system (CNS) lesion profiles in recipient rodents. The sporadic CJD M1 strain was isolated from MM1 and MV1 inocula, the M2 strain from MM2, the V1 strain from VV1, and the V2 strain from MV2 and VV2 sCJD inocula.
We report on the characterization of an additional subtype of CJD that showed unique properties in transmission studies in bank voles and TgHu mice compared with previously isolated strains (10, 11). We also show that a single PrPTSE contains multiple conformers, which propagate with different transmission properties.
RESULTS
The clinical, molecular, and pathological features of the atypical CJD patient have been previously described (12). Briefly, the patient was a 69-year-old woman carrying the heterozygous MV polymorphism at codon 129 of the PRNP gene and no point or insert mutations. PrPTSE showed aberrant glycosylation (MVAG) and peculiar neuropathological features, such as preferential intraneuronal and intra-axonal PrPTSE deposition (12). We previously reported that the patient had distinctive features from variably protease-sensitive prionopathy (VPSPr) and familial prion diseases associated with altered ratios of the three PrP glycoforms (12–15). The patient had been treated for several years with phospholipids extracted from bovine brains (12).
To provide a consistent comparative analysis, positive controls for biochemical and transmission studies included brain tissues from codon 129 PrP-heterozygous sCJD patients with either type 1 (sCJD-MV1) or type 2 (sCJD-MV2) PrPTSE.
The PrPTSE MVAG glycotype shows molecular properties distinct from those of MV1 and MV2 in human brain samples.
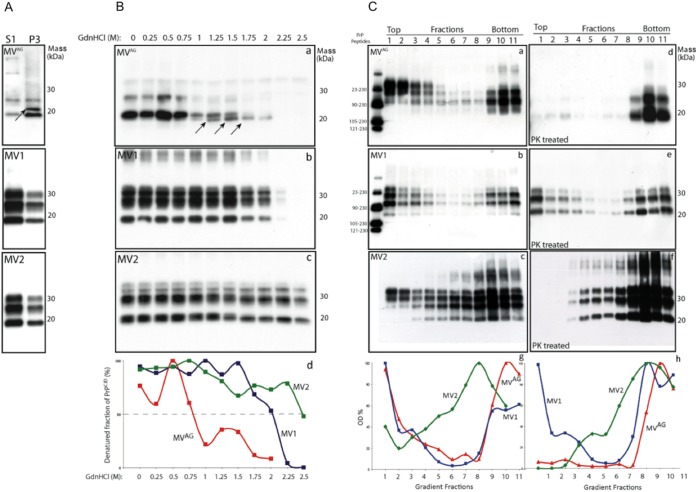
Immunoblot analyses of PrPTSE following high-speed centrifugation of CJD-MVAG brain homogenate revealed in the supernatant mono- and unglycosylated band, while in the detergent-insoluble fractions (P3), the unglycosylated isoform separated as two bands migrating at 23 and 19 kDa (Fig. 1A), representing two major PrPTSE PK-resistant fragments. The 23-kDa band was detected only in the insoluble fraction from 300 mg of brain tissue, indicating a relatively small amount compared with the 19-kDa band (Fig. 1A, P3, arrow). These bands correspond to PrPTSE peptides with N-terminal endings at residues 67 to 71 and 90 (12). In contrast, PrPTSE from sCJD-MV1 and sCJD-MV2 brain samples comigrated in both S1 and P3 fractions (Fig. 1A).
FIG 1.
Biochemical and conformational properties of PrPTSE in MVAG, MV1, and MV2 brain samples. (A) Characterization of PrPTSE soluble and insoluble in nondenaturing detergents. Shown are detergent-soluble (S1) and detergent-insoluble (P3) fractions obtained from MVAG, MV1, and MV2 frontal cortices following ultracentrifugation and proteinase K digestion. In MVAG cortices, the S1 fraction PrPTSE is represented by a monoglycosylated and an unglycosylated band, while in the P3 fraction, the unglycosylated band (arrow) shows an upper band of 23 and a lower band of 19 kDa. In contrast, PrPTSE in MV1 and MV2 cortices shows three bands representing the differently glycosylated isoforms of PrPTSE, and the unglycosylated band migrates at 19 and 21 kDa, respectively. The membranes were probed with 3F4. (B) Conformational stability assay in MVAG, MV1, and MV2 brain homogenates. Western blot analyses of total brain homogenate following denaturation with various concentrations of GdnHCl from 0 to 2.5 M. In MVAG homogenates, PrPTSE was digested at 2.0 M GdnHCl, and the unglycosylated band separated as two bands (arrows) with distinct conformational stabilities. The conformational stability of PrPTSE was analyzed by plotting the fraction of PrPTSE as a function of the GdnHCl concentration. The GdnHCl1/2 value for MVAG was 0.8 M; for MV1, 2.0 M; and for MV2, 2.5 M. The membranes were probed with 3F4. (C) Fractionation of PrPTSE aggregates in MVAG, MV1, and MV2 brain homogenates. The brain homogenates were sedimented in a 10 to 60% sucrose gradient. (Right) After sedimentation, half samples were PK treated (MVAG, MV1, and MV2). PrPTSE MVAG was distributed across all the fractions, albeit concentrated in the top (lanes 1 to 4) and bottom (lanes 9 to 11) fractions. A similar separation pattern was observed for PrPTSE MV1. In contrast, PrPTSE MV2 sedimented in the lower fractions. Following PK treatment, PrPTSE MVAG was composed of large aggregates, while PrPTSE MV1 was mainly concentrated in the upper and lower fractions and PrPTSE MV2 at the bottom (the PrP synthetic peptides in the first lane were used as PrP size markers). The representations of PrPTSE size aggregates were produced by plotting the amount of PrP as a function of the sucrose concentration. The membranes were probed with 3F4.
Next, we compared the conformational stabilities of PrPTSE in brain tissue from CJD-MVAG, sCJD-MV1, and sCJD-MV2 by determining the concentration of guanidine hydrochloride (GdnHCl) that solubilizes 50% of the PrPTSE (GdnHCl1/2). Western blot analysis showed that the GdnHCl1/2 concentration was 0.8 M for CJD-MVAG (Fig. 1B, a), 2.0 M for sCJD-MV1, and 2.5 M for sCJD-MV2 (Fig. 1B, b and c), suggesting less conformation stability of MVAG than of control positive samples. At 1.0 M GdnHCl, the CJD-MVAG unglycosylated band separated as a doublet with different resistances to proteolysis; the upper band was completely digested at 1.75 M GdnHCl, while the lower band was detectable at a 1.0 M concentration of GdnHCl. This band reached a peak at 1.5 M and progressively disappeared at 2.25 M (Fig. 1B, a, arrows). As expected, the relatively small amount of the 23-kDa band in total brain homogenate precluded its detection. These findings indicate that PrPTSE CJD-MVAG contains multiple conformers with different conformational stabilities.
Further evidence on distinct conformational properties of PrPTSE in sCJD-MV subtypes was determined by measuring the sizes of PrPTSE aggregates by sedimentation velocity in sucrose gradients before and after PK digestion (Fig. 1C). Before PK digestion, CJD-MVAG and sCJD-MV1 PrPTSE were mainly detected in the top (fractions 1 to 4) and bottom (fractions 9 to 11) sucrose gradient fractions, while in sCJD-MV2, PrPTSE gradually increased toward the bottom fractions (Fig. 1C, c).
After PK digestion, CJD-MVAG PrPTSE was confined to the bottom fractions (Fig. 1C, d), sCJD-MV1 PrPTSE had a bimodal distribution, and sCJD-MV2 PrPTSE showed increased amounts from the top to the bottom of sucrose fractions (Fig. 1C, e and f). Thus, conformational assays indicated that PrPTSE associated with sCJD-MV1, sCJD-MV2, and CJD-MVAG has distinct biochemical properties, suggesting that distinct prion conformers characterize each sCJD-MV subtype.
Transmission studies in TgHu mice indicate that MVAG behaves as a distinct sCJD-MV strain.
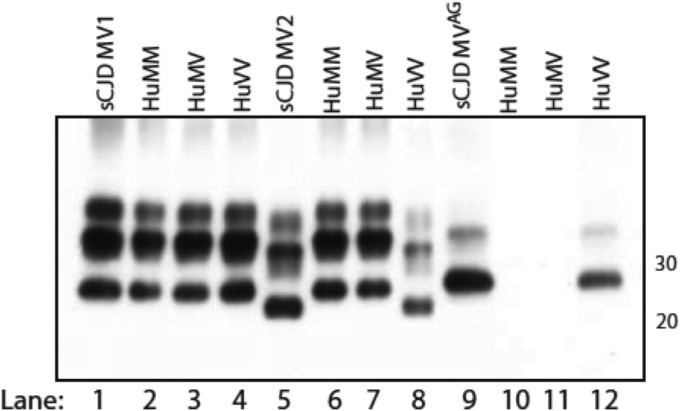
TgHu mice carrying the MM, MV, or VV genotype at codon 129 did not develop clinical disease after intracerebral inoculation of CJD-MVAG brain tissue. Neuropathological examination of TgHu mice did not show spongiform changes or PrPTSE deposition by immunohistochemistry (IHC). In contrast, in 4 of 18 HuVV-infected mice, Western blot analysis revealed an unglycosylated dominant PrPTSE glycotype reproducing the atypical pattern of the inoculum (Fig. 2, lane 12 versus 9). Western blot analyses of brain samples from CJD-MVAG-inoculated TgHuMM and TgHuMV mice were negative (Fig. 2, lanes 10 and 11).
FIG 2.
Western blot comparison of PrPTSE patterns in the brains of the three TgHu mouse genotypes and in the corresponding CJD inocula. Mice inoculated with sCJD-MV1 (lanes 2 to 4) produced a PrPTSE type identical to that of the original inoculum (lane 1) regardless of the genotype. In the MV2 group (lanes 5 to 8), only the HuVV genotype (lane 8) reproduced the original type 2 PrPTSE (lane 5). Atypical CJD PrPTSE MVAG (lane 9) with features identical to those of the original inoculum was observed only in HuVV mice (lane 12). No PrPTSE was detected in HuMM and HuMV mice injected with CJD-MVAG (lanes 10 and 11). Approximate molecular masses (kilodaltons) are on the right. The membranes were probed with 3F4.
As positive and relevant controls of CJD-MVAG, we inoculated one sCJD-MV1 and two sCJD-MV2 brain homogenate samples into the three lines of TgHu mice (MM, MV, and VV). The sCJD-MV1 inoculum induced disease in all three lines of TgHu mice with relatively similar attack rates and survival times (Table 1). The attack rate of the sCJD-MV2 inocula was between 80 and 100% in all the lines of TgHu mice, but the mean survival time was much shorter in TgHuVV mice than in TgHuMM and -MV mice (Table 1).
TABLE 1.
Survival times and rates of transmission of different CJD-MV inocula in TgHu mice
| Inoculum from human CJD | TgHu genotype | Survival time of PrPTSE-positive animals (days) (mean ± SD) | No. (%) PrPTSE-positive/no. animals tested | PrPTSE type |
|---|---|---|---|---|
| sCJD-MV1 | MM | 425 ± 71 | 12/15 (80) | 1 |
| VV | 542 ± 102 | 9/11 (82) | 1 | |
| MV | 501 ± 99 | 16/17 (94) | 1 | |
| sCJD-MV2 no. 1 | MM | 502 ± 88 | 16/16 (100) | 1 |
| VV | 229 ± 18 | 20/20 (100) | 2 | |
| MV | 519 ± 100 | 17/17 (100) | 1 | |
| sCJD-MV2 no. 2 | MM | 510 ± 62 | 13/16 (81) | 1 |
| VV | 258 ± 21 | 20/20 (100) | 2 | |
| MV | 541 ± 99 | 17/19 (89) | 1 | |
| CJD-MVAG | MM | −a | 0/14 (0) | |
| VV | 626 ± 29 | 4/18 (22) | AG | |
| MV | −a | 0/14° (0) |
These animals were observed for ≥500 days.
Western blot analysis of brain samples from TgHu mice inoculated with sCJD-MV1 showed identical migration (type 1) of PrPTSE unglycosylated fragments in all samples (Fig. 2, lanes 1 to 4). In sCJD-MV2-inoculated TgHuMM and TgHuMV mice, PrPTSE showed electrophoretic migration corresponding to PrPTSE type 1 (Fig. 2, lanes 6 and 7), while in TgHuVV mice, PrPTSE comigrated with that of the inoculum (type 2) (Fig. 2, lanes 8 and 5).
These data suggest that TgHu mice inoculated with CJD-MVAG show a disease phenotype distinct from that of sCJD-MV1 and sCJD-MV2, thus representing a novel human prion isolate.
Experimental transmission of CJD-MVAG, sCJD-MV1, and sCJD-MV2 to voles reveals distinct transmission phenotypes.
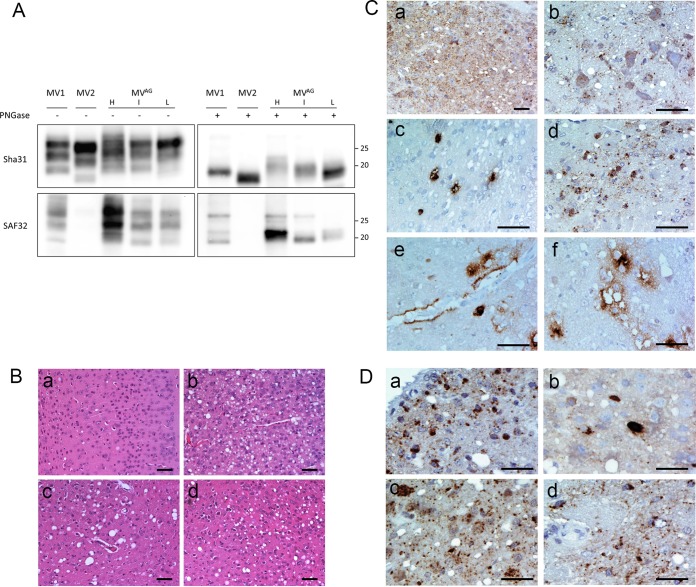
Voles inoculated with CJD-MVAG succumbed to prion disease with a 93% attack rate, but with a wide range of survival times spanning from 430 to 913 days (Table 2). As positive controls, voles injected with an MV2 sample had a much lower attack rate (33%) but a similar mean survival time (544 ± 56 days), while voles injected with an sCJD-MV1 sample (10) succumbed to disease (100%) with much shorter survival times (Table 2). Western blot analyses of recipient CJD-MVAG voles showed fully glycosylated PrPTSE with variable PrP fragment sizes after PK digestion (Fig. 3A). Of the 22 brain samples from CJD-MVAG-infected voles analyzed by Western blotting, 13 showed the PrPTSE unglycosylated band migrating between type 1 and type 2 fragment sizes (Fig. 3A, lanes I), 7 had a “preferentially high” (H) pattern characterized by the presence of fragments with an electrophoretic migration higher than that in sCJD-MV1-inoculated voles (Fig. 3A, lanes H), and 2 showed a “preferentially low” (L) PrPTSE similar to that observed in sCJD-MV2-inoculated voles (Fig. 3A, lanes L). These findings indicate the presence of multiple core fragments in PrPTSE of CJD-MVAG voles.
TABLE 2.
Survival times and rates of transmission of different CJD-MV inocula in bank voles
| Inoculum from human CJD-MV | Survival time of PrPTSE-positive/neuropathologically confirmed animals (days) (mean ± SD) | No. PrPTSE-positive or neuropathologically confirmed/no. animals tested (%) | PrPTSE typea |
|---|---|---|---|
| sCJD-MV1b | 179 ± 10 | 20/20 (100) | A |
| sCJD-MV2 | 544 ± 56 | 5/15 (33) | C |
| CJD-MVAG | 575 ± 120c | 25/27 (93) | Variable (n = 22) |
Type A and type C for voles infected with sCJD-MV1 and sCJD-MV2 as described by Pirisinu et al. (40). The variable type includes 7 animals showing a prevalent high pattern (H), with a mean survival time of 592 ± 162 days; 13 animals had a PrPTSE unglycosylated band migrating between type A and type C fragment size (I), with a survival time of 572 ± 98 days; 2 animals showed a prevalent low pattern (L), with survival times of 546 and 595 days.
Data from Nonno et al. (10).
Incomplete data for only 2 animals were previously reported by Zanusso et al. (12).
FIG 3.
Biochemical and histopathological findings observed in bank voles inoculated with CJD-MVAG. (A) Western blot analysis of proteinase K-resistant PrPTSE in brains of individual voles infected with CJD-MVAG in comparison with representative voles infected with sCJD-MV1 (type A in voles) (40) or sCJD-MV2 (type C in voles) (40). Samples were loaded as indicated above the blots either before or after treatment with PNGase to remove N-linked oligosaccharides. Replica blots were revealed with MAb Sha31 (top) (the Sha31 epitope is in the core of the PrP fragments), which was expected to bind all protease-resistant C-terminal PrP fragments, or with SAF32 (bottom) (the SAF32 epitope is in the octarepeat region of PrP at the extreme N-terminal end of PK-resistant PrP fragments), which was expected to bind only PrP fragments cleaved N terminally to the last octarepeat. Three individual voles from the CJD-MVAG group were selected because they were representative of the electrophoretic variability of PK-resistant PrP fragments observed in the whole group, encompassing cases displaying the preferentially high (H), preferentially low (L), or intermediate (I) pattern (see the text). Note that with Sha31, upon deglycosylation, the unglycosylated PK-resistant PrP fragments from MVAG appeared as less defined bands than MV1 and MV2, suggesting the presence of PrP fragments of variable size. Among these, the H pattern included PrP fragments similar to or higher than MV1, the I pattern encompassed apparent MWs between those of MV1 and MV2, and the L pattern seemed similar to that of MV2. With SAF32, which binds to MV1 but not MV2 PK-resistant PrP, high-MW PrP fragments were evident in H-type patterns and with decreasing intensity also in types I and L, suggesting that in all samples from MVAG there was copresence of PrP fragments with different N-terminal cleavages. (B) Histopathological analysis of the brains of two representative voles with low (a and c) or high (b and d) cortical involvement. The occipital cortex was relatively spared (a) or severely affected (b), while the thalamus was similarly affected in both voles (c and d). Bars, 40 μm. (C) Immunohistochemical examination of voles' infected brains revealed different PrPTSE deposition patterns, which included synaptic/punctate (a), intraneuronal (b and c), intra-astrocytic and intramicroglial (d), perivascular (e), and perivacuolar (f). Bars, 20 μm. (D) Magnification of peculiar intraneuronal engulfment of PrPTSE observed in colliculus (a), external capsule (b and d), and vestibular nucleus (c). Bars, 25 μm.
Neuropathological evaluation of CJD-MVAG-infected voles (n = 15) showed intense and uniform spongiosis and gliosis in the superior colliculus, hippocampus, and thalamus in all the animals. Cortical areas were variably affected, showing severe neuropathologic changes in some voles while others were relatively spared (Fig. 3B). Four of five voles with mild spongiform changes in cortices showed a preferentially high-type PrPTSE biochemical pattern in Western blot analysis (survival time, 651 ± 183 days [mean and standard deviation {SD}]), while severe cortical spongiform degeneration was observed in two voles with preferentially low-type PrPTSE (survival time, 546 and 595 days). All the other voles showed moderate and variable spongiform degeneration in the cerebral cortices that was unrelated to PrPTSE Western blot patterns (survival time, 606 ± 98 days [mean and SD; n = 9]). Immunohistochemical analysis in the brains of CJD-MVAG-affected voles (n = 15) highlighted the presence of different PrPTSE deposition patterns, such as synaptic/punctate, intraneuronal, intra-astrocytic, intramicroglial, perivascular, and perivacuolar types of PrPTSE deposition (Fig. 3C, a to f). Four voles showed a preferentially synaptic/punctate type (survival time, 582 ± 95 days [mean and SD]), while the other 11 showed dominant intraneuronal, astrocytic, or microglial PrP staining (survival time, 624 ± 126 days [mean and SD]). Six voles belonging to the second group had a peculiar intraneuronal engulfment of PrPTSE in several areas of the brain reminiscent of the PrPTSE deposition pattern observed in the brain of the CJD-MVAG patient (Fig. 3D). Perivascular and perivacuolar deposition types were rarely observed. No correlation was found between PrPTSE deposition types, biochemical and histopathological features, and survival time, making it difficult to conclude whether there was more than one strain in the inoculum.
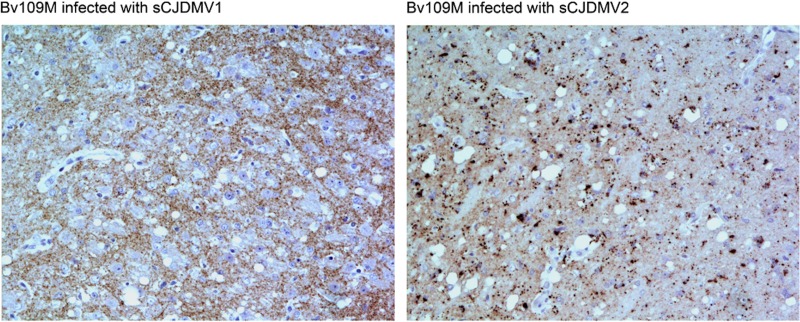
In contrast, voles inoculated with positive controls, sCJD-MV1 and sCJD-MV2, showed remarkably different neuropathological and immunohistochemical patterns. In voles affected with sCJD-MV1, small and medium vacuoles were found in several areas of the gray matter, mainly involving the thalamus, striatum, hippocampus, geniculate nucleus, and cerebral cortex. By IHC, a synaptic-punctate PrPTSE pattern was observed in the same areas involved in spongiform degeneration, but rarely intraneuronal deposits (Fig. 4). Conversely, medium and large vacuolation changes scattered in the thalamus, pons, and medulla were observed in voles inoculated with sCJD-MV2. In the same brains, granular intraglial and intraneuronal patterns were extensively observed (Fig. 4).
FIG 4.
Representative immunohistochemistry of bank voles inoculated with sCJD-MV1 and sCJD-MV2. Shown are patterns of PrPTSE observed by immunohistochemistry in the thalamus of voles inoculated with positive controls, sCJD-MV1 and sCJD-MV2. In the brains of sCJD-MV1-affected voles, there were extracellular punctate PrPTSE deposits, while in sCJD-MV2-affected voles, there were remarkable granular intracellular depositions in glia and neurons in the same area.
These data suggest that the prion isolate from CJD-MVAG differs from those of sCJD-MV1 and sCJD-MV2. Finally, the multiple biochemical and immunohistochemical PrP patterns observed in voles inoculated with CJD-MVAG suggest the presence of more than a single prion strain. Alternatively, it is possible that this phenomenon corresponds to strain instability, revealing itself upon interspecies transmission.
DISCUSSION
We report several lines of evidence that indicate that the prion strain responsible for this atypical CJD (12) has biological properties distinct from those of other human isolates, including sCJD-MV1 and sCJD-MV2.
The first evidence comes from biochemical studies indicating that PrPTSE CJD-MVAG has properties distinct from those of classical type 1 and type 2 PrPTSE observed in sCJD-MV patients. Conformation stability assays (CSA) under denaturing conditions showed that PrPTSE sedimentation velocities and GdnHCl1/2 concentrations relative to CJD-MVAG were unique and distinct from those observed in sCJD-MV1 and sCJD-MV2 isolates. Because these techniques have been widely used in the past to distinguish sCJD-MM1 from sCJD-MM2, or protease-sensitive prionopathy from GSS A117V or sCJD-VV1 (13, 15, 16), it is reasonable to assume that the unique properties of PrPTSE observed in CJD-MVAG indicate a novel isolate of the prion. In addition, CSA shows less stability of PrPTSE aggregates in CJD-MVAG than in other sCJD subtypes, including variant CJD, further indicating that PrPTSE has conformational properties distinct from those of other CJD forms (17).
The second line of evidence comes from studies of transmission of CJD-MVAG to TgHu mice. None of the inoculated TgHu mice developed clinical signs of disease, irrespective of their genotype, while PrPTSE with the same abnormal glycosylation of the inoculum was detected in only 22% of injected TgHuVV mice. This is in contrast to the present and previously published data on the successful transmission of sCJD-MV1 and sCJD-MV2 subtypes in TgHu mice (11). Sporadic CJD-MV1 and sCJD-MV2 inocula always propagated PrPTSE type 1 in TgHu recipient mice, except for TgHuVV mice, which reproduced PrPTSE type 2 after challenge with sCJD-MV2 samples. Interestingly, TgHuVV mice were the only ones that also faithfully reproduced the abnormally glycosylated pattern of PrPTSE of the CJD-MVAG inoculum, suggesting that valine-homozygous individuals are more susceptible than other genotypes to inocula containing at least one allele of PrP in valine. Lack of clinical signs and neuropathological lesions in TgHu mice injected with CJD brain samples is not unique to CJD-MVAG. Sporadic CJD MM2-type TgHu mice used in our study did not show any clinical signs or neuropathological lesions, despite the fact that PrPTSE was detected in only a few TgHuMV and TgHuVV mice (11).
The third line of evidence results from the transmission of CJD-MVAG to voles. These rodents are susceptible to a variety of human and animal prions with an efficacy that is often higher than that observed in Tg mice carrying a PrP sequence matching that of the inoculum (10, 18–20). The mean survival time of voles intracerebrally injected with CJD-MVAG was similar to that observed after challenge with sCJD-MV2 (more than 500 days), but the transmission rate (93%) was almost 3 times higher than with sCJD-MV2 (33%) and approached that observed with the sCJD-MV1 inoculum (100%) (10). Although incubation periods and attack rates might be influenced by differences in the infectivity titers of field isolates, the unique combination of survival times and rate of transmission for CJD-MVAG suggests that the prion has independent biological properties. The biochemical and pathological analyses of brain tissues from recipient voles confirmed the atypical transmission features of CJD-MVAG compared to sCJD-MV1 and sCJD-MV2. In particular, biochemical studies evidenced that CJD-MVAG-infected voles had a complex pattern of PrPTSE with multiple core fragments that was not found in vole brains infected with classical CJD samples. In addition, the neuropathological and immunohistochemical studies highlighted peculiar transmission features of the CJD-MVAG isolate, not observed in sCJD-MV1 and sCJD-MV2 samples, that resemble some phenotypical features observed in the brain of the patient.
Finally, the findings that CJD-MVAG-infected voles showed either PrPTSE aggregates with intense intraneuronal accumulation or synaptic punctate depositions with no intraneuronal PrPTSE and two or possibly three patterns of PrPTSE migration on Western blots is suggestive of the presence of multiple prion strains in the original inoculum (21). The finding that the intraneuronal PrPTSE accumulations occurred in brain tissues of CJD-MVAG and voles argues that part of the neuropathological phenotype was faithfully reproduced in the rodent model. This evidence represents a specific signature of a distinct strain contained in the human isolate. The evidence that a PrP conformer reproduces the pathological phenotype in the recipients indicates that the biological properties of infecting prions are encoded in specific PrPTSE conformers.
The coexistence of multiple prion strains in the same host is a well-known phenomenon in scrapie-affected sheep (22), in mink with transmissible mink encephalopathy (23), and in sCJD patients carrying both type 1 and type 2 PrPTSE in the brain. However, the isolation of a given prion strain occurs only after several passages in the same host species, which depends on the preferential propagation of one or another strain dictated by the host tropism (24).
The origin of this atypical and novel strain of CJD remains unknown. However, because the patient received treatment with hypothalamic phospholipids extracted from bovine brains for several years (12), it is conceivable that she was infected with one of the three known prion strains of bovine spongiform encepahlopathy (BSE) despite the fact that validation experiments had convincingly shown that the extraction of brain phospholipids strongly inactivated prion infectivity (25). Potential exposure to classical BSE (C-BSE) is unlikely because the neuropathological lesions and PrPTSE glycotype did not show the characteristic features observed in variant CJD, including the last reported 129 MV case (26). Moreover, studies of transmissions in TgHu mice and voles infected with CJD-MVAG were strikingly different from those observed in variant CJD-inoculated rodents (27, 28). Epidemiological evidence of transmission of atypical BSE (L-BSE and H-BSE) to humans has never been reported (29), and therefore, there are no data on the phenotypes of these atypical BSE strains in rodents after passage in humans. However, Tg mice overexpressing human PrP with methionine at codon 129 develop prion disease after inoculation with L-BSE, but not with H-BSE (30), suggesting that L-BSE represents a higher zoonotic risk than H-BSE. These and other data in Tg humanized mice (31) and nonhuman primates (32–34) have indicated that L-BSE-affected humans might show a disease phenotype resembling sporadic rather than variant CJD. On no occasion, however, was a PrPTSE signature similar to that observed in the CJD-MVAG patient and faithfully replicated in TgHuVV mice reported. It is therefore likely that the CJD-MVAG patient represents a novel subtype of sporadic CJD rather than an infectious form of CJD caused by a classical or atypical BSE strain, although the experimental evidence is insufficient to draw a convincing conclusion. Further in vivo studies are ongoing to address this issue.
What remains unexplained is the discrepancy between PrPTSE glycotypes observed in voles and TgHuVV mice. Previous studies postulated that PrPTSE acts as a template for its amplification, while others showed that the host PrP dictates the glycosylation status of de novo-generated PrPTSE (35–38). A unique feature of PrPTSE MVAG is that it acts as a genuine aberrant glycosylated PrPTSE template and is not generated in experimental models following the manipulation of host PrPC glycosylation. Tuzi and colleagues (38) showed that the inoculation of wild-type mice with mono- or unglycosylated PrPTSE, generated in scrapie-infected transgenic mice with defective glycosylation of PrP, resulted in the production of fully glycosylated PrPTSE in the brains of recipient wild-type animals. In contrast, in vitro experiments showed that exogenous PrPTSE dictates the glycosylation profile of the host PrPTSE, suggesting that the glycosylation pattern of PrPTSE in vivo is the result of the glycosylation state of the PrPC pool present in the cellular environment where PrP conversion occurs (37). Our results showed that both mechanisms seem to be involved. In TgHuVV mice, PrPTSE acts as a template dictating PrPTSE glycosylation, while in voles the host PrPC restores the fully glycosylated PrPTSE.
Taken together, these results indicate that in different animal models of transmission, PrPTSE replication is either host or strain dependent and is not strictly influenced by PrP homology.
MATERIALS AND METHODS
Patients and inocula.
Patients were recruited through the National CJD surveillance unit of the Istituto Superiore di Sanità (ISS).
The disease phenotype of the atypical CJD case, CJD-MVAG, had been previously reported (12), while the sCJD cases heterozygous at codon 129 (sCJD-MV1 and sCJD-MV2K) showed typical disease phenotypes, as described by Parchi et al. (3, 9).
Immunoblot analysis.
Brain samples were homogenized in 9 volumes of lysis buffer (100 mM NaCl, 10 mM EDTA, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 10 mM Tris, pH 7.4) and clarified by centrifugation at 1,000 × g for 10 min. The supernatant (S1) was stored at −80°C until it was used, and the pellet was discarded. S1 was centrifuged at 100,000 × g for 90 min at 4°C. The supernatant (S2) was saved, and the pellet was resuspended in lysis buffer and centrifuged as described above. The final pellet (P3) was designated detergent insoluble, whereas the detergent-soluble fraction referred to the final combination of S2 and S3. Protease resistance was assayed by incubating 10% brain sample aliquots with 100 μg/ml of PK (Boehringer Mannheim, Germany) at 37°C for 60 min. The reaction was stopped by the addition of protease inhibitor (5 mM phenylmethylsulfonyl fluoride [PMSF]). Proteins were dissolved in sample buffer (3% SDS, 3% beta-mercaptoethanol, 2 mM EDTA, 10% glycerol, 62.5 mM Tris, pH 6.8) and boiled for 5 min. An equivalent of 0.4 mg of wet tissue was loaded on SDS-12% PAGE gels, and the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon P; Millipore) for 2 h at 60 V. The membranes were blocked with 1% nonfat dry milk in TBST (10 mM Tris, 150 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 h at 37°C and incubated overnight at 4°C with the following anti-human PrP monoclonal antibodies (MAb): 3F4 (Signet; 1:10,000), 6H4 (Prionics; 1:5,000), and 4G11 (kindly donated by L. Capucci; recognizing human PrP residues 199 to 212; 1:500). The blots were developed with an enhanced chemiluminescence (ECL) system (Amersham), and PrP was visualized on autoradiography film (Hyperfilm; Amersham). The films were scanned with a GS-200 (Bio-Rad) densitometer.
Guanidine hydrochloride denaturation.
Brain homogenates were incubated for 1 h with different concentrations of GdnHCl, starting from 0.25 M and gradually increasing to 2.25 M. Samples were then diluted with 1× phosphate-buffered saline (PBS) to a final concentration of GdnHCl of 0.1 M. The diluted samples were PK digested as described above. The conformational stability of PrPTSE in CJD samples was based on GdnHCl1/2, i.e., the concentration of GdnHCl required to make half of the PrPTSE susceptible to protease digestion.
Sedimentation velocity in a sucrose gradient.
The S1 fractions prepared by centrifugation of 20% brain homogenates at 8,000 × g for 5 min at 4°C were incubated with an equal volume of 2% sarcosyl for 30 min on ice. Samples were loaded atop a 10 to 60% step sucrose gradient and centrifuged at 200,000 × g for 1 h at 4°C, as described by Tzaban et al. (39). Eleven fractions were collected from the top of the tube and processed for Western blot analysis of PrP.
Experimental transmission. (i) Ethics statement.
The National CJD surveillance unit of the ISS received approval from the ethical committee to collect samples obtained during routine clinical work from patients referred to the unit and to use them for research purposes (decree numbers CE-ISS/09/266 and CE-ISS/15/263).
The research protocol, approved by the Service for Biotechnology and Animal Welfare of the Istituto Superiore di Sanità and authorized by the Italian Ministry of Health, adhered to the guidelines contained in Italian Legislative Decree 116/92, based on European Directive 86/609/EEC on Laboratory Animal Protection (decree numbers 84/12.B and 255/2012.B).
(ii) Transgenic humanized mice.
Brain samples were collected from areas showing pathological changes and PrP accumulation; homogenized at 10% (wt/vol) in sterile PBS with new, sterile glass/Teflon Potter tissue grinders; and temporarily stored at −80°C. On the day of inoculation, the brain homogenates were thawed, vortexed, and diluted to 1% (wt/vol) in sterile PBS before intracerebral injection into mice.
TgHu mice of both sexes expressing different human genotypes (129 MM, MV, and VV) were obtained from the colony established at the ISS with founders provided by professor Jean Manson, Roslin Institute, University of Edinburgh.
Groups of 18 to 22 mice belonging to each of the three PRNP genotypes were anesthetized and injected in the left cerebral hemisphere with 20 μl of the relevant homogenate. The mice, divided by sex, were housed with food and water ad libitum and examined twice a week. Animals were culled with carbon dioxide when their ability to drink and feed adequately was compromised due to TSE disease, to senescence, or to prion-unrelated disease (e.g., intercurrent infection). Culled or dead animals were autopsied, and the brain hemispheres were separated for biochemical (left hemisphere) and histological (right hemisphere) purposes. Clinical TSE signs observed in TgHu mice were kyphosis, hypoactivity, lowering of the hind back, and ruffled fur, and transmission of CJD was considered successful when PrPTSE was detected in the brain tissue by both IHC and Western blotting.
(iii) Bank voles.
Groups of 15 to 27 8-week-old bank voles coding for methionine at codon 109 (Bv109M) were obtained from the breeding colony at the ISS. The voles were inoculated with human brain homogenates at 10% (wt/vol) in PBS. The inoculation procedures were identical to those reported in TgHu mice.
(iv) Histopathology.
Histology and IHC analyses were performed on formalin-fixed tissues as previously described (10, 18). Briefly, brains were trimmed at standard coronal levels, embedded in paraffin wax, and cut at 6 μm for hematoxylin and eosin staining and IHC. Sections were randomly mixed and coded for blind pathological assessment. IHC was performed as described previously (18), using the SAF84 MAb (Millipore; 1.5 μg/ml) for TgHu and the 6C2 MAb (kindly donated by Jan Langeveld) for voles, on the same standard coronal levels used for pathological assessment.
(v) Western blot analysis.
Brain homogenates (10% [wt/vol]) from individual animals were prepared in 100 mM Tris-HCl, pH 7.4, containing 2% sarcosyl; incubated for 20 min at room temperature; and then digested with PK (50 μg/ml) for 1 h at 37°C with gentle shaking. Protease treatment was stopped with 3 mM PMSF. Electrophoresis and Western blotting were performed as described above.
ACKNOWLEDGMENTS
We gratefully acknowledge J. Manson (Roslin Institute, University of Edinburgh) for providing TgHu mouse breeding couples and J. Langeveld and L. Capucci for providing antibodies.
We also thank M. Venditti, N. Bellizzi, M. Bonanno, I. Itro, E. Laconi, and P. Frassanito for animal care; C. D'Agostino for clinical evaluation; G. Riccardi for technical support; and A. Garozzo, A. Caggiano, and C. Gasparrini for administrative support.
REFERENCES
- 1.Caughey B, Chesebro B. 1997. Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biol 7:56–62. doi: 10.1016/S0962-8924(96)10054-4. [DOI] [PubMed] [Google Scholar]
- 2.Pocchiari M, Puopolo M, Croes EA, Budka H, Gelpi E, Collins S, Lewis V, Sutcliffe T, Guilivi A, Delasnerie-Laupretre N, Brandel JP, Alperovitch A, Zerr I, Poser S, Kretzschmar HA, Ladogana A, Rietvald I, Mitrova E, Martinez-Martin P, de Pedro-Cuesta J, Glatzel M, Aguzzi A, Cooper S, Mackenzie J, van Duijn CM, Will RG. 2004. Predictors of survival in sporadic Creutzfeldt-Jakob disease and other human transmissible spongiform encephalopathies. Brain 127:2348–2359. doi: 10.1093/brain/awh249. [DOI] [PubMed] [Google Scholar]
- 3.Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H, Kopp N, Piccardo P, Poser S, Rojiani A, Streichemberger N, Julien J, Vital C, Ghetti B, Gambetti P, Kretzschmar H. 1999. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46:224–233. [PubMed] [Google Scholar]
- 4.Cali I, Castellani R, Yuan J, Al-Shekhlee A, Cohen ML, Xiao X, Moleres FJ, Parchi P, Zou WQ, Gambetti P. 2006. Classification of sporadic Creutzfeldt-Jakob disease revisited. Brain 129:2266–2277. doi: 10.1093/brain/awl224. [DOI] [PubMed] [Google Scholar]
- 5.Cardone F, Liu QG, Petraroli R, Ladogana A, D'Alessandro M, Arpino C, Di Bari M, Macchi G, Pocchiari M. 1999. Prion protein glycotype analysis in familial and sporadic Creutzfeldt-Jakob disease patients. Brain Res Bull 49:429–433. doi: 10.1016/S0361-9230(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 6.Zanusso G, Farinazzo A, Prelli F, Fiorini M, Gelati M, Ferrari S, Righetti PG, Rizzuto N, Frangione B, Monaco S. 2004. Identification of distinct N-terminal truncated forms of prion protein in different Creutzfeldt-Jakob disease subtypes. J Biol Chem 279:38936–38942. doi: 10.1074/jbc.M405468200. [DOI] [PubMed] [Google Scholar]
- 7.Zanusso G, Fiorini M, Ferrari S, Meade-White K, Barbieri I, Brocchi E, Ghetti B, Monaco S. 2014. Gerstmann-Sträussler-Scheinker disease and “anchorless prion protein” mice share prion conformational properties diverging from sporadic Creutzfeldt-Jakob disease. J Biol Chem 289:4870–4881. doi: 10.1074/jbc.M113.531335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parchi P, Zou W, Wang W, Brown P, Capellari S, Ghetti B, Kopp N, Schulz-Schaeffer WJ, Kretzschmar HA, Head MW, Ironside JW, Gambetti P, Chen SG. 2000. Genetic influence on the structural variations of the abnormal prion protein. Proc Natl Acad Sci U S A 97:10168–10172. doi: 10.1073/pnas.97.18.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parchi P, Strammiello R, Notari S, Giese A, Langeveld JP, Ladogana A, Zerr I, Roncaroli F, Cras P, Ghetti B, Pocchiari M, Kretzschmar H, Capellari S. 2009. Incidence and spectrum of sporadic Creutzfeldt-Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neuropathol 118:659–671. doi: 10.1007/s00401-009-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonno R, Di Bari MA, Cardone F, Vaccari G, Fazzi P, Dell'Omo G, Cartoni C, Ingrosso L, Boyle A, Galeno R, Sbriccoli M, Lipp HP, Bruce M, Pocchiari M, Agrimi U. 2006. Efficient transmission and characterization of Creutzfeldt-Jakob disease strains in bank voles. PLoS Pathog 2:e12. doi: 10.1371/journal.ppat.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop MT, Will RG, Manson JC. 2010. Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc Natl Acad Sci U S A 107:12005–12010. doi: 10.1073/pnas.1004688107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanusso G, Polo A, Farinazzo A, Nonno R, Cardone F, Di Bari M, Ferrari S, Principe S, Gelati M, Fasoli E, Fiorini M, Prelli F, Frangione B, Tridente G, Bentivoglio M, Giorgi A, Schininà ME, Maras B, Agrimi U, Rizzuto N, Pocchiari M, Monaco S. 2007. Novel prion protein conformation and glycotype in Creutzfeldt-Jakob disease. Arch Neurol 64:595–599. doi: 10.1001/archneur.64.4.595. [DOI] [PubMed] [Google Scholar]
- 13.Gambetti P, Dong Z, Yuan J, Xiao X, Zheng M, Alshekhlee A, Castellani R, Cohen M, Barria MA, Gonzalez-Romero D, Belay ED, Schonberger LB, Marder K, Harris C, Burke JR, Montine T, Wisniewski T, Dickson DW, Soto C, Hulette CM, Mastrianni JA, Kong Q, Zou WQ. 2008. A novel human disease with abnormal prion protein sensitive to protease. Ann Neurol 63:697–708. doi: 10.1002/ana.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao X, Yuan J, Haïk S, Cali I, Zhan Y, Moudjou M, Li B, Laplanche JL, Laude H, Langeveld J, Gambetti P, Kitamoto T, Kong Q, Brandel JP, Cobb BA, Petersen RB, Zou WQ. 2013. Glycoform-selective prion formation in sporadic and familial forms of prion disease. PLoS One 8:e58786. doi: 10.1371/journal.pone.0058786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou WQ, Puoti G, Xiao X, Yuan J, Qing L, Cali I, Shimoji M, Langeveld JP, Castellani R, Notari S, Crain B, Schmidt RE, Geschwind M, Dearmond SJ, Cairns NJ, Dickson D, Honig L, Torres JM, Mastrianni J, Capellari S, Giaccone G, Belay ED, Schonberger LB, Cohen M, Perry G, Kong Q, Parchi P, Tagliavini F, Gambetti P. 2010. Variably protease-sensitive prionopathy: a new sporadic disease of the prion protein. Ann Neurol 68:162–172. doi: 10.1002/ana.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirisinu L, Di Bari M, Marcon S, Vaccari G, D'Agostino C, Fazzi P, Esposito E, Galeno R, Langeveld J, Agrimi U, Nonno R. 2010. A new method for the characterization of strain-specific conformational stability of protease-sensitive and protease-resistant PrP. PLoS One 5:e12723. doi: 10.1371/journal.pone.0012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cescatti M, Saverioni D, Capellari S, Tagliavini F, Kitamoto T, Ironside J, Giese A, Parchi P. 2016. Analysis of conformational stability of abnormal prion protein aggregates across the spectrum of Creutzfeldt-Jakob disease prions. J Virol 90:6244–6254. doi: 10.1128/JVI.00144-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Bari MA, Chianini F, Vaccari G, Esposito E, Conte M, Eaton SL, Hamilton S, Finlayson J, Steele PJ, Dagleish MP, Reid HW, Bruce M, Jeffrey M, Agrimi U, Nonno R. 2008. The bank vole (Myodes glareolus) as a sensitive bioassay for sheep scrapie. J Gen Virol 89:2975–2985. doi: 10.1099/vir.0.2008/005520-0. [DOI] [PubMed] [Google Scholar]
- 19.Di Bari MA, Nonno R, Castilla J, D'Agostino C, Pirisinu L, Riccardi G, Conte M, Richt J, Kunkle R, Langeveld J, Vaccari G, Agrimi U. 2013. Chronic wasting disease in bank voles: characterisation of the shortest incubation time model for prion diseases. PLoS Pathog 9:e1003219. doi: 10.1371/journal.ppat.1003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrimi U, Nonno R, Dell'Omo G, Di Bari MA, Conte M, Chiappini B, Esposito E, Di Guardo G, Windl O, Vaccari G, Lipp HP. 2008. Prion protein amino acid determinants of differential susceptibility and molecular feature of prion strains in mice and voles. PLoS Pathog 4:e1000113. doi: 10.1371/journal.ppat.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langenfeld KA, Shikiya RA, Kincaid AE, Bartz JC. 2016. Incongruity between prion conversion and incubation period following coinfection. J Virol 90:5715–5723. doi: 10.1128/JVI.00409-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong A, Kennedy I, Goldmann W, Green A, González L, Jeffrey M, Hunter N. 2015. Archival search for historical atypical scrapie in sheep reveals evidence for mixed infections. J Gen Virol 96:3165–3178. doi: 10.1099/jgv.0.000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimberlin RH, Cole S, Walker CA. 1986. Transmissible mink encephalopathy (TME) in Chinese hamsters: identification of two strains of TME and comparisons with scrapie. Neuropathol Appl Neurobiol 12:197–206. doi: 10.1111/j.1365-2990.1986.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 24.Kimberlin RH, Walker CA. 1978. Evidence that the transmission of one source of scrapie agent to hamsters involves separation of agent strains from a mixture. J Gen Virol 39:487–496. doi: 10.1099/0022-1317-39-3-487. [DOI] [PubMed] [Google Scholar]
- 25.Di Martino A, Safar J, Gibbs CJ Jr. 1994. The consistent use of organic solvents for purification of phospholipids from brain tissue effectively removes scrapie infectivity. Biologicals 22:221–225. doi: 10.1006/biol.1994.1032. [DOI] [PubMed] [Google Scholar]
- 26.Mok T, Jaunmuktane Z, Joiner S, Campbell T, Morgan C, Wakerley B, Golestani F, Rudge P, Mead S, Jäger HR, Wadsworth JD, Brandner S, Collinge J. 2017. Variant Creutzfeldt-Jakob disease in a patient with heterozygosity at PRNP codon 129. N Engl J Med 376:292–294. doi: 10.1056/NEJMc1610003. [DOI] [PubMed] [Google Scholar]
- 27.Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, Thomson V, Tuzi NL, Head MW, Ironside JW, Will RG, Manson JC. 2006. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol 5:393–398. doi: 10.1016/S1474-4422(06)70413-6. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa JC, Nonno R, Di Bari M, Aguilar-Calvo P, Pirisinu L, Fernández-Borges N, Vanni I, Vaccari G, Marín-Moreno A, Frassanito P, Lorenzo P, Agrimi U, Torres JM. 2016. PrPc governs susceptibility to prion strains in bank vole, while other host factors modulate strain features. J Virol 90:10660–10669. doi: 10.1128/JVI.01592-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown P, McShane LM, Zanusso G, Detwiler L. 2006. On the question of sporadic or atypical bovine spongiform encephalopathy and Creutzfeldt-Jakob disease. Emerg Infect Dis 12:1816–1821. doi: 10.3201/eid1212.060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Béringue V, Herzog L, Reine F, Le Dur A, Casalone C, Vilotte JL, Laude H. 2008. Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerg Infect Dis 14:1898–1901. doi: 10.3201/eid1412.080941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong Q, Zheng M, Casalone C, Qing L, Huang S, Chakraborty B, Wang P, Chen F, Cali I, Corona C, Martucci F, Iulini B, Acutis P, Wang L, Liang J, Wang M, Li X, Monaco S, Zanusso G, Zou WQ, Caramelli M, Gambetti P. 2008. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol 82:3697–3701. doi: 10.1128/JVI.02561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comoy EE, Casalone C, Lescoutra-Etchegaray N, Zanusso G, Freire S, Marcé D, Auvré F, Ruchoux MM, Ferrari S, Monaco S, Salès N, Caramelli M, Leboulch P, Brown P, Lasmézas CI, Deslys JP. 2008. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PLoS One 3:e3017. doi: 10.1371/journal.pone.0003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono F, Tase N, Kurosawa A, Hiyaoka A, Ohyama A, Tezuka Y, Wada N, Sato Y, Tobiume M, Hagiwara K, Yamakawa Y, Terao K, Sata T. 2011. Atypical L-type bovine spongiform encephalopathy (L-BSE) transmission to cynomolgus macaques, a non-human primate. Jpn J Infect Dis 64:81–84. [PubMed] [Google Scholar]
- 34.Mestre-Francés N, Nicot S, Rouland S, Biacabe AG, Quadrio I, Perret-Liaudet A, Baron T, Verdier JM. 2012. Oral transmission of L-type bovine spongiform encephalopathy in primate model. Emerg Infect Dis 18:142–145. doi: 10.3201/eid1801.111092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 36.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 37.Vorberg I, Priola SA. 2002. Molecular basis of scrapie strain glycoform variation. J Biol Chem 277:36775–36781. doi: 10.1074/jbc.M206865200. [DOI] [PubMed] [Google Scholar]
- 38.Tuzi NL, Cancellotti E, Baybutt H, Blackford L, Bradford B, Plinston C, Coghill A, Hart P, Piccardo P, Barron RM, Manson JC. 2008. Host PrP glycosylation: a major factor determining the outcome of prion infection. PLoS Biol 6:e100. doi: 10.1371/journal.pbio.0060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzaban S, Friedlander G, Schonberger O, Horonchik L, Yedidia Y, Shaked G, Gabizon R, Taraboulos A. 2002. Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 41:12868–12875. doi: 10.1021/bi025958g. [DOI] [PubMed] [Google Scholar]
- 40.Pirisinu L, Marcon S, Di Bari MA, D'Agostino C, Agrimi U, Nonno R. 2013. Biochemical characterization of prion strains in bank voles. Pathogens 2:446–456. doi: 10.3390/pathogens2030446. [DOI] [PMC free article] [PubMed] [Google Scholar]