ABSTRACT
Influenza A virus (IAV) RNA packaging signals serve to direct the incorporation of IAV gene segments into virus particles, and this process is thought to be mediated by segment-segment interactions. These packaging signals are segment and strain specific, and as such, they have the potential to impact reassortment outcomes between different IAV strains. Our study aimed to quantify the impact of packaging signal mismatch on IAV reassortment using the human seasonal influenza A/Panama/2007/99 (H3N2) and pandemic influenza A/Netherlands/602/2009 (H1N1) viruses. Focusing on the three most divergent segments, we constructed pairs of viruses that encoded identical proteins but differed in the packaging signal regions on a single segment. We then evaluated the frequency with which segments carrying homologous versus heterologous packaging signals were incorporated into reassortant progeny viruses. We found that, when segment 4 (HA) of coinfecting parental viruses was modified, there was a significant preference for the segment containing matched packaging signals relative to the background of the virus. This preference was apparent even when the homologous HA constituted a minority of the HA segment population available in the cell for packaging. Conversely, when segment 6 (NA) or segment 8 (NS) carried modified packaging signals, there was no significant preference for homologous packaging signals. These data suggest that movement of NA and NS segments between the human H3N2 and H1N1 lineages is unlikely to be restricted by packaging signal mismatch, while movement of the HA segment would be more constrained. Our results indicate that the importance of packaging signals in IAV reassortment is segment dependent.
IMPORTANCE Influenza A viruses (IAVs) can exchange genes through reassortment. This process contributes to both the highly diverse population of IAVs found in nature and the formation of novel epidemic and pandemic IAV strains. Our study sought to determine the extent to which IAV packaging signal divergence impacts reassortment between seasonal IAVs. Our knowledge in this area is lacking, and insight into the factors that influence IAV reassortment will inform and strengthen ongoing public health efforts to anticipate the emergence of new viruses. We found that the packaging signals on the HA segment, but not the NA or NS segments, restricted IAV reassortment. Thus, the packaging signals of the HA segment could be an important factor in determining the likelihood that two IAV strains of public health interest will undergo reassortment.
KEYWORDS: evolution, influenza virus, packaging, reassortment, segment mismatch
INTRODUCTION
Influenza A virus (IAV) is a member of the Orthomyxoviridae family and contains a negative-sense, single-stranded RNA genome with eight distinct gene segments (1). During coinfection of a cell with two or more IAVs, the multipartite nature of its genome allows the virus to undergo reassortment (gene segment exchange), resulting in the production of progeny viruses that are genetically distinct from both of the parental viruses (2). Reassortment events gave rise to the last three IAV pandemic strains (3–5), resulting in high mortality as well as increased economic burdens (6–8). In addition, reassortment within an individual IAV subtype, termed intrasubtype reassortment, contributes to the epidemiology and evolution of seasonal IAVs (9–11).
Although the potential for reassortment in nature is high (2, 12–18), when coinfecting viruses are dissimilar, reassortment is constrained by incompatibilities between heterologous viral components, a phenomenon termed segment mismatch (19, 20). RNA mismatch can limit the number of genotypically distinct viruses that emerge following a coinfection event, while protein mismatch manifests following spread of reassortant progeny viruses to new cells. Several studies focusing on the outcomes of reassortment between phylogenetically distinct IAVs report biases in the genotypes detected and fitness defects associated with a wide range of reassortant genotypes. This body of work includes examination of reassortment between human H3N2 or H1N1 viruses and avian influenza viruses of the H2N2, H5N1, H7N7, and H9N2 subtypes (21–30); between human IAVs and equine or swine IAVs (31–34); and between human H1N1 and human H3N2 viruses (35, 36). In all cases, reduced fitness of certain reassortant genotypes relative to parental viruses is noted. Common sources of mismatch at the protein level include imbalance in hemagglutinin (HA) attachment and neuraminidase (NA) release functions and incompatibility among polymerase components (34, 37, 38). Conversely, when coinfecting parental viruses are highly homologous, reassortment occurs readily (39).
RNA packaging signals are segment- and strain-specific regions of IAV viral RNAs (vRNAs) that are comprised of both the untranslated region (UTR) and various amounts of coding region at both the 3′ and 5′ ends of each segment, and their purpose is to direct the incorporation of IAV segments into newly forming virus particles (40–45). It has been proposed that the packaging signal regions encompass two distinct signals: a genome incorporation signal, which allows for packaging of that individual segment, and a genome bundling signal, which allows for the incorporation of all eight IAV segments en masse (46). Bundling signals are thought to mediate specific interactions among vRNA segments that ensure subsequent incorporation of eight distinct segments into virions (47–52). Since the packaging signals on any given segment are not fully conserved across different IAV strains, the hypothesis arises that RNA packaging signal divergence may impact IAV reassortment outcomes. During a reassortment event, segments that contain compatible RNA packaging signals might be favored in order to maintain optimal segment-segment interactions, thus resulting in a loss of reassortment efficiency.
Two elegant studies have provided proof of principle that incompatibility between RNA packaging signals is sufficient to prevent the formation of particular reassortant genotypes (53, 54). Additionally, it has been shown that rewiring the packaging signals between two segments of a single IAV strain prevents its reassortment with a wild-type (WT) IAV (55). However, there is a lack of data on (i) the quantitative effects of packaging signal mismatch and (ii) which segments are subject to this type of mismatch. Therefore, our study aimed to determine the extent to which packaging signal mismatch interferes with reassortment between a seasonal H3N2 and a 2009 pandemic H1N1 strain, with a focus on the three segments with the least-conserved packaging signal regions: HA, NA, and nonstructural protein (NS). To accomplish this aim, we adapted our previously described system for measuring reassortment (39). This system relies on the use of two A/Panama/2007/99 (P99)-based parental viruses that differ only by silent nucleotide changes in each segment. These changes between WT and silently mutated segments (VAR, for variant) allow their differentiation using high-resolution melt (HRM) analysis. Virus isolates can thus be fully genotyped using HRM, eliminating the need for partial or full sequencing of each isolate in order to detect and characterize reassortant progeny. To measure the impact of packaging signals on reassortment, we modified the terminal regions of a single segment in each parental virus so that the WT virus had heterologous packaging signals derived from pandemic influenza A/Netherlands/602/2009 (H1N1) (NL) virus and the VAR virus had homologous, P99, packaging signals. Both viruses encoded wild-type P99 proteins, so that observed phenotypes could be attributed to the effects of packaging signals alone. Using this well-controlled system, we show that sequence divergence in the packaging signals of the HA segment, but not the NA or NS segments, significantly influences reassortment.
RESULTS
Generation of chimeric IAV segments with identical protein coding regions but differing packaging signal regions.
Broad regions of each of the eight vRNAs that support efficient incorporation into virions have been mapped and include the UTRs as well as various amounts of 3′ and 5′ coding regions, depending on the segment (40–45). To evaluate the impact of heterologous packaging signals on IAV reassortment, we generated WT-VAR virus pairs in the P99 background in which the WT virus carried NL packaging signals on a single segment. By retaining all P99 open reading frames (ORFs), we aimed to ensure that any bias in reassortment was due to packaging signals rather than incompatibilities occurring at the protein level. In addition, to control for any effects of the engineering that we performed, we produced control segments that carried P99 packaging signals flanking the corresponding P99 ORF. Our chimeric segment design was informed by the work of Gao et al. (56) and included the following three features (Fig. 1). (i) The first 60 nucleotides (nt) of the 3′ end and the last 60 nt of the 5′ end of the P99 ORF were modified via silent mutation of every codon (excluding start, stop, Met, and Trp codons). This step was demonstrated by Gao et al. to be necessary to disrupt native packaging signal function. (ii) The ORFs were flanked with packaging signal regions from either P99 or NL. The terminal sequences added onto the HA, NA, and NS segments were at least 100 nt in length at either end and were selected based on conservative estimates of the packaging signal locations as mapped by others (see reference 57 for a review). (iii) The ATG codons upstream of the true start codon were disrupted via silent mutation, so that translation would result in production of wild-type P99 protein. Each of these chimeric segments was incorporated into a P99 strain background via reverse genetics to produce a total of nine viruses (Fig. 2). For each of the segments examined, three viruses were made: (i) a WT virus with P99 packaging signals on the segment of interest (e.g., P99wt HA_P99PS), (ii) a WT virus with NL packaging signals on the segment of interest (e.g., P99wt HA_NLPS), and (iii) a VAR virus with P99 packaging signals on the segment of interest (e.g., P99var15 HA_P99PS) (Fig. 2). Labeling as WT or VAR refers to the absence or presence, respectively, of silent genetic tags in each segment (39).
FIG 1.
Chimeric IAV segments have the same protein coding regions but different packaging signal regions. HA, NA, and NS segments with either NL (H1N1) (A) or P99 (H3N2) (B) packaging signals were constructed around the P99 open reading frame (ORF) for that segment. Silent mutations were introduced into the packaging signal regions of the ORF so that packaging would be directed only by the sequences flanking the 3′ and 5′ ends of the ORF. Start codons within the added 3′ packaging signal region were mutated so that translation would result in the production of full-length wild-type protein. A variant (VAR) version of the construct in panel B was generated via silent nucleotide changes in the ORF (C). Segments are shown in positive sense for clarity. Chimeric segments were then cloned into the pDP2002 ambisense vector to allow for recovery of infectious virus from cDNA.
FIG 2.
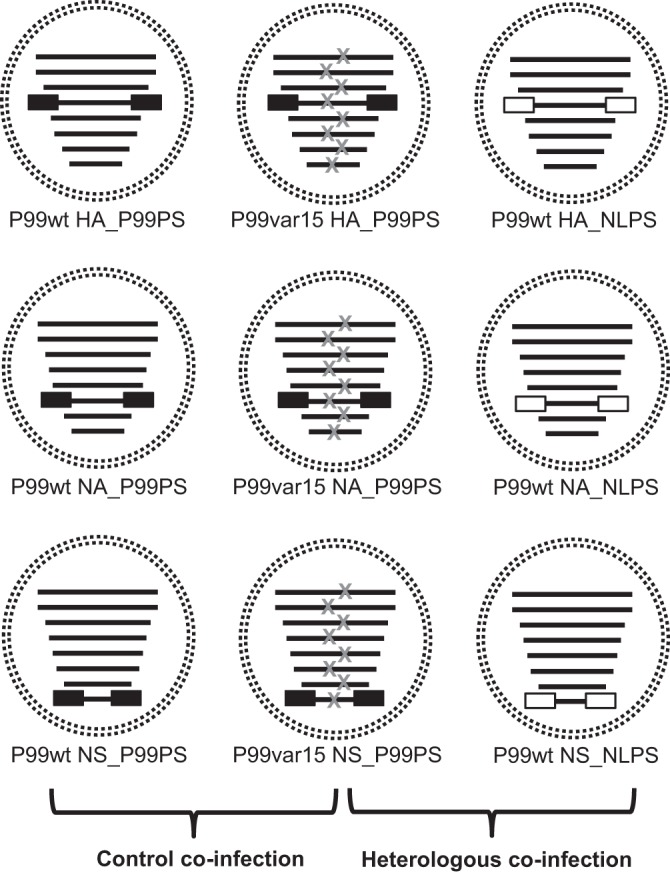
Viruses generated to evaluate the effects of packaging signal divergence on IAV reassortment. Viruses were rescued in a P99 background (black segments) with either P99 packaging signals (black boxes) or NL packaging signals (white boxes) on the HA, NA, or NS segments. Each virus contained one segment with modified packaging signals, and three viruses were rescued for each segment examined. Within each set of three viruses, one carried the silent VAR mutations that act as genetic tags for genotyping (gray x's), and the remaining two were WT (no genetic tags). Brackets indicate the virus pairs used for the control and heterologous coinfections. The same VAR virus was part of both virus pairings.
Viruses with altered packaging signal regions exhibited similar growth kinetics.
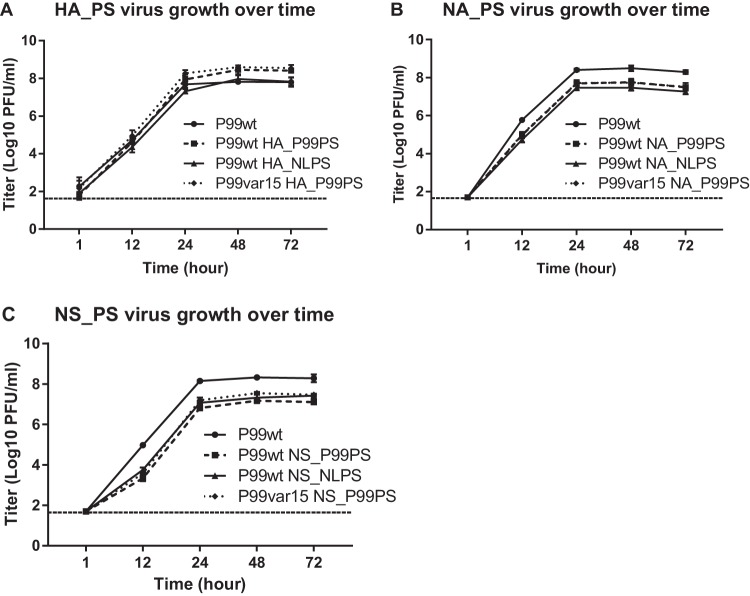
To assess whether introduced mutations and added packaging signals affected virus growth, we performed multicycle growth analyses. As fitness differences within each set of three viruses could skew reassortment outcomes, we wanted to ensure that each set of viruses exhibited growth comparable to the others. The P99 wild-type virus (recombinant A/Panama/2007/99 [P99wt]) was included as a control in parallel with each set of three chimeric packaging signal viruses rescued per segment. Triplicate wells of Madin-Darby canine kidney (MDCK) cells were infected with each virus, and output titers over time were determined by plaque assay. Compared to the P99wt control, we saw no attenuation of growth due to modification of the HA segment, and at the later time points, two of the modified HA_PS viruses produced higher titers than the control (Fig. 3A). In contrast, compared to the P99wt control, we saw ∼10-fold attenuation in growth for all of the NA_PS viruses (Fig. 3B) and NS_PS viruses (Fig. 3C). However, since all three of the modified viruses for each segment exhibited comparable growth phenotypes, we concluded that fitness differences would not bias our coinfection experiments.
FIG 3.
P99-based viruses with chimeric segments exhibited comparable growth phenotypes. Viruses containing chimeric HA (A), NA (B), or NS (C) segments were used to infect triplicate wells of MDCK cells at an MOI of 0.01 PFU/cell, and output titers over time were compared to the P99 wild-type (P99wt) control virus. Data are represented as means ± 1 SD. The limit of detection was 50 PFU/ml and is indicated by a dashed line.
Heterologous segments showed various efficiencies of replication.
We next addressed how efficiently the heterologous segments within each set of viruses were replicated. The growth curves in Fig. 3 suggested that the viruses had approximately equal growth properties; however, (i) these experiments involved single infections done under multicycle growth conditions, whereas experiments to assess reassortment would use coinfection under single-cycle conditions, and (ii) growth curves are not a sensitive way to assess replication of a single segment. We therefore applied reverse transcription-droplet digital PCR (RT ddPCR) to quantify the vRNA copy number of each chimeric segment in the context of coinfection with WT NLPS and VAR P99PS viruses. RNA was extracted from the inoculum (0 h postinfection [p.i.]) and from the coinfected cells (12 h p.i.), yielding samples representative of the input vRNA and the output vRNA, respectively. RT ddPCR with primers specific for each chimeric segment (see Table 3) was then used to quantify segment copy numbers in each sample. To evaluate replication efficiency, the fold increase was calculated as the copy number of output vRNA/input vRNA. For the heterologous HA segments, HA_P99PS was replicated ∼2.1-fold more efficiently than HA_NLPS (Fig. 4A). The heterologous NA segments were replicated with comparable efficiency: NA_NLPS had an ∼1.2-fold advantage over NA_P99PS (Fig. 4B). For the heterologous NS segments, NS_P99PS was replicated ∼2.1-fold more efficiently than NS_NLPS (Fig. 4C).
TABLE 3.
Nucleotide sequences of primers used for RT ddPCR
| Segment | Sequence of primer: |
|
|---|---|---|
| Forward | Reverse | |
| HA_P99PS | AAGACTATCATTGCTTTGAGCTAC | GTTGCCGTGCTGTTGTAAT |
| HA_NLPS | GTTCTGCTATATACATTTGCAACC | GTGTCTACAGTGTCTGTTGAAT |
| NA_P99PS | CGATTGGCTCTGTTTCTCTC | TGCTTGAAATGCAATGTTACAG |
| NA_NLPS | CCATTGGTTCGGTCTGTTTG | CCAAGTTGAATTGAGTGGCTAA |
| NS_P99PS | CCAACACTGTGTCAAGTTTC | AACACTCAGTTCTTGGTCTAC |
| NS_NLPS | CAACACCTTGTCAAGCTTT | GCAACACCCAATCCAATG |
FIG 4.
Segments with different packaging signal regions were replicated with various efficiencies. MDCK cells were coinfected at a high MOI with P99-based viruses containing chimeric HA (A), NA (B), or NS (C) segments. RNA was extracted from both the inoculum (0 h p.i.) and the cells (12 h p.i.) and reverse transcribed into cDNA, which was used as the template in ddPCR with primers specific for the 3′ packaging signal region of each segment of interest. The copy number of each segment in the cells (output) was divided by the copy number of each segment in the inoculum (input). Data were analyzed using Student's t test. Data are represented as means ± 1 SD for 2 to 4 biological replicates performed in triplicate.
Although the differences in replication for the HA_PS and NS_PS segments are moderate, they could potentially impact reassortment outcomes. For example, if less of the NLPS-containing segment was present in coinfected cells, then the P99PS segment might predominate in reassortant progeny viruses due to its greater availability rather than a preference for homologous packaging signals during genome assembly. As indicated below, we corrected for unequal replication of P99PS and NLPS segments where necessary by altering the input ratio of the coinfecting viruses such that equal or greater amounts of the NLPS segment were present in cells at 12 h p.i. In this way, if the P99PS segment was favored for incorporation, then the phenotype could be confidently attributed to a packaging defect of the NLPS segment and not to a difference in availability.
HA segments containing matched packaging signals relative to the background of the virus were significantly favored for incorporation into new virus particles.
To assess whether the packaging signals on the HA segment affected IAV reassortment outcomes, we performed coinfections in MDCK cells with (i) P99wt HA_P99PS and P99var15 HA_P99PS viruses as a control or (ii) P99var15 HA_P99PS and P99wt HA_NLPS viruses (Fig. 2). For the control coinfection, 5 PFU/cell of each virus was used. Due to the differences in replication of HA_P99PS and HA_NLPS segments outlined above, the inoculum for the heterologous coinfection was formulated to contain 5 PFU/cell of the P99var15 HA_P99PS virus and >5 PFU/cell of the P99wt HA_NLPS virus. Both coinfections were performed in triplicate on two separate occasions and limited to a single round of replication by adding ammonium chloride to the medium. At 12 h p.i., the cell culture supernatants and the infected cell monolayers were harvested. The cells were used to quantify the total number of each modified HA segment available within coinfected cells at 12 h p.i. Plaque isolates derived from the collected supernatants were genotyped to evaluate reassortment outcomes. Genotype data averaged across all six replicate coinfections are presented in Fig. 5A. For each segment, the percentage of virus isolates containing a WT segment was plotted. Reassortment efficiency for these experiments was high: for the heterologous coinfections, 94 to 100% of isolates had reassortant genotypes.
FIG 5.
HA segments with matched packaging signals were preferentially incorporated into new virus particles. (A) MDCK cells were coinfected at a high MOI with P99-based viruses containing chimeric HA segments, and the genotypes of progeny viruses collected at 12 h p.i. after one cycle of replication were determined. The percentage of viruses that contained a WT segment (as opposed to VAR) is plotted on the y axis. The WT HA segment has matched packaging signals for the control coinfection (black bars) and mismatched packaging signals for the heterologous coinfection (gray bars). For all other segments, both WT and VAR contain matched packaging signals. *, P < 0.0001 compared to all other values using two-way ANOVA with Tukey's multiple comparisons. Data are represented as means ± 1 SD for two biological replicates performed in triplicate. (B) RT ddPCR was used to quantify the number of each HA segment available in the coinfected cells at 12 h p.i. using primers specific for the 3′ packaging signal region of each segment. The total number of HA copies in each well was calculated and graphed. Data are represented as means ± 1 SD.
Since the viruses used in the control coinfection contained P99 packaging signals on all eight segments, we expected approximately a 50:50 distribution of WT and VAR for each segment. We did indeed see this distribution in our data set (black bars, Fig. 5A). Similarly, all segments in the viruses used in the heterologous coinfection with the exception of the HA segment had P99 packaging signals. We therefore also expected to see approximately equal incorporation of WT and VAR versions of these seven segments in reassortant progeny, and again this expected result was observed (gray bars, Fig. 5A). In the heterologous coinfection, the WT HA segment had NL packaging signals and the VAR HA segment had P99 packaging signals. Our data show that 15% of isolates genotyped contained a WT HA_NLPS segment (Fig. 5A). Conversely, in the control coinfection, 60% of isolates genotyped contained a WT HA_P99PS segment. Figure 5B shows that there was on average 3.9-fold more HA_NLPS than HA_P99PS available in the cells at 12 h p.i. (gray bars, Fig. 5A). Taken together, these data show a clear preference for incorporation of HA segments containing packaging signals that match those of the other seven segments, and this phenotype is due to inefficient incorporation of the HA_NLPS segment and not due to lower availability of the HA_NLPS segment in the cell.
The NA segment with heterologous packaging signals is not disfavored during reassortment.
Based on low sequence identity in the packaging signal regions (see Table 1), we hypothesized that introduction of NLPS sequences onto the P99 NA segment would disfavor its incorporation, as was seen for HA. However, contrary to our hypothesis, we did not see a significant difference in the incorporation of NA_P99PS versus NA_NLPS (Fig. 6). Coinfections of MDCK cells, genotyping of virus isolates, and enumeration of the NA_PS segments present in the cells at 12 h p.i. were carried out as described for the HA segment above. For the NA_PS viruses, a 1:1 input ratio of VAR to WT viruses in the control coinfection yielded approximately a 50:50 distribution of WT and VAR for each segment as expected (black bars in Fig. 6). However, a 1:1 input ratio of VAR to WT viruses in the heterologous coinfection showed an overrepresentation of VAR across all eight segments (gray bars, Fig. 6A), even though there were equal amounts of NA_P99PS and NA_NLPS segments available in the cells at 12 h p.i. (Fig. 6B). As explained above, we expected to see approximately a 50:50 distribution of WT and VAR in all unmodified segments for the heterologous coinfection, since all unmodified segments have P99 packaging signals. Therefore, we repeated the heterologous coinfection experiments using a 1:1.5 input ratio of VAR to WT viruses. After this correction, we saw approximately a 50:50 distribution of WT and VAR across the unmodified segments as expected (gray bars, Fig. 6C). Focusing on the NA segment, we observed a slight preference for incorporation of the segment containing matched packaging signals, but this bias was not significant (Fig. 6C). We observed 1.5-fold more NA_NLPS than NA_P99PS available in the cells at 12 h p.i. (Fig. 6D), reflecting the adjusted WT input. Taken together, these data suggest that packaging signal divergence on the NA segment does not significantly affect reassortment outcomes between the IAV strains tested.
TABLE 1.
Percent nucleotide identity in packaging signal regions, P99 (H3N2) versus NL (H1N1)a
| Segment | % nucleotide identity | PS length (UTR + coding) (nt) |
|
|---|---|---|---|
| 3′ | 5′ | ||
| PB2 | 89.7 | 177 | 184 |
| PB1 | 96.9 | 174 | 193 |
| PA | 89.8 | 174 | 208 |
| HA | 50.0 | 136 | 136 |
| NP | 87.5 | 195 | 174 |
| NA | 56.6 | 150 | 200 |
| M | 90.1 | 275 | 270 |
| NS | 83.3 | 200 | 200 |
Regions analyzed are conservative estimates of packaging signal (PS) lengths, based on data compiled by Hutchinson et al. (57). Data in bold indicate the segments examined in the present study.
FIG 6.
NA segments with matched packaging signals were not significantly favored for incorporation. (A and C) MDCK cells were coinfected at a high MOI with P99-based viruses containing chimeric NA segments in either a 1:1 input ratio of VAR to WT (A) or a 1:1 input ratio of VAR to WT for the control coinfection and a 1:1.5 input ratio of VAR to WT for the heterologous coinfection (C). The genotypes of progeny viruses collected at 12 h p.i. after a single cycle of replication were then analyzed via HRM analysis. The percentage of viruses that contained a WT segment (as opposed to VAR) is plotted on the y axis. The WT NA segment has matched packaging signals for the control coinfection (black bars) and mismatched packaging signals for the heterologous coinfection (gray bars). For all other segments, both WT and VAR contain matched packaging signals. n.s., not significant using two-way ANOVA with Tukey's multiple comparisons. Data are represented as means ± 1 SD for two biological replicates performed in triplicate for each condition. The data sets used for the control coinfections in panels A and C are the same. (B and D) RT ddPCR was used to quantify the number of each NA segment available in the coinfected cells at 12 h p.i. using primers specific for the 3′ packaging signal region of each segment. The total number of NA copies in each well was calculated and graphed. Input ratio of VAR to WT, 1:1 (B) and 1:1.5 (D). Data are represented as means ± 1 SD.
Packaging signals on the NS segment do not impact IAV reassortment outcomes.
Compared to the HA and NA segments, the NS segment has a higher percent nucleotide identity in the packaging signal regions of P99 and NL viruses (see Table 1). However, it is important to note that the precise nucleotides within these regions that are important for IAV segment incorporation have not been identified; thus, the 17% difference in nucleotide sequence could impact NS segment packaging in the context of a heterologous coinfection. The MDCK cell coinfections, genotyping of virus isolates, and quantification of the NS_PS segments present in coinfected cells at 12 h p.i. were performed as described above for the HA segment. Somewhat similarly to the NA segment coinfections, we found that a 1:1 input ratio of VAR to WT viruses resulted in an overrepresentation of VAR across all eight segments in the control coinfection as well as across all unmodified segments in the heterologous coinfection (Fig. 7A). However, even under these conditions, it was clear that the NS_NLPS segment was packaged efficiently, as the percent WT for the NS segment was one of the higher values calculated (Fig. 7A). Interestingly, this phenotype was observed even though there was less of the NS_NLPS segment available in the cells at 12 h p.i. than of the NS_P99PS segment (Fig. 7B).
FIG 7.
NS segments with heterologous packaging signals assorted randomly. (A and C) MDCK cells were coinfected at a high MOI with P99-based viruses containing chimeric NS segments in either a 1:1 input ratio of VAR to WT (A) or a 1:1.25 input ratio of VAR to WT for the control coinfection and a 1:1.5 input ratio of VAR to WT for the heterologous coinfection (C). The genotypes of progeny viruses collected at 12 h p.i. after one cycle of replication were then analyzed via HRM analysis. The percentage of viruses that contained a WT segment (as opposed to VAR) is plotted on the y axis. The WT NS segment has matched packaging signals for the control coinfection (black bars) and mismatched packaging signals for the heterologous coinfection (gray bars). For all other segments, both WT and VAR contain matched packaging signals. n.s., not significant using two-way ANOVA with Tukey's multiple comparisons. Data are represented as means ± 1 SD for two biological replicates performed in triplicate for each condition. (B and D) RT ddPCR was used to quantify the number of each NS segment available in the coinfected cells at 12 h p.i. using primers specific for the 3′ packaging signal region of each segment. The total number of NS copies in each well was calculated and graphed. Input ratio of VAR to WT, 1:1 (B) and 1:1.5 (D). Data are represented as means ± 1 SD.
To verify these data, we repeated the coinfection experiments, this time increasing the amount of WT virus used in both the control (25% increase) and heterologous (50% increase) coinfections but keeping the amount of VAR virus constant. Under these conditions, we observed approximately a 50:50 distribution of WT and VAR for all segments in the control coinfection and for all unmodified segments in the heterologous coinfection as expected (Fig. 7C). This second set of coinfections confirmed our original findings: the heterologous NS_NLPS segment was not underrepresented compared to the unmodified segments of the same coinfection (gray bars, Fig. 7C) or to the NS_P99PS counterpart in the control coinfection. In this repeat experiment, approximately equal amounts of the heterologous NS segments were available in the cells at 12 h p.i. (Fig. 7D). Taken together, our data show that packaging signal divergence on the NS segment does not impact IAV reassortment, at least for the strains tested.
DISCUSSION
This study aimed to quantify the impact of packaging signal divergence on IAV reassortment. Toward generating results relevant for currently circulating IAVs, we used human IAV strains of the seasonal H3N2 and pandemic H1N1 lineages. We focused on the HA, NA, and NS segments due to the degree of divergence in the packaging signal regions, between the two strains selected for our experiments and also more generally among IAVs (58). Our knowledge to date on how incompatibilities among vRNAs affect IAV reassortment is limited, in part due to the challenge of finding a system that allows for examination of RNA mismatch in the absence of other confounding factors. To overcome this problem, we applied our previously described WT-VAR system to isolate the effects of mismatch among RNA packaging signals from those of protein mismatch (39).
Our results show that HA segments containing homologous packaging signals were preferentially packaged into P99-based viruses. Under single-cycle conditions, 85% of progeny viruses were found to have incorporated the homologous HA_P99PS segment, while 15% of progeny viruses incorporated the heterologous HA_NLPS segment. This preference was observed even when HA segments with heterologous packaging signals constituted a majority of the HA segment pool in the coinfected cells, indicating that the favored incorporation was directed by packaging signals and not by abundance in the cell. Our data are consistent with the observations of Essere et al. (53) and confirm that heterologous packaging signals can limit reassortment. These findings are of particular interest given the relevance of HA segment reassortment for the formation of antigenically novel IAVs.
In contrast to the HA segment, introduction of NL packaging signals onto the NA or NS segment of P99 viruses did not result in a detectable bias in reassortment patterns. These results were unexpected based on our own findings for HA and those of Essere et al. (53) and the low sequence identity between P99 and NL viruses in NS and particularly NA packaging signal regions. Several possible explanations exist. The first is that, despite differences in nucleotide sequence over the broad terminal regions examined, the RNA signals on NA and NS that mediate segment bundling may not differ substantially between NL and P99 viruses. For NS, which has ∼83% nucleotide identity between P99 and NL viruses in the packaging signal regions, it is reasonable to propose that the particular nucleotides involved in packaging are conserved (59). For NA, which has ∼57% identity in the packaging signal regions, this explanation is less satisfactory. Potentially, however, secondary structures formed by the RNA are critical for packaging, and nucleotide differences between P99 and NL may not disrupt these structures in the NA and NS segments. It has been proposed that interactions between segments during packaging occur through complementary sequences found on local RNA structures, or RNA loops, which are exposed from the viral ribonucleoprotein complexes (vRNPs) (60–63). If the molecular cues for genome assembly found on certain segments are conserved among divergent IAVs, it follows that these signals would be unlikely to restrict heterologous reassortment.
A second possible explanation for the absence of a packaging phenotype for NA and NS is the existence of packaging signals that are outside the currently mapped terminal regions. A recent study demonstrated that the influenza A/Udorn/307/72 virus PB1 and NA segments were preferentially copackaged during reassortment with A/Puerto Rico/8/34 virus. The interaction between these two segments was mapped to an internal region of PB1 comprising nucleotides 1776 to 2070 (64, 65). Additionally, NS and PB1 from influenza A/Finch/England/2051/91 virus have been shown to interact via complementary sequences that are located within internal regions of both segments (51). As our study examined only terminal packaging signal regions, we cannot discount the possibility that the P99 NA and/or NS assemble with other segments via sequences within the inner coding region. Similarly, it is formally possible that serial mutation within the terminal ∼60 nt of the P99 ORF did not disrupt the nucleotides important for NA and NS packaging, such that P99 sequences directed incorporation of segments, rather than NL-derived terminal sequences. However, Gao et al. demonstrated that a similar strategy was sufficient to disrupt packaging in a PR8 background (56). It is also important to note that multiple reports have documented a key role for the terminal regions in packaging of NA and NS gene segments (41, 45, 49).
A third reason why heterologous packaging signals on NA or NS segments were not found to bias reassortment could be that these two segments are relatively unimportant in the selective process that results in segment bundling. The existence of a hierarchy in which the packaging signals of certain segments have a dominant role in genome assembly has been noted (56, 66). Gao et al. were furthermore able to rescue a seven-segmented IAV that expressed an influenza C virus glycoprotein flanked with HA-specific packaging signals in the place of the HA and NA segments. This virus was stable over multiple passages in eggs despite the lack of NA packaging signals, suggesting that the NA sequences are not critical for incorporation of the other seven segments (67). A subsequent study demonstrated that deleting the packaging signals of the NS segment had no effect on recombinant virus growth, implying that NS packaging signals might also be of lesser importance during genome packaging (56).
It is now well accepted that packaging of the IAV genome is a selective process (19, 50, 68–71). Since an IAV particle typically does not carry more than eight segments and must carry one copy of each segment to be infectious, high-fidelity packaging is needed to ensure viability (52, 72). However, there is a tradeoff between infectivity and evolutionary capacity: a very stringent selective packaging mechanism would limit reassortment among IAVs that differ in their packaging signals. In other words, a more flexible packaging mechanism may offer an evolutionary advantage by allowing greater genetic diversification through reassortment (52). Our findings show that movement of the antigenically important HA segment between two human IAV subtypes was disfavored at the level of packaging, while the movement of NS and NA (which also has antigenic properties) was relatively free. These results suggest that packaging is relatively flexible but that this property varies with segment (and likely also virus strain). Although our data revealed a preference for the HA segment containing homologous packaging signals, the HA segment with heterologous packaging signals was present in 15% of P99-based progeny viruses. In other words, packaging signal mismatch reduced but did not completely block HA reassortment. Low levels of reassortment are expected to be epidemiologically significant if certain reassortant genotypes have a selective advantage, such as immunological novelty (73).
It will be of interest to determine whether the reassortment patterns observed here extend to other strain pairings. Detailed knowledge of the segment interactions that occur during packaging is currently limited, and importantly, the extent to which the process of IAV genome assembly is conserved among strains remains unclear. As discussed above, sequences or structures involved in packaging may differ between divergent IAVs. In the HA and NA segments in particular, the degree of sequence conservation within mapped packaging signal regions among subtypes is low. In addition, the order in which segments are bundled and the pairwise interactions that make up the full network of eight vRNPs may not be conserved. Without this detailed knowledge, it is difficult to predict how packaging might shape reassortment between a given pairing of parental viruses. A key question is whether the preference for matched packaging signals on HA holds true across subtypes. The compatibility of zoonotic H5 and H7 subtype strains with seasonal human IAVs is of particular relevance since a reassortment event could lead to the formation of a pandemic strain. Our system is well suited to give quantitative answers to these important questions.
In summary, the data presented here suggest that movement of the NA and NS segments from viruses of the 2009 pandemic H1N1 lineage into a seasonal H3N2 background would not be significantly restricted by packaging signal mismatch, while movement of the H1 HA segment would be constrained at the level of genome assembly. Thus, our data reveal that the impact of packaging signals on reassortment is segment dependent.
MATERIALS AND METHODS
Cells and viruses.
Madin-Darby canine kidney (MDCK) cells were maintained in minimum essential medium (Gibco) supplemented with penicillin-streptomycin and 10% fetal bovine serum (FBS). Human embryonic kidney cells (293T) were maintained in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% FBS. Reverse genetics-derived influenza A/Panama/2007/99 (H3N2) virus (referred to as P99) was used for this study, and packaging signal regions were taken from P99 as well as from influenza A/Netherlands/602/2009 (H1N1) virus (referred to as NL). Virus stocks were generated in 9- to 11-day-old embryonated eggs from hens (Hy-Line). Viruses with modified HA packaging signals were passaged through eggs once, viruses with modified NA packaging signals were passaged through eggs twice, and virus stocks with modified NS packaging signals were generated by infecting eggs with a plaque isolate derived in MDCK cells from the egg passage 1 stock.
Design of chimeric segments.
The three segments examined in this study (HA, NA, and NS) had the lowest percent nucleotide identity in the packaging signal regions of the viruses used (Table 1) and were selected for this reason. Chimeric segments were designed as follows and then synthesized through Genewiz. For HA, NA, and NS, the full P99 open reading frame (ORF) was flanked by 3′ and 5′ packaging signal regions taken from either P99 or NL. For HA, packaging signals regions constituted 136 nt at each of the 3′ and 5′ ends; for NA, packaging signal regions constituted 150 nt at the 3′ end and 200 nt at the 5′ end; and for NS, packaging signal regions constituted 200 nt at each of the 3′ and 5′ ends. To disrupt the native packaging signals in each segment, the first and last 60 nt of the ORF were modified via silent mutation of every codon (excluding start, stop, Met, and Trp codons). To ensure translation initiation at the appropriate start codon, all ATGs in the added 3′ packaging signal region were removed via silent mutation. The constructs were then flanked with BsmBI restriction sites to allow for ligation into the pDP2002 ambisense vector (a generous gift of Daniel Perez, University of Georgia). Construct design was informed by the work of Gao et al. (56), and packaging signal lengths were based on literature compiled by Hutchinson et al. (57).
Generation of modified viruses.
The constructs described above were each ligated into the pDP2002 reverse genetics vector (74). A variant (VAR) version of each construct with P99 packaging signals was then generated by introducing up to six silent mutations into the ORF via site-directed mutagenesis (Fig. 1C). The mutations introduced correspond to those used in the Pan/99var15 virus described previously (75). These silent mutations allow differentiation of VAR from WT gene segments using HRM analysis and were previously shown to have no detectable effect on viral fitness (39, 75). Plasmids encoding chimeric segments were then used with a pPOL1 P99 reverse genetics system to rescue viruses in a P99 background, according to standard procedures (76, 77). Briefly, a 15-plasmid rescue system based on pPOL1 and pCAGGS helper plasmids (PB2, PB1, PA, HA, NP, NA, and NS) was used. At 16 to 24 h posttransfection, 293T cells were injected into 9- to 11-day-old embryonated eggs from hens and incubated for 48 h to generate virus stocks. Stock titers were then determined via plaque assay on MDCK cells. Chimeric WT segments were rescued in a full P99 WT background, and chimeric VAR segments were rescued in a full P99 VAR background. The VAR mutations for all eight segments have been described previously (39, 75) (Pan/99var15 was used in the present study). All rescued viruses contained only one segment with modified packaging signal regions, as follows. One WT virus and one VAR virus containing P99 packaging signals were generated for each of the three modified segments of interest (HA, NA, or NS), and one WT virus containing NL packaging signals was generated for each of the three modified segments of interest (HA, NA, or NS). Therefore, a total of nine viruses were rescued as illustrated in Fig. 2.
Sequencing of virus stocks and genotype verification.
The full genomes of all nine virus stocks used in this study were sequenced via Illumina next-generation sequencing through the Emory Integrated Genomics Core (EIGC). Sequences of the grafted packaging signals as well as the serially mutated P99 ORF regions of each modified segment were confirmed via Sanger sequencing (Genewiz), as analysis of the deep sequencing data revealed an inability to map reads within the pseudoduplicated regions.
Viral growth kinetics.
The multicycle growth properties of each virus were assessed as follows. Triplicate wells of MDCK cells were infected at a multiplicity of infection (MOI) of 0.01 PFU/cell alongside the P99 wild-type virus as a control. After 1 h at 33°C, virus inoculum was removed and medium containing tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin was added. A sample of medium was collected from each well at 1 h, 12 h, 24 h, 48 h, and 72 h postinfection (p.i.) and stored at −80°C, and subsequently titers were determined via plaque assay on MDCK cells. Mean titers of the three replicates ± 1 standard deviation (SD) were plotted against time. The limit of detection was 50 PFU/ml.
Coinfection of MDCK cells with modified viruses.
Triplicate wells of MDCK cells were coinfected at an MOI of 5 PFU/cell for each virus (unless otherwise specified) for a final MOI of 10 PFU/cell to ensure high levels of coinfection. Previously published work from our lab using the same A/Panama/2007/99 WT-VAR system reported a high frequency of coinfected cells (∼85% of infected cells) at this MOI (78). The control coinfections used WT and VAR viruses with matched packaging signals on the segment of interest (P99 for both), and the heterologous coinfections used WT and VAR viruses with mismatched packaging signals on the segment of interest (NL and P99, respectively) as shown in Fig. 2. Inoculations were performed on ice, and after a 45-min incubation period at 4°C to allow for virus attachment, the virus inoculum was removed and the cells were washed 3 times with cold phosphate-buffered saline (PBS) to remove unattached virus. Warm medium was then added to allow for synchronized virus entry, and the cells were incubated at 33°C for 3 h. After 3 h, the medium was removed from the cells and replaced with medium supplemented with 1 M ammonium chloride and HEPES buffer. The absence of TPCK-treated trypsin and the presence of ammonium chloride ensure a single cycle of viral replication. Cells were incubated for an additional 9 h; then, at 12 h p.i., supernatants were collected and frozen at −80°C for use in reassortment analyses and cells were harvested in RNAprotect cell reagent (Qiagen) for subsequent analysis of RNA by reverse transcription-droplet digital PCR (RT ddPCR).
Genotyping viral isolates from coinfected cells.
Viruses present in the coinfection supernatants were sampled randomly via plaque purification on MDCK cells. Well-separated plaque isolates were placed in 150 μl PBS, one isolate per PBS aliquot. Twenty-one virus isolates were picked for each supernatant sample. In total for each set of like coinfections performed, a minimum of 126 plaque isolates were picked. RNA was extracted from the plaque isolates using the Zymo Research ZR-96 viral RNA kit according to the manufacturer's instructions with the following modification: 40 μl water was used for the elution step. A 12.8-μl amount of RNA was reverse transcribed using Maxima reverse transcriptase (Fermentas) according to the manufacturer's instructions. The resultant cDNA was then diluted in water and used in quantitative PCR (qPCR) with Precision Melt Supermix (Bio-Rad) and primers specific for each of the eight segments of P99 virus (Table 2). These primers bind to sequences that do not differ between WT and VAR viruses, but the ∼100-bp amplicons produced contain the region altered in VAR gene segments. qPCR mixtures were set up in white, thin-walled, 384-well plates (Bio-Rad), and the reactions were run in a CFX384 real-time PCR detection system (Bio-Rad). PCR amplification was followed immediately by a melt curve analysis. The conditions of PCR amplification and melt analysis followed the protocol provided with the Precision Melt Supermix. Data were analyzed using Precision Melt Analysis software (Bio-Rad) and comparison to WT and VAR cDNA controls. Thus, the genotype of each segment in each virus isolate was determined to be either WT or VAR depending on which control the melt curves clustered with in the software. Genotype data were analyzed by determining the proportion of genomes that carried the WT version of each of the eight segments. Only reassortant virus isolates were included in these analyses; parental genotypes were excluded due to the possibility that parental-type progeny were produced by singly infected, rather than coinfected, cells. The data were analyzed using two-way analysis of variance (ANOVA) with Tukey's multiple comparisons, and means ± 1 SD were graphed. All coinfections were performed in triplicate two independent times.
TABLE 2.
Nucleotide sequences of P99-specific primers used for HRM analysis
| Segment | Sequence of primer: |
|
|---|---|---|
| Forward | Reverse | |
| PB2 | TGGAATAGAAATGGACCTGTGA | GGTTCCATGTTTTAACCTTTCG |
| PB1 | AGGCTAATAGATTTCCTCAAGGATG | ACTCTCCTTTTTCTTTGAAAGTGTG |
| PA | TGCAACACTACTGGAGCTGAG | CTCCTTGTCACTCCAATTTCG |
| HA | CCTTGATGGAGAAAACTGCAC | CAACAAAAAGGTCCCATTCC |
| NP | CAACATACCAGAGGACAAGAGC | ACCTTCTAGGGAGGGTCGAG |
| NA | TCATGCGATCCTGACAAGTG | TGTCATTTGAATGCCTGTTG |
| M | GTTTTGGCCAGCACTACAGC | CCATTTGCCTGGCCTGACTA |
| NS | ACCTGCTTCGCGATACATAAC | AGGGGTCCTTCCACTTTTTG |
Determining heterologous segment availability at 12 h p.i.
The coinfected cells that had been harvested at 12 h p.i. in RNAprotect cell reagent were either used immediately postharvest or stored for <24 h at 4°C prior to use. RNA was extracted from the cells using the RNeasy Plus minikit (Qiagen) according to the manufacturer's instructions with the following modifications: homogenization was achieved via vortexing for 2 to 3 min, 40 μl water was used for the elution step and allowed to incubate on the membranes of the columns for 1 to 2 min at room temperature, and RNA was eluted by spinning the tubes at 11,000 rpm for 2 min in a Beckman Coulter Microfuge 22R centrifuge. Two micrograms of RNA was then reverse transcribed using Maxima reverse transcriptase (Fermentas). A no–reverse-transcriptase control was run in parallel. cDNA was diluted in water and used in droplet digital PCR (ddPCR). An overview of the ddPCR protocol is outlined in reference 79. Briefly, cDNAs were diluted to achieve working concentrations within the linear range, and 8.8 μl of diluted cDNA per sample was combined with 13.2 μl master mix (11 μl QX200 ddPCR EvaGreen Supermix [Bio-Rad] plus 2.2 μl 4 μM primer set). The primers used were specific for the 3′ packaging signal region so that the P99 and NL versions of the modified segment present in coinfected cells could be differentiated. All primers used showed no cross-priming (data not shown). A list of the primers used for ddPCR can be found in Table 3. Twenty microliters of each PCR was transferred to a DG8 cartridge (Bio-Rad) alongside 70 μl droplet generation oil for EvaGreen (Bio-Rad), and droplets were generated using a QX200 droplet generator (Bio-Rad). Forty microliters of the generated droplets was transferred to a Twin-Tec 96-well PCR plate (Eppendorf), and after heat sealing the plate with foil, 40 cycles of PCR were run in a C1000 Touch Thermal Cycler (Bio-Rad) with the following parameters: 95°C for 5 min, 40 cycles of 95°C for 30 s followed by 53.7°C for 1 min, 4°C for 5 min, 90°C for 5 min, and a final hold at 4°C. The ramp rate for all steps was set at 2°C/s as recommended by the manufacturer. The plate was then read in a QX200 droplet reader (Bio-Rad). No-reverse-transcriptase control samples were included in the ddPCR analysis as negative controls. ddPCR was run with two technical replicates per sample. The copy number of each segment of interest (segments with modified packaging signal regions) in each sample was determined using QuantaSoft software (Bio-Rad). The total copy number of each segment present in each well of coinfected cells was then calculated, and data were graphed as means ± 1 SD. For HA and NS, ddPCR was performed in parallel with the reassortment analyses (i.e., the same coinfections were used); for NA, ddPCR was performed using coinfections that had been set up under the same conditions and using the same virus stocks as those used to generate the reassortment data.
Replication efficiencies of heterologous segments.
In order to calculate the replication efficiencies of the chimeric packaging signal segments, coinfected cells were harvested at 12 h p.i. and processed for RT ddPCR as described above. In addition, viral RNA was extracted from the inoculum used for the coinfections immediately following infection using the QIAamp viral RNA kit (Qiagen) according to the manufacturer's instructions with the following modifications: carrier RNA was not added to the AVL buffer, 40 μl water was used for the elution, and tubes were spun for 2 min instead of 1 min in a Beckman Coulter Microfuge 22R centrifuge to elute the RNA. Twelve microliters of RNA was reverse transcribed using Maxima reverse transcriptase (Fermentas). A no-reverse-transcriptase control was included in parallel. cDNA was diluted in water and used in ddPCR as described above with the primers listed in Table 3. The copy number of each segment of interest in both the inoculum (input) and the cellular extracts (output) was determined using QuantaSoft software (Bio-Rad), taking into account the dilutions performed to ensure that each cDNA was within the linear range of the assay. The ratio of output to input values for each replicate was then determined, and the means ± 1 SD across 2 to 4 biological replicates performed in triplicate were graphed. Data were analyzed using Student's t test.
Statistical analyses.
All statistical analyses were performed in GraphPad Prism v7.02.
ACKNOWLEDGMENTS
We thank Daniel Perez for the kind gift of the pDP2002 plasmid. We thank Christopher Scharer, Bhanu Gandham, and Ramya Govindarajan for their assistance and expertise with the analysis of the whole-genome sequencing data.
This study was supported in part by the Emory Integrated Genomics Core (EIGC), which is subsidized by the Emory University School of Medicine and is one of the Emory Integrated Core Facilities. This work was funded in part by the NIAID Centers of Excellence in Influenza Research and Surveillance (CEIRS), contract number HHSN272201400004C to A.C.L. and J.S., and by R01 grants AI125268 and AI099000 to A.C.L.
We declare no conflicts of interest.
REFERENCES
- 1.Shaw ML, Palese P. 2013. Orthomyxoviridae, p 1151–1185. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Desselberger U, Nakajima K, Alfino P, Pedersen FS, Haseltine WA, Hannoun C, Palese P. 1978. Biochemical evidence that “new” influenza virus strains in nature may arise by recombination (reassortment). Proc Natl Acad Sci U S A 75:3341–3345. doi: 10.1073/pnas.75.7.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawaoka Y, Krauss S, Webster RG. 1989. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol 63:4603–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RAM, Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Boxrud D, Sambol AR, Abid SH, St. George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilbourne ED. 2006. Influenza pandemics of the 20th century. Emerg Infect Dis 12:9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viboud C, Simonsen L, Fuentes R, Flores J, Miller MA, Chowell G. 2016. Global mortality impact of the 1957–1959 influenza pandemic. J Infect Dis 213:738–745. doi: 10.1093/infdis/jiv534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ, GlaMOR Collaborating Teams. 2013. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR Project: a modeling study. PLoS Med 10:e1001558. doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen-Van-Tam JS, Hampson AW. 2003. The epidemiology and clinical impact of pandemic influenza. Vaccine 21:1762–1768. doi: 10.1016/S0264-410X(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 9.Nelson MI, Viboud C, Simonsen L, Bennett RT, Griesemer SB, St. George K, Taylor J, Spiro DJ, Sengamalay NA, Ghedin E, Taubenberger JK, Holmes EC. 2008. Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLoS Pathog 4:e1000012. doi: 10.1371/journal.ppat.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes EC, Ghedin E, Miller N, Taylor J, Bao Y, St. George K, Grenfell BT, Salzberg SL, Fraser CM, Lipman DJ, Taubenberger JK. 2005. Whole-genome analysis of human influenza A virus reveals multiple persistent lineages and reassortment among recent H3N2 viruses. PLoS Biol 3:e300. doi: 10.1371/journal.pbio.0030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westgeest KB, de Graaf M, Fourment M, Bestebroer TM, van Beek R, Spronken MI, de Jong JC, Rimmelzwaan GF, Russell CA, Osterhaus AD, Smith GJ, Smith DJ, Fouchier RA. 2012. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolution. J Gen Virol 93:1996–2007. doi: 10.1099/vir.0.043059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wille M, Tolf C, Avril A, Latorre-Margalef N, Wallerstrom S, Olsen B, Waldenstrom J. 2013. Frequency and patterns of reassortment in natural influenza A virus infection in a reservoir host. Virology 443:150–160. doi: 10.1016/j.virol.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Deng G, Tan D, Shi J, Cui P, Jiang Y, Liu L, Tian G, Kawaoka Y, Li C, Chen H. 2013. Complex reassortment of multiple subtypes of avian influenza viruses in domestic ducks at the Dongting Lake Region of China. J Virol 87:9452–9462. doi: 10.1128/JVI.00776-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abolnik C, Gerdes GH, Sinclair M, Ganzevoort BW, Kitching JP, Burger CE, Romito M, Dreyer M, Swanepoel S, Cumming GS, Olivier AJ. 2010. Phylogenetic analysis of influenza A viruses (H6N8, H1N8, H4N2, H9N2, H10N7) isolated from wild birds, ducks, and ostriches in South Africa from 2007 to 2009. Avian Dis 54:313–322. doi: 10.1637/8781-040109-Reg.1. [DOI] [PubMed] [Google Scholar]
- 15.Steel J, Lowen AC. 2014. Influenza A virus reassortment. Curr Top Microbiol Immunol 385:377–401. doi: 10.1007/82_2014_395. [DOI] [PubMed] [Google Scholar]
- 16.Vijaykrishna D, Poon LL, Zhu HC, Ma SK, Li OT, Cheung CL, Smith GJ, Peiris JS, Guan Y. 2010. Reassortment of pandemic H1N1/2009 influenza A virus in swine. Science 328:1529. doi: 10.1126/science.1189132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson MI, Detmer SE, Wentworth DE, Tan Y, Schwartzbard A, Halpin RA, Stockwell TB, Lin X, Vincent AL, Gramer MR, Holmes EC. 2012. Genomic reassortment of influenza A virus in North American swine, 1998–2011. J Gen Virol 93:2584–2589. doi: 10.1099/vir.0.045930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dugan VG, Chen R, Spiro DJ, Sengamalay N, Zaborsky J, Ghedin E, Nolting J, Swayne DE, Runstadler JA, Happ GM, Senne DA, Wang R, Slemons RD, Holmes EC, Taubenberger JK. 2008. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog 4:e1000076. doi: 10.1371/journal.ppat.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubeck MD, Palese P, Schulman JL. 1979. Nonrandom association of parental genes in influenza A virus recombinants. Virology 95:269–274. doi: 10.1016/0042-6822(79)90430-6. [DOI] [PubMed] [Google Scholar]
- 20.Greenbaum BD, Li OT, Poon LL, Levine AJ, Rabadan R. 2012. Viral reassortment as an information exchange between viral segments. Proc Natl Acad Sci U S A 109:3341–3346. doi: 10.1073/pnas.1113300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai LQ, Sedyaningsih ER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A 103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson S, Van Hoeven N, Chen LM, Maines TR, Cox NJ, Katz JM, Donis RO. 2009. Reassortment between avian H5N1 and human H3N2 influenza viruses in ferrets: a public health risk assessment. J Virol 83:8131–8140. doi: 10.1128/JVI.00534-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LM, Davis CT, Zhou H, Cox NJ, Donis RO. 2008. Genetic compatibility and virulence of reassortants derived from contemporary avian H5N1 and human H3N2 influenza A viruses. PLoS Pathog 4:e1000072. doi: 10.1371/journal.ppat.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatta M, Halfmann P, Wells K, Kawaoka Y. 2002. Human influenza a viral genes responsible for the restriction of its replication in duck intestine. Virology 295:250–255. doi: 10.1006/viro.2002.1358. [DOI] [PubMed] [Google Scholar]
- 25.Kimble JB, Sorrell E, Shao H, Martin PL, Perez DR. 2011. Compatibility of H9N2 avian influenza surface genes and 2009 pandemic H1N1 internal genes for transmission in the ferret model. Proc Natl Acad Sci U S A 108:12084–12088. doi: 10.1073/pnas.1108058108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrauwen EJ, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Fouchier RA, Herfst S. 2013. Reassortment between avian H5N1 and human influenza viruses is mainly restricted to the matrix and neuraminidase gene segments. PLoS One 8:e59889. doi: 10.1371/journal.pone.0059889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cline TD, Karlsson EA, Freiden P, Seufzer BJ, Rehg JE, Webby RJ, Schultz-Cherry S. 2011. Increased pathogenicity of a reassortant 2009 pandemic H1N1 influenza virus containing an H5N1 hemagglutinin. J Virol 85:12262–12270. doi: 10.1128/JVI.05582-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Hatta M, Nidom CA, Muramoto Y, Watanabe S, Neumann G, Kawaoka Y. 2010. Reassortment between avian H5N1 and human H3N2 influenza viruses creates hybrid viruses with substantial virulence. Proc Natl Acad Sci U S A 107:4687–4692. doi: 10.1073/pnas.0912807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Octaviani CP, Ozawa M, Yamada S, Goto H, Kawaoka Y. 2010. High level of genetic compatibility between swine-origin H1N1 and highly pathogenic avian H5N1 influenza viruses. J Virol 84:10918–10922. doi: 10.1128/JVI.01140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhang Q, Kong H, Jiang Y, Gao Y, Deng G, Shi J, Tian G, Liu L, Liu J, Guan Y, Bu Z, Chen H. 2013. H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science 340:1459–1463. doi: 10.1126/science.1229455. [DOI] [PubMed] [Google Scholar]
- 31.Ma W, Liu Q, Qiao C, del Real G, Garcia-Sastre A, Webby RJ, Richt JA. 2014. North American triple reassortant and Eurasian H1N1 swine influenza viruses do not readily reassort to generate a 2009 pandemic H1N1-like virus. mBio 5:e00919-13. doi: 10.1128/mBio.00919-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma J, Shen H, Liu Q, Bawa B, Qi W, Duff M, Lang Y, Lee J, Yu H, Bai J, Tong G, Hesse RA, Richt JA, Ma W. 2015. Pathogenicity and transmissibility of novel reassortant H3N2 influenza viruses with 2009 pandemic H1N1 genes in pigs. J Virol 89:2831–2841. doi: 10.1128/JVI.03355-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dlugolenski D, Jones L, Howerth E, Wentworth D, Tompkins SM, Tripp RA. 2015. Swine influenza virus PA and neuraminidase gene reassortment into human H1N1 influenza virus is associated with an altered pathogenic phenotype linked to increased MIP-2 expression. J Virol 89:5651–5667. doi: 10.1128/JVI.00087-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Hatta M, Watanabe S, Neumann G, Kawaoka Y. 2008. Compatibility among polymerase subunit proteins is a restricting factor in reassortment between equine H7N7 and human H3N2 influenza viruses. J Virol 82:11880–11888. doi: 10.1128/JVI.01445-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schrauwen EJ, Herfst S, Chutinimitkul S, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, Kuiken T, Fouchier RA. 2011. Possible increased pathogenicity of pandemic (H1N1) 2009 influenza virus upon reassortment. Emerg Infect Dis 17:200–208. doi: 10.3201/eid1702.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song MS, Pascua PN, Lee JH, Baek YH, Park KJ, Kwon HI, Park SJ, Kim CJ, Kim H, Webby RJ, Webster RG, Choi YK. 2011. Virulence and genetic compatibility of polymerase reassortant viruses derived from the pandemic (H1N1) 2009 influenza virus and circulating influenza A viruses. J Virol 85:6275–6286. doi: 10.1128/JVI.02125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner R, Matrosovich M, Klenk H. 2002. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol 12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- 38.Octaviani CP, Goto H, Kawaoka Y. 2011. Reassortment between seasonal H1N1 and pandemic (H1N1) 2009 influenza viruses is restricted by limited compatibility among polymerase subunits. J Virol 85:8449–8452. doi: 10.1128/JVI.05054-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall N, Priyamvada L, Ende Z, Steel J, Lowen AC. 2013. Influenza virus reassortment occurs with high frequency in the absence of segment mismatch. PLoS Pathog 9:e1003421. doi: 10.1371/journal.ppat.1003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang Y, Hong Y, Parslow TG. 2005. cis-Acting packaging signals in the influenza virus PB1, PB2, and PA genomic RNA segments. J Virol 79:10348–10355. doi: 10.1128/JVI.79.16.10348-10355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii Y, Goto H, Watanabe T, Yoshida T, Kawaoka Y. 2003. Selective incorporation of influenza virus RNA segments into virions. Proc Natl Acad Sci U S A 100:2002–2007. doi: 10.1073/pnas.0437772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe T, Watanabe S, Noda T, Fujii Y, Kawaoka Y. 2003. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J Virol 77:10575–10583. doi: 10.1128/JVI.77.19.10575-10583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozawa M, Fujii K, Muramoto Y, Yamada S, Yamayoshi S, Takada A, Goto H, Horimoto T, Kawaoka Y. 2007. Contributions of two nuclear localization signals of influenza A virus nucleoprotein to viral replication. J Virol 81:30–41. doi: 10.1128/JVI.01434-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozawa M, Maeda J, Iwatsuki-Horimoto K, Watanabe S, Goto H, Horimoto T, Kawaoka Y. 2009. Nucleotide sequence requirements at the 5′ end of the influenza A virus M RNA segment for efficient virus replication. J Virol 83:3384–3388. doi: 10.1128/JVI.02513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujii K, Fujii Y, Noda T, Muramoto Y, Watanabe T, Takada A, Goto H, Horimoto T, Kawaoka Y. 2005. Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions. J Virol 79:3766–3774. doi: 10.1128/JVI.79.6.3766-3774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goto H, Muramoto Y, Noda T, Kawaoka Y. 2013. The genome-packaging signal of the influenza A virus genome comprises a genome incorporation signal and a genome-bundling signal. J Virol 87:11316–11322. doi: 10.1128/JVI.01301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noda T, Sugita Y, Aoyama K, Hirase A, Kawakami E, Miyazawa A, Sagara H, Kawaoka Y. 2012. Three-dimensional analysis of ribonucleoprotein complexes in influenza A virus. Nat Commun 3:639. doi: 10.1038/ncomms1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fournier E, Moules V, Essere B, Paillart J-C, Sirbat J-D, Isel C, Cavalier A, Rolland J-P, Thomas D, Lina B, Marquet R. 2012. A supramolecular assembly formed by influenza A virus genomic RNA segments. Nucleic Acids Res 40:2197–2209. doi: 10.1093/nar/gkr985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fournier E, Moules V, Essere B, Paillart JC, Sirbat JD, Cavalier A, Rolland JP, Thomas D, Lina B, Isel C, Marquet R. 2012. Interaction network linking the human H3N2 influenza A virus genomic RNA segments. Vaccine 30:7359–7367. doi: 10.1016/j.vaccine.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 50.Chou YY, Vafabakhsh R, Doganay S, Gao Q, Ha T, Palese P. 2012. One influenza virus particle packages eight unique viral RNAs as shown by FISH analysis. Proc Natl Acad Sci U S A 109:9101–9106. doi: 10.1073/pnas.1206069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gavazzi C, Yver M, Isel C, Smyth RP, Rosa-Calatrava M, Lina B, Moulès V, Marquet R. 2013. A functional sequence-specific interaction between influenza A virus genomic RNA segments. Proc Natl Acad Sci U S A 110:16604–16609. doi: 10.1073/pnas.1314419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakatsu S, Sagara H, Sakai-Tagawa Y, Sugaya N, Noda T, Kawaoka Y. 2016. Complete and incomplete genome packaging of influenza A and B viruses. mBio 7:e01248-16. doi: 10.1128/mBio.01248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Essere B, Yver M, Gavazzi C, Terrier O, Isel C, Fournier E, Giroux F, Textoris J, Julien T, Socratous C, Rosa-Calatrava M, Lina B, Marquet R, Moules V. 2013. Critical role of segment-specific packaging signals in genetic reassortment of influenza A viruses. Proc Natl Acad Sci U S A 110:E3840–E3848. doi: 10.1073/pnas.1308649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker SF, Nogales A, Finch C, Tuffy KM, Domm W, Perez DR, Topham DJ, Martinez-Sobrido L. 2014. Influenza A and B virus intertypic reassortment through compatible viral packaging signals. J Virol 88:10778–10791. doi: 10.1128/JVI.01440-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao Q, Palese P. 2009. Rewiring the RNAs of influenza virus to prevent reassortment. Proc Natl Acad Sci U S A 106:15891–15896. doi: 10.1073/pnas.0908897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao Q, Chou Y-Y, Doğanay S, Vafabakhsh R, Ha T, Palese P. 2012. The influenza A virus PB2, PA, NP, and M segments play a pivotal role during genome packaging. J Virol 86:7043–7051. doi: 10.1128/JVI.00662-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hutchinson EC, von Kirchbach JC, Gog JR, Digard P. 2010. Genome packaging in influenza A virus. J Gen Virol 91:313–328. doi: 10.1099/vir.0.017608-0. [DOI] [PubMed] [Google Scholar]
- 58.Chen R, Holmes EC. 2010. Hitchhiking and the population genetic structure of avian influenza virus. J Mol Evol 70:98–105. doi: 10.1007/s00239-009-9312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gog JR, Afonso EDS, Dalton RM, Leclercq I, Tiley L, Elton D, von Kirchbach JC, Naffakh N, Escriou N, Digard P. 2007. Codon conservation in the influenza A virus genome defines RNA packaging signals. Nucleic Acids Res 35:1897–1907. doi: 10.1093/nar/gkm087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerber M, Isel C, Moules V, Marquet R. 2014. Selective packaging of the influenza A genome and consequences for genetic reassortment. Trends Microbiol 22:446–455. doi: 10.1016/j.tim.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Gultyaev AP, Spronken MI, Richard M, Schrauwen EJ, Olsthoorn RC, Fouchier RA. 2016. Subtype-specific structural constraints in the evolution of influenza A virus hemagglutinin genes. Sci Rep 6:38892. doi: 10.1038/srep38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baudin F, Bach C, Cusack S, Ruigrok RW. 1994. Structure of influenza virus RNP. I. Influenza virus nucleoprotein melts secondary structure in panhandle RNA and exposes the bases to the solvent. EMBO J 13:3158–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arranz R, Coloma R, Chichon FJ, Conesa JJ, Carrascosa JL, Valpuesta JM, Ortin J, Martin-Benito J. 2012. The structure of native influenza virion ribonucleoproteins. Science 338:1634–1637. doi: 10.1126/science.1228172. [DOI] [PubMed] [Google Scholar]
- 64.Cobbin JC, Ong C, Verity E, Gilbertson BP, Rockman SP, Brown LE. 2014. Influenza virus PB1 and neuraminidase gene segments can cosegregate during vaccine reassortment driven by interactions in the PB1 coding region. J Virol 88:8971–8980. doi: 10.1128/JVI.01022-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilbertson B, Zheng T, Gerber M, Printz-Schweigert A, Ong C, Marquet R, Isel C, Rockman S, Brown L. 2016. Influenza NA and PB1 gene segments interact during the formation of viral progeny: localization of the binding region within the PB1 gene. Viruses 8:E238. doi: 10.3390/v8080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marsh GA, Rabadan R, Levine AJ, Palese P. 2008. Highly conserved regions of influenza a virus polymerase gene segments are critical for efficient viral RNA packaging. J Virol 82:2295–2304. doi: 10.1128/JVI.02267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao Q, Brydon EW, Palese P. 2008. A seven-segmented influenza A virus expressing the influenza C virus glycoprotein HEF. J Virol 82:6419–6426. doi: 10.1128/JVI.00514-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakajima K, Sugiura A. 1977. Three-factor cross of influenza virus. Virology 81:486–489. doi: 10.1016/0042-6822(77)90165-9. [DOI] [PubMed] [Google Scholar]
- 69.Bergmann M, Muster T. 1995. The relative amount of an influenza A virus segment present in the viral particle is not affected by a reduction in replication of that segment. J Gen Virol 76:3211–3215. doi: 10.1099/0022-1317-76-12-3211. [DOI] [PubMed] [Google Scholar]
- 70.Smith GL, Hay AJ. 1982. Replication of the influenza virus genome. Virology 118:96–108. doi: 10.1016/0042-6822(82)90323-3. [DOI] [PubMed] [Google Scholar]
- 71.Inagaki A, Goto H, Kakugawa S, Ozawa M, Kawaoka Y. 2012. Competitive incorporation of homologous gene segments of influenza A virus into virions. J Virol 86:10200–10202. doi: 10.1128/JVI.01204-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enami M, Sharma G, Benham C, Palese P. 1991. An influenza virus containing nine different RNA segments. Virology 185:291–298. doi: 10.1016/0042-6822(91)90776-8. [DOI] [PubMed] [Google Scholar]
- 73.Ince WL, Gueye-Mbaye A, Bennink JR, Yewdell JW. 2013. Reassortment complements spontaneous mutation in influenza A virus NP and M1 genes to accelerate adaptation to a new host. J Virol 87:4330–4338. doi: 10.1128/JVI.02749-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen H, Ye J, Xu K, Angel M, Shao H, Ferrero A, Sutton T, Perez DR. 2012. Partial and full PCR-based reverse genetics strategy for influenza viruses. PLoS One 7:e46378. doi: 10.1371/journal.pone.0046378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tao H, Li L, White MC, Steel J, Lowen AC. 2015. Influenza A virus coinfection through transmission can support high levels of reassortment. J Virol 89:8453–8461. doi: 10.1128/JVI.01162-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, García-Sastre A. 1999. Rescue of influenza A virus from recombinant DNA. J Virol 73:9679–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steel J, Lowen AC, Mubareka S, Palese P. 2009. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog 5:e1000252. doi: 10.1371/journal.ppat.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fonville JM, Marshall N, Tao H, Steel J, Lowen AC. 2015. Influenza virus reassortment is enhanced by semi-infectious particles but can be suppressed by defective interfering particles. PLoS Pathog 11:e1005204. doi: 10.1371/journal.ppat.1005204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwartz SL, Lowen AC. 2016. Droplet digital PCR: anovel method for detection of influenza virus defective interfering particles. J Virol Methods 237:159–165. doi: 10.1016/j.jviromet.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]