ABSTRACT
Antibodies recognizing conserved CD4-induced (CD4i) epitopes on human immunodeficiency virus type 1 (HIV-1) Env and able to mediate antibody-dependent cellular cytotoxicity (ADCC) have been shown to be present in sera from most HIV-1-infected individuals. These antibodies preferentially recognize Env in its CD4-bound conformation. CD4 downregulation by Nef and Vpu dramatically reduces exposure of CD4i HIV-1 Env epitopes and therefore reduce the susceptibility of HIV-1-infected cells to ADCC mediated by HIV-positive (HIV+) sera. Importantly, this mechanism of immune evasion can be circumvented with small-molecule CD4 mimetics (CD4mc) that are able to transition Env into the CD4-bound conformation and sensitize HIV-1-infected cells to ADCC mediated by HIV+ sera. However, HIV-1 developed additional mechanisms to avoid ADCC, including Vpu-mediated BST-2 antagonism, which decreases the overall amount of Env present at the cell surface. Accordingly, BST-2 upregulation in response to alpha interferon (IFN-α) was shown to increase the susceptibility of HIV-1-infected cells to ADCC despite the activity of Vpu. Here we show that BST-2 upregulation by IFN-β and interleukin-27 (IL-27) also increases the surface expression of Env and thus boosts the ability of CD4mc to sensitize HIV-1-infected cells to ADCC by sera from HIV-1-infected individuals.
IMPORTANCE HIV-1 evolved sophisticated strategies to conceal Env epitopes from ADCC-mediating antibodies present in HIV+ sera. Vpu-mediated BST-2 downregulation was shown to decrease ADCC responses by limiting the amount of Env present at the cell surface. This effect of Vpu was shown to be attenuated by IFN-α treatment. Here we show that in addition to IFN-α, IFN-β and IL-27 also affect Vpu-mediated BST-2 downregulation and greatly enhance ADCC responses against HIV-1-infected cells in the presence of CD4mc. These findings may inform strategies aimed at HIV prevention and eradication.
KEYWORDS: HIV-1, BST-2, envelope glycoproteins, gp120, CD4, CD4-bound conformation, nonneutralizing antibodies, ADCC, CD4 mimetics, IFN-α, Env, IL-27, interferons
INTRODUCTION
Antibodies that preferentially recognize the CD4-bound conformation of human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins (Env) can eliminate HIV-1-infected cells through antibody-dependent cellular cytotoxicity (ADCC) responses (1–4). These antibodies are present in serum (1, 5), breast milk (5), and cervicovaginal lavage fluid (2, 5) samples from HIV-1-infected individuals and have been proposed to be part of the pressure exerted on HIV-1 to efficiently downregulate CD4 from the cell surface (6). Accordingly, Nef- and Vpu-mediated CD4 downregulation conceal the exposure of Env epitopes recognized by these antibodies (1, 3, 7). In addition, HIV-1 decreases ADCC responses by diminishing the overall amount of Env present at the cell surface. This is achieved through Vpu-mediated BST-2 (tetherin/CD317/HM1.24) downregulation (7–9), which allows for efficient release of viral particles (10, 11), and also through efficient Env internalization mediated by an endocytosis motif in the cytoplasmic tail of gp41 (12).
A better understanding of the importance that the CD4-bound conformation of HIV-1 envelope glycoproteins has on ADCC responses prompted us to “force” this Env conformation on the surface of infected cells using small-molecule CD4 mimetics (CD4mc). CD4mc induction of the CD4-bound conformation results in enhanced recognition of HIV-1-infected cells by serum, breast milk, and cervicovaginal fluid samples from HIV-1-infected subjects. Most importantly, CD4mc sensitizes HIV-1-infected cells to ADCC responses mediated by these biological fluids (4, 5, 13).
The effect of CD4mc on ADCC responses may be influenced by the amount of Env available at the cell surface. Only limited amounts of Env are presented at the cell surface due to efficient Env internalization (12) and Vpu-mediated BST-2 downregulation (7–9); this places an upper limit on the amount of Env that can be rendered susceptible to ADCC by CD4mc. Interestingly, two BST-2 isoforms possessing distinct biological properties have been described (14, 15). While the long isoform of BST-2 (L-BST-2) contains a cytoplasmic tyrosine motif mediating endocytic recycling, sensitivity to HIV-1 M Vpu and innate immune sensing, the short isoform of BST-2 (S-BST-2) lacks this motif due to the utilization of an alternative start codon (14, 15). How these two isoforms modulate Env recognition on the surface of HIV-1-infected cells by HIV-positive (HIV+) sera and how this affects the activity of CD4mc remain unknown.
Type I interferons (IFNs) are an important part of the early host immune response observed during acute HIV-1 infection (16). The antiviral effect exerted by IFN is highlighted by the observation that transmitted/founder HIV-1 strains that initiate host infection have been shown to be more resistant to type I IFN responses than HIV-1 strains found during the chronic phase of infection (17–19). Furthermore, Vpu enhances viral replication particularly during early stages of infection, probably by counteracting the IFN-inducible restriction factor BST-2 (18, 20). The induction of BST-2 expression by type I IFN treatment was also shown to sensitize infected cells to ADCC (8). In addition, interleukin-27 (IL-27) also enhances BST-2 levels on the surface of human monocytes and CD4 T cells (21). IL-27 is a member of the IL-12 family of cytokines and drives the differentiation of Th1 CD4 T cells (22, 23). Interestingly, IL-27 induces an antiviral gene expression profile similar to that induced by alpha interferon (IFN-α), including the apobec3g gene (24). Furthermore, IL-27 inhibited the replication of HIV-1 in cultures of primary CD4+ T cells and monocytes/macrophages through the induction of APOBEC (apolipoprotein B mRNA-editing, enzyme-catalytic, polypeptide-like) proteins (24, 25). Notably, IL-27-mediated BST-2 upregulation was shown to be independent from type I IFN responses (21). However, the effect of IL-27 on ADCC responses during viral infection has not been determined.
Here we evaluated the role of BST-2 on Env accumulation on the surface of HIV-1-infected cells and tested whether type I IFNs or IL-27 could be exploited in conjunction with CD4mc to further enhance ADCC responses mediated by HIV-positive (HIV+) sera.
RESULTS
BST-2 expression modulates Env accumulation on the surface of HIV-1-infected cells and its recognition by HIV+ sera in the presence of CD4mc.
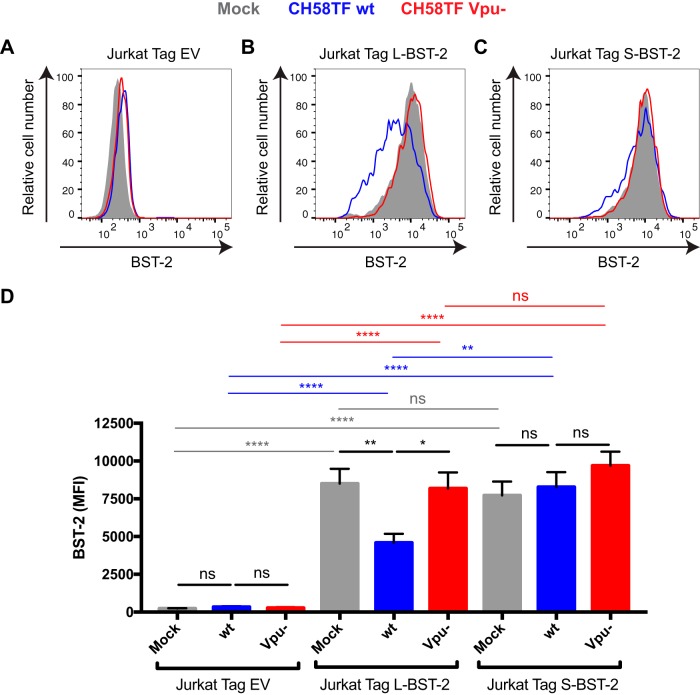
In the absence of Vpu, Env accumulates at the plasma membrane of HIV-1-infected cells (7–9) in large part due to the inhibitory effects of BST-2 on virus release (10, 11). This surface accumulation results in increased susceptibility of HIV-1-infected cells to ADCC (7–9). To further evaluate the role of BST-2 on Env surface expression, we infected Jurkat cell lines expressing no BST-2 (Jurkat Tag) or expressing the long isoform of BST-2 (Jurkat Tag L-BST-2) or the short isoform of BST-2 (Jurkat Tag S-BST-2) (15). Cells were infected with the transmitted/founder virus CH58 (CH58 TF) (5) expressing the Vpu accessory protein (wild-type [wt] CH58 TF) or containing a vpu deletion (Vpu−). Forty-eight hours postinfection, BST-2 and Env levels were evaluated by cell surface staining followed by intracellular p24 staining to identify infected (p24-positive [p24+]) cells. As expected, while BST-2 was not detected on the surface of Jurkat Tag cells (Fig. 1A and D), it was equivalently detected on the surface of uninfected (mock) Jurkat Tag L-BST-2 and S-BST-2 cells, indicating that these two cell lines express similar levels of BST-2 (Fig. 1B to D). However, in agreement with previous reports, HIV-1 infection significantly decreased expression of L-BST-2 but not that of S-BST-2. The S-BST-2 isoform lacks 12 residues of the cytoplasmic tail required for Vpu group M-mediated BST-2 endosomal degradation (14, 15) (Fig. 1C and D). As expected, a virus lacking Vpu (Vpu−) was unable to decrease cell surface levels of BST-2 (Fig. 1B to D).
FIG 1.
Differential sensitivity of BST-2 isoforms to HIV-1 Vpu in Jurkat cell lines. Jurkat Tag cells (A and D) expressing no BST-2 (Jurkat Tag EV [empty vector]) or stably expressing the L-BST2 (B and D) or S-BST-2 (C and D) were mock infected or infected with the transmitted/founder virus HIV-1 CH58 (CH58TF) expressing Vpu (wild-type CH58TF [CH58TF wt]) or not expressing Vpu (CH58TF Vpu−). Forty-eight hours postinfection, cells were stained with anti-BST-2 Ab, followed with appropriate secondary Abs. (A to C) Histograms depicting representative staining; (D) mean fluorescence intensity (MFI) obtained in at least six independent experiments. Values are means plus standard error of the means (SEM) (error bars). Statistical significance was tested using an unpaired t test (*, P < 0.05; **, P < 0.01, ****, P < 0.0001; ns, nonsignificant).
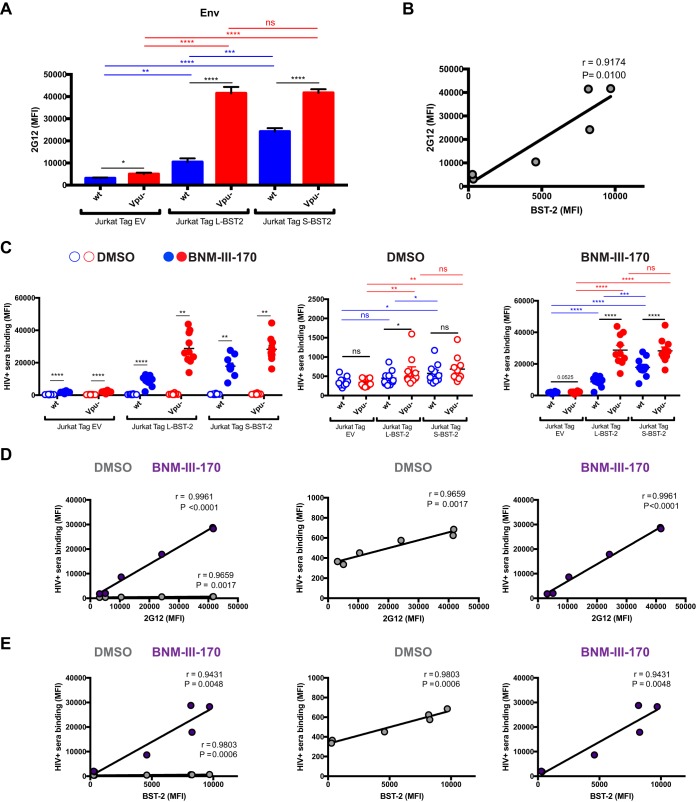
When we evaluated Env levels on the surface of infected cells with the conformation-independent 2G12 antibody (Fig. 2A), we observed a significant correlation with BST-2 levels (Fig. 2B). This supports previous observations indicating that BST-2 modulates the overall amount of Env on the surfaces of infected cells (7, 8). We then assessed whether enhanced accumulation of Env affected recognition of HIV-1-infected cells by HIV+ sera. Despite different amounts of BST-2 and Env present on the surface of Jurkat cell lines expressing S-BST-2, L-BST-2, or no BST-2, cells infected with a wild-type virus were barely recognized by HIV+ sera (Fig. 2C). This is believed to reflect the ability of HIV-1 to downregulate CD4 in infected cells such that only closed Env trimers remain (1, 3, 5, 7, 26). Antibodies present in HIV+ sera preferentially recognize Env in its CD4-bound conformation (1, 27). Infection with a vpu− virus led to a small increase in recognition of HIV-1-infected cells by HIV+ sera (Fig. 2C). In order to expose epitopes recognized by antibodies present in HIV+ sera, infected cells were incubated in parallel with the potent CD4mc BNM-III-170 which forces Env to adopt a CD4-bound-like conformation (4, 28) and in conjunction with coreceptor binding site antibodies (CoRBS) efficiently expose anti-cluster A epitopes (4). CD4mc addition enhanced recognition of all three infected cell lines (Fig. 2C). In agreement with decreased sensitivity of S-BST-2 to Vpu-mediated downregulation, Jurkat Tag S-BST-2 cells infected with a wild-type virus responded significantly better to CD4mc than Jurkat Tag L-BST-2 cells infected with the same virus. This likely results from an enhanced Env accumulation on the surfaces of Jurkat Tag S-BST-2 cells due to the inability of Vpu to downregulate S-BST-2 (Fig. 2A and C). Likely due to the absence of BST-2 in the Jurkat Tag empty vector (EV) cell line, infection with a vpu− virus has a minor effect on Env levels (as evaluated by 2G12) and therefore recognition of infected cells by HIV+ sera in the absence of CD4mc. Infection with a Vpu− virus of Jurkat Tag S-BST-2 and L cells led to a slightly better recognition of infected cells in the absence of CD4mc. Upon addition of CD4mc, however, all infected cells were significantly better recognized by HIV+ sera. This finding confirms previous observations indicating that antibodies present in HIV+ sera preferentially recognize Env in the CD4-bound conformation (1). Of note, the difference in HIV+ serum recognition in the presence of CD4mc between wild-type and vpu− virus-infected cells was higher in Jurkat L-BST-2 cells that express the BST-2 isoform susceptible to Vpu action (Fig. 2C). Accumulation of Env (as measured by 2G12) correlated significantly with recognition of infected cells by HIV+ sera (Fig. 2D). Recognition of infected cells by HIV+ sera also correlated with BST-2 expression (Fig. 2E).
FIG 2.
BST-2 expression correlates with cell surface Env level and recognition of HIV-1-infected cells by HIV+ sera. Jurkat Tag cells expressing no BST-2 (Jurkat Tag EV [empty vector]) or stably expressing L-BST-2 or S-BST-2 were mock infected or infected with the transmitted/founder virus HIV-1 CH58 (CH58 T/F) expressing Vpu (wild-type CH58 T/F [wt]) or not expressing Vpu (Vpu−). Forty-eight hours postinfection, cells were stained with the anti-Env Ab 2G12 (A and B) or with sera from 10 HIV-1-infected individuals (C) in the presence of the CD4mc BNM-III-170 (50 μM) or equivalent volume of DMSO, followed with appropriate secondary Abs. (A) Mean fluorescence intensity (MFI) of 2G12 binding obtained in at least six independent experiments; (B) correlation between 2G12 binding and BST-2 level. (C) MFIs obtained with all the different sera in the presence of CD4mc BNM-III-170 or DMSO; (D and E) correlation between HIV+ serum binding and 2G12 level or BST-2 level, respectively, in the presence of 50 μM CD4mc BNM-III-17 or DMSO. Values are means plus standard error of the means (SEM) (error bars). Statistical significance was tested using an unpaired t test (A), a Pearson correlation test (B, D, and E), or a paired t test or Wilcoxon matched-pair signed-rank test based on statistical normality (C) (*, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001; ns, nonsignificant).
BST-2 levels regulate Env accumulation and its recognition by HIV+ sera on the surface of HIV-1-infected primary CD4+ T cells.
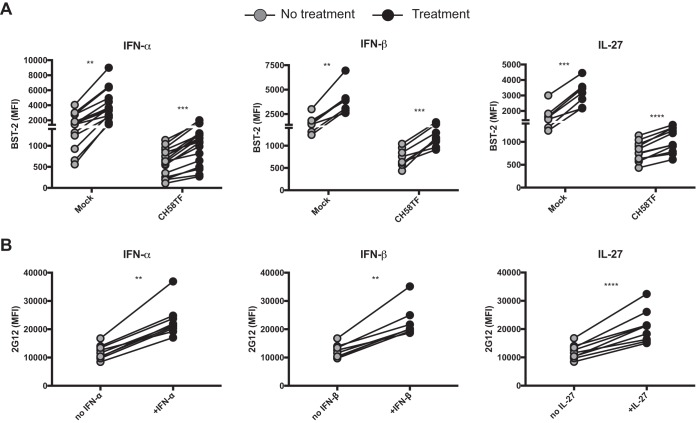
Figures 1 and 2 showed that BST-2 levels, and its sensitivity to Vpu downregulation, dictated Env accumulation on the surfaces of HIV-1-infected cell lines. Moreover, Env accumulation on the surfaces of infected cells increased the amount of Env available to engage CD4mc and henceforth sample the CD4-bound conformation, which is preferentially recognized by HIV+ sera (1, 27). IFN-α treatment has been shown to enhance BST-2 levels, resulting in an accumulation of Env on the surfaces of HIV-1-infected cells and thus increasing the sensitivity of HIV-1-infected cells to ADCC (8). Similar observations were recently reported (29). Therefore, we decided to take advantage of the type 1 interferon responsiveness of BST-2 (10, 11). Primary CD4+ T cells from healthy HIV-1 uninfected individuals were mock infected or infected with HIV-1 (CH58 TF), and BST-2 levels were modulated by stimulation with type 1 IFNs (IFN-α and IFN-β) or with IL-27 (21). As expected, BST-2 levels were significantly higher in uninfected than HIV-1-infected cells. Interestingly, IFN-α, IFN-β, and IL-27 treatment enhanced BST-2 detection in both uninfected and HIV-1-infected cells (Fig. 3A). BST-2 upregulation resulted in Env accumulation on the surfaces of infected cells, as measured with the 2G12 antibody (Fig. 3B).
FIG 3.
Treatment with type I IFN or IL-27 enhances Env levels on the surface of HIV-1-infected cells through BST-2 upregulation. Primary CD4+ T cells were mock infected or infected with the transmitted/founder virus CH58 (CH58TF) and either treated for 24 h with type I IFN (IFN-α and IFN-β) or IL-27 or not treated. Forty-eight hours postinfection, cells were stained with anti-BST-2 Ab (A) or anti-Env Ab 2G12 (B), followed with appropriate secondary Abs. The graphs shown represent the mean fluorescence intensities obtained for at least eight independent experiments. Statistical significance was tested using a paired t test or Wilcoxon matched-pair signed-rank test based on statistical normality (**, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
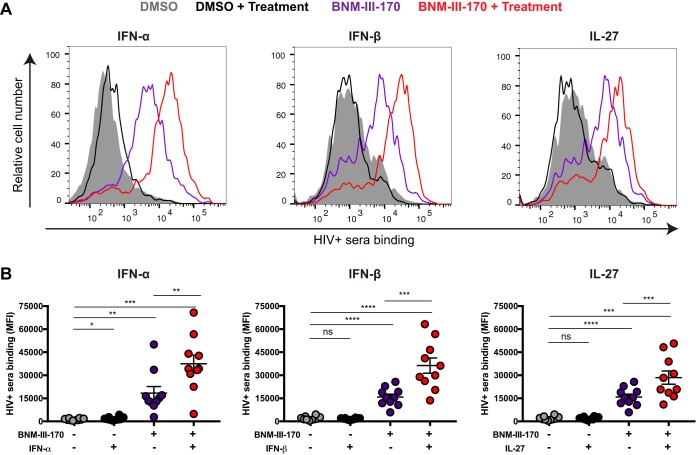
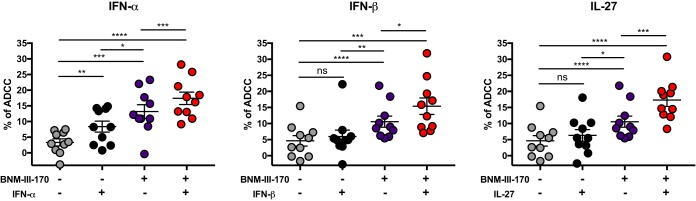
We then evaluated whether IFN-α, IFN-β, and IL-27 treatment enhanced recognition of HIV-1-infected cells by HIV+ sera. Despite a significant increase in Env accumulation on the surfaces of infected cells (Fig. 3B), treatment with IFN-β and IL-27 failed to enhance recognition of infected cells by HIV+ sera, and the effect of IFN-α treatment was relatively minor (Fig. 4). However, addition of the CD4mc BNM-III-170 significantly increased recognition of HIV-1-infected cells by HIV+ sera; these results are in agreement with previous reports demonstrating the ability of HIV+ sera to recognize CD4i epitopes on primary HIV-1 Env that are not spontaneously exposed (1, 3) and the capacity of CD4mc to promote the CD4-bound conformation of Env on the surfaces of HIV-1-infected cells (4, 5, 13, 30). Remarkably, the combination of IFN-α, IFN-β, or IL-27 with BNM-III-170 further increased recognition of HIV-1-infected cells by all sera tested compared to any one of these treatments (Fig. 4).
FIG 4.
Treatment with type I IFN or IL-27 enhances recognition of HIV-1-infected cells by sera from HIV-1-infected individuals in the presence of CD4mc. Primary CD4+ T cells were mock infected or infected with the transmitted/founder virus HIV-1 CH58 (CH58 T/F) and either treated for 24 h with type I IFN (IFN-α and IFN-β) or IL-27 or not treated. Forty-eight hours postinfection, cells were stained with sera from 10 HIV-1-infected individuals in the presence of the CD4mc BNM-III-170 (50 μM) or an equivalent volume of DMSO, followed with appropriate secondary Abs. (A) Histograms depicting representative staining; (B) mean fluorescence intensities (MFI) obtained with all the different sera. Values are means ± standard error of the means (SEM) (error bars). Statistical significance was tested using a paired t test or Wilcoxon matched-pair signed-rank test based on statistical normality (*, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001; ns, nonsignificant).
BST-2 upregulation boosts the capacity of CD4mc to sensitize HIV-1-infected cells to ADCC mediated by HIV+ sera.
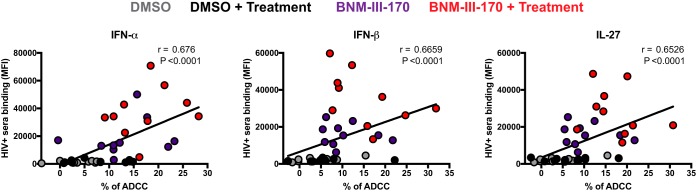
To evaluate whether the enhanced recognition of HIV-1-infected cells induced by the combination of IFN-α, IFN-β, and IL-27 treatments and BNM-III-170 would result in enhanced ADCC killing, we infected primary CD4+ T cells with HIV-1 CH58 TF and evaluated their susceptibility to ADCC mediated by autologous peripheral blood mononuclear cells (PBMCs) using a previously described fluorescence-activated cell sorting (FACS)-based assay (5, 31). As reported (5, 13), CD4mc BNM-III-170 significantly increased ADCC mediated by all HIV+ sera tested (Fig. 5). In agreement with the recognition of infected cells by HIV+ sera (Fig. 4), IFN-α treatment alone had a minor but significant effect on ADCC responses (Fig. 5), but IFN-β and IL-27 treatment failed to do so (Fig. 5). Remarkably, addition of BNM-III-170 further enhanced the susceptibility of infected cells to ADCC for cells treated with IFN-α, IFN-β, or IL-27 (Fig. 5). As expected, enhanced recognition of HIV-1-infected cells by HIV+ sera positively correlated with enhanced ADCC responses (Fig. 6). These results highlight the potential of combining type I IFNs and IL-27 with CD4mc to sensitize HIV-1-infected cells to ADCC.
FIG 5.
Treatment with type I IFN or IL-27 boosts CD4mc sensitization of HIV-1-infected cells to ADCC. Primary CD4+ T cells infected with the transmitted/founder virus CH58 (CH58 T/F), either treated for 24 h with type I IFN (IFN-α and IFN-β) or IL-27 or not treated, were used as target cells, and autologous PBMCs were used as effector cells in our FACS-based ADCC assay. Shown are the percentages of ADCC-mediated killing obtained with sera from 10 HIV-1-infected individuals in the presence of the CD4mc BNM-III-170 (50 μM) or an equivalent volume of DMSO. Values are means ± standard error of the means (SEM) (error bars). Statistical significance was tested using a paired t test or Wilcoxon matched-pair signed-rank test based on statistical normality (*, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001; ns, nonsignificant).
FIG 6.
Enhanced recognition of HIV-1-infected cells positively correlates with enhanced ADCC responses. A positive correlation was observed between the recognition of primary CD4 T cells infected with the transmitted/founder virus CH58 (CH58 T/F) by HIV+ sera and their ability to mediate an ADCC response. The correlations obtained for the different treatments (IFN-α, IFN-β, or IL-27) are shown. Statistical analysis was tested utilizing a Spearman rank correlation.
DISCUSSION
Increasing evidence suggests that Fcγ receptor-dependent functions of antibodies play a role in controlling human immunodeficiency virus type 1 (HIV-1) infection and replication (32–40). Analysis of the correlates of protection in the RV144 vaccine trial suggested that decreased HIV-1 acquisition was linked to increased ADCC activity in protected vaccinees (41). ADCC-mediating antibodies (Abs) targeting anti-cluster A epitopes were isolated from some RV144 vaccinees (42) and were shown to preferentially recognize the HIV-1 envelope glycoproteins sampling the CD4-bound conformation (7). CD4i antibodies represent a significant portion of the anti-Env Abs elicited during natural HIV-1 infection (1, 27, 43). This elicitation of CD4i Abs could result from transitional exposure of CD4i Env epitope during viral entry (44) or, most likely, after binding of shed gp120 with CD4 on uninfected bystander cells (30). However, not all CD4i antibodies are able to mediate ADCC against HIV-1-infected cells. While anti-cluster A antibodies have been shown to mediate potent ADCC responses against infected cells exposing Env in the CD4-bound conformation (3, 4, 7, 45), CD4i antibodies targeting the coreceptor binding site appear to be unable to do so (3, 4, 45, 46). While the reasons for these differences are not fully understood, the angle of approach of the antibody toward Env might differentially expose the Fc region which must be engaged by the Fcγ receptor in order to activate effector cells. Nevertheless, to limit the exposure of anti-cluster A epitopes that are exposed in the CD4-bound conformation of Env on the surfaces of infected cells, HIV-1 evolved sophisticated mechanisms to efficiently internalize Env (12) to counteract the host restriction factor BST-2 with the viral Vpu protein (7–9) and to downregulate CD4 by Nef and Vpu (1, 7). The requirement to evade ADCC provides one plausible explanation of why the vast majority of circulating HIV-1 strains worldwide express functional Nef and Vpu proteins, which limit the exposure of CD4i Env epitopes on the surfaces of infected cells.
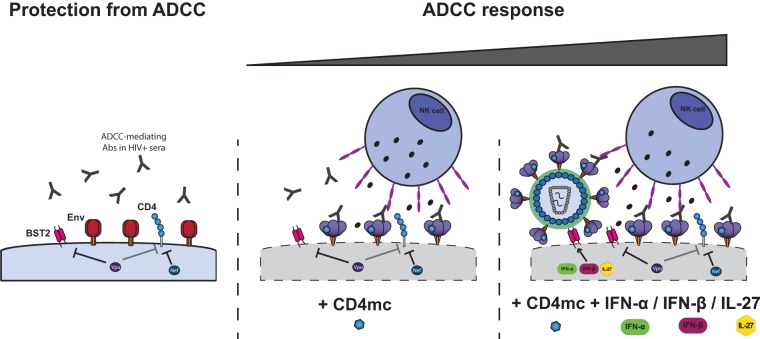
In agreement with the necessity for HIV-1 to avoid exposing the CD4-bound conformation of Env, we recently showed that forcing Env to adopt this conformation with CD4mc sensitizes HIV-1-infected cells to ADCC by sera from HIV-1-infected subjects (5). Here we show that increasing the amounts on Env at the cell surface, once this Env is induced by CD4mc to adopt the CD4-bound conformation, results in increased recognition of HIV-1-infected cells by HIV+ sera. We found that enhanced recognition of infected cells by HIV+ sera translates into enhanced susceptibility of infected cells to ADCC. This was achieved by exploiting the type 1 interferon responsiveness of the restriction factor BST-2, known to trap mature viral particles on the surfaces of infected cells. IFN-α and -β enhance BST-2 levels on the surfaces of infected cells, which translates into enhanced levels of Env potentially able to be targeted by ADCC after engaging the CD4mc. Interestingly, similar results were obtained using IL-27, a cytokine known to modulate BST-2 levels in an IFN-independent manner. Altogether, our results suggest a model (Fig. 7) where the conformation and availability of Env at the cell surface dictates the sensitivity of HIV-1-infected cells to ADCC. HIV-1 limits the amount of Env present at the cell surface and tightly controls its conformation. By preventing Env from assuming the CD4-bound conformation, HIV-1 avoids Env recognition by CD4i ADCC-mediating Abs present in the sera of the majority of HIV-1-infected individuals. Small CD4mc sensitize HIV-1-infected cells to ADCC by forcing Env to expose CD4i anti-cluster A-mediating epitopes. IFN-α, IFN-β, and IL-27 treatment, through upregulation of BST-2, increases the total amounts of Env available for CD4mc to induce ADCC-susceptible Env conformations on the surfaces of infected cells. While HIV-1-infected cells are protected from ADCC responses, we recently demonstrated that uninfected bystander CD4+ T cells bind gp120 shed from productively infected cells and are efficiently recognized by ADCC-mediating antibodies (30). Importantly, we also demonstrated that this phenomenon can be blocked by CD4mc that abrogates the binding of gp120 to uninfected cells and effectively redirects the immune system to infected cells. Therefore, the combination of CD4mc and type I IFN or IL-27 would represent an effective strategy to specifically target and eliminate HIV-1-infected cells by ADCC.
FIG 7.
Env conformation and its accumulation at the cell surface dictates sensitivity of HIV-1-infected cells to ADCC. ADCC-mediating Abs present in sera from HIV-1-infected individuals preferentially recognize Env in its CD4-bound conformation (1). To limit the exposure of this conformation, HIV-1 has evolved sophisticated mechanisms to counteract the host restriction factor BST-2 with the viral Vpu protein (7–9) and to downregulate CD4 by Nef and Vpu (7). Nef and Vpu decrease the accumulation of Env and its interaction with CD4 at the cell surface, two factors that determine the susceptibility of HIV-1-infected cells to ADCC. Small CD4 mimetics sensitize HIV-1-infected cells to ADCC mediated by HIV+ sera by forcing Env to sample its CD4-bound conformation (5). Type I IFN or IL-27 treatment, through upregulation of BST-2 despite Vpu activity, boosts the ability of CD4mc by increasing the amounts of CD4mc-sensitized Env available on the cell surface.
Robust type I interferon responses are among the earliest host immune defenses observed during acute HIV-1 infection (16). Accordingly, transmitted/founder viruses, including those used in the present study, were found to be more resistant to IFN treatment than viruses from chronic HIV-1 infection (17–19). In that context, Vpu counteraction of BST-2 was recently identified as a major determinant of this IFN resistance (18, 20) and was found to play a crucial role in enhancing virus replication and release in human CD4+ T cells, particularly in the presence of IFN (18). Here we found that IFN-α, IFN-β, or IL-27 treatment enhanced BST-2 levels and, in combination with CD4mc, similarly sensitized HIV-1-infected cells to ADCC. However, there are many other IFN-α subtypes, and some of them inhibit HIV-1 replication more efficiently in vitro and in animal models than IFN-α2 (47, 48). Thus, it will be important to evaluate to what extent the different IFN-α subtypes sensitize HIV-1-infected cells to ADCC in the presence of CD4mc.
CD4mc were recently shown to enhance the viral neutralization and ADCC activities of antibodies elicited in nonhuman primates (NHP) by several different Env immunogens (49), suggesting that combining a vaccine with a small-molecule CD4mc, administered orally or in a microbicide formulation, might be useful as a prophylactic strategy against HIV-1 transmission. Interestingly, mucosal application of IFN-β protected macaques from intrarectal and intravaginal simian-human immunodeficiency virus (SHIV) challenges (50). Similarly, IFN-α2 treatment of rhesus macaques prevented systemic infection by simian immunodeficiency virus (SIV) (51). Whereas a combination of IFNs or IL-27 with CD4mc might further limit HIV-1 transmission or help decrease the size of the viral reservoir in HIV-1-infected individuals remains to be evaluated, our results support performing future experiments aimed at evaluating whether sensitization of HIV-infected cells to ADCC could affect viral transmission and/or replication in animal models.
MATERIALS AND METHODS
Cell lines and isolation of primary cells.
HEK293T human embryonic kidney (obtained from ATCC) and primary cells were grown as previously described (7, 52). Peripheral blood mononuclear cells (PBMCs) were obtained by leukapheresis. All participants provided informed written consent prior to enrollment in accordance with Institutional Review Board approval. CD4 T lymphocytes were purified from resting PBMCs by negative selection and activated as previously described (5). Jurkat Tag cells stably expressing the long isoform of BST-2 (L-BST-2) or the short isoform of BST-2 (S-BST-2) and the Jurkat Tag empty vector (EV) cell line expressing no BST-2 were previously described (15).
Viral production, infections, and detection of infected cells.
In order to achieve the same level of infection between wild-type (wt) and Vpu− viruses, vesicular stomatitis virus G (VSVG)-pseudotyped HIV-1 replicating competent viruses were produced. Briefly, proviral vectors and a VSVG-encoding plasmid were cotransfected in 293T cells by standard calcium phosphate transfection. Two days after transfection, cell supernatants were harvested, clarified by low-speed centrifugation (5 min at 1,500 rpm), and concentrated by ultracentrifugation for 1 h at 4°C at 100,605 × g over a 20% sucrose cushion. Pellets were resuspended in fresh RPMI 1640 medium, and aliquots were stored at −80°C until use (1). Viruses were then used to infect Jurkat Tag cell lines or primary CD4 T cells from healthy donors by spin infection at 800 × g for 1 h in 96-well plates at 25°C.
CD4 mimetic, type I IFN, or IL-27 treatments.
The CD4mc BNM-III-170 was synthesized as described previously (28). BNM-III-170 was dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 10 mM, aliquoted, and stored at −20°C. BNM-III-170 was then diluted to 50 μM in phosphate-buffered saline (PBS) for cell surface staining or in complete RPMI 1640 medium for ADCC assays. IFN-α (PBL Assay Science) was reconstituted in complete RPMI 1640 medium at 1 × 107 U/ml, aliquoted, and stored at −80°C. IFN-α was then added to the cells at 1,000 U/ml. IFN-β (Rebif; EMD Serono Inc.) (19) was added to the cells at 1 ng/ml. IL-27 (R&D Systems) was reconstituted at 100 μg/ml in sterile PBS containing 0.1% bovine serum albumin and stored at −80°C. IL-27 was then added to the cells at 100 ng/ml. Type I IFN or IL-27 was added to the cells 24 h postinfection, 24 h before cell surface staining or ADCC assays.
Antibodies and sera.
The following antibodies (Abs) were used as the primary Abs for cell surface staining: 5 μg/ml of human anti-HIV-1 Env monoclonal antibody (MAb) 2G12 (National Institutes of Health [NIH] AIDS and Research and Reference Reagent Program), 2 μg/ml rabbit anti-BST-2 Ab (sc-99191; Santa Cruz), or sera from HIV-1-infected individuals (1:1,000 dilution), whereas 1 μg/ml of either Alexa Fluor 647-labeled goat anti-human MAbs (Invitrogen, San Diego, CA, USA) or Brilliant Violet 421-labeled donkey anti-rabbit MAbs (Biolegend, San Diego, CA, USA) were used as secondary Abs, and AquaVivid (Invitrogen, San Diego, CA, USA) was used as a viability dye. All sera were heat inactivated for 30 min at 56°C and stored at 4°C until ready to use in subsequent experiments. Written informed consent was obtained from all study participants (the Montreal Primary HIV Infection Cohort [53, 54] and the Canadian Cohort of HIV Infected Slow Progressors [55–57]), and research adhered to the ethical guidelines of Centre de Recherche du CHUM (CRCHUM) and was reviewed and approved by the CRCHUM institutional review board (ethics committee). A random-number generator (QuickCalcs; GraphPad) was used to randomly select a number of sera for experiments.
Plasmids.
The plasmid encoding the HIV-1 transmitted founder (T/F) CH58 was previously described (5, 17, 58–60).
Flow cytometry analysis of cell surface staining and ADCC responses.
Cell surface staining was performed as previously described (1, 5). Binding of HIV-1-infected cells by HIV+ sera, anti-Env MAbs (2G12) or anti-BST-2 MAbs was performed 48 h after infection, 24 h after treatment with type I IFN or IL-27, in the presence or absence of BNM-III-170 (50 μM) or an equivalent volume of vehicle (DMSO). Detection of p24+ infected cells was performed as described previously (5). The percentage of infected cells (p24+ cells) was determined by gating the living cell population based on the viability dye staining (Aqua Vivid; Invitrogen). Samples were analyzed on a LSRII cytometer (BD Biosciences, Mississauga, ON, Canada), and data analysis was performed using FlowJo vX.0.7 (Tree Star, Ashland, OR, USA).
Measurement of ADCC-mediated killing was performed with a previously described assay (5). Briefly, primary CD4+ T cells infected for 48 h and treated for 24 h with type I IFN or IL-27 or not treated with type I IFN or IL-27 were incubated with autologous PBMCs (effector/target cell ratio of 10:1) in the presence or absence of HIV+ sera (1:1,000), in the presence of CD4mc BNM-III-170 (50 μM), or with an equivalent volume of vehicle (DMSO). The percentage of cytotoxicity was calculated as described previously (5).
Statistical analyses.
Statistics were analyzed using GraphPad Prism version 6.01 (GraphPad, San Diego, CA, USA). Every data set was tested for statistical normality, and this information was used to apply the appropriate (parametric or nonparametric) statistical test. P values of <0.05 were considered significant; significance values are indicated as follows: *, P < 0.05; **, P < 0.01, ***, P < 0.001; ****, P < 0.0001.
ACKNOWLEDGMENTS
We thank Elizabeth Carpelan for help with manuscript preparation. We thank Dominique Gauchat from the CRCHUM Flow Cytometry Platform for technical assistance and Mario Legault for cohort coordination and clinical samples.
This work was supported by CIHR foundation grant 352417 and by amfAR Innovation Grant 109343-59-RGRL with support from FAIR to A.F. and by a FRQS AIDS and Infectious Diseases Network grant to J.R. and A.F. A.F. is the recipient of a Canada Research Chair on Retroviral Entry. J.R. is the recipient of a CIHR Fellowship Award 135349. D.E.K. is supported by a Research Scholar Career Award of the Quebec Health Research Fund (FRQS). This study was also supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health and by the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID), grants UM1-AI100645 and AI100663, by the National Institutes of Health grant GM56550, the late William F. McCarty-Cooper, NIH R01AI116274, HL-092565, R01 AI114266, and by NIH grants AI121135, AI098485, and AI095098 to D.T.E. F.K. is supported by the DFG and an ERC Advanced grant, and D.S. is supported by the junior professorship program of the state Baden-Wuerttemberg, Germany.
Our funding sources had no role in data collection, analysis or interpretation and were not involved in the writing of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We have no conflicts of interest to report.
REFERENCES
- 1.Veillette M, Coutu M, Richard J, Batraville LA, Dagher O, Bernard N, Tremblay C, Kaufmann DE, Roger M, Finzi A. 2015. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 89:545–551. doi: 10.1128/JVI.02868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batraville LA, Richard J, Veillette M, Labbe AC, Alary M, Guedou F, Kaufmann DE, Poudrier J, Finzi A, Roger M. 2014. Anti-HIV-1 envelope immunoglobulin Gs in blood and cervicovaginal samples of Beninese commercial sex workers. AIDS Res Hum Retroviruses 30:1145–1149. doi: 10.1089/aid.2014.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding S, Veillette M, Coutu M, Prevost J, Scharf L, Bjorkman PJ, Ferrari G, Robinson JE, Sturzel C, Hahn BH, Sauter D, Kirchhoff F, Lewis GK, Pazgier M, Finzi A. 2015. A highly conserved residue of the HIV-1 gp120 inner domain is important for antibody-dependent cellular cytotoxicity responses mediated by anti-cluster A antibodies. J Virol 90:2127–2134. doi: 10.1128/JVI.02779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richard J, Pacheco B, Gohain N, Veillette M, Ding S, Alsahafi N, Tolbert WD, Prevost J, Chapleau JP, Coutu M, Jia M, Brassard N, Park J, Courter JR, Melillo B, Martin L, Tremblay C, Hahn BH, Kaufmann DE, Wu X, Smith AB III, Sodroski J, Pazgier M, Finzi A. 2016. Co-receptor binding site antibodies enable CD4-mimetics to expose conserved anti-cluster A ADCC epitopes on HIV-1 envelope glycoproteins. EBioMedicine 12:208–218. doi: 10.1016/j.ebiom.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, Pazgier M, Schon A, Freire E, Routy JP, Smith AB III, Park J, Jones DM, Courter JR, Melillo BN, Kaufmann DE, Hahn BH, Permar SR, Haynes BF, Madani N, Sodroski JG, Finzi A. 2015. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 112:E2687–E2694. doi: 10.1073/pnas.1506755112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veillette M, Richard J, Pazgier M, Lewis GK, Parsons MS, Finzi A. 2016. Role of HIV-1 envelope glycoproteins conformation and accessory proteins on ADCC responses. Curr HIV Res 14:9–23. doi: 10.2174/1570162X13666150827093449. [DOI] [PubMed] [Google Scholar]
- 7.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, Rakasz EG, Evans DT. 2014. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 111:6425–6430. doi: 10.1073/pnas.1321507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, Fierer DS, Simon V, Chen BK. 2014. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol 88:6031–6046. doi: 10.1128/JVI.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 11.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Bredow B, Arias JF, Heyer LN, Gardner MR, Farzan M, Rakasz EG, Evans DT. 2015. Envelope glycoprotein internalization protects human and simian immunodeficiency virus-infected cells from antibody-dependent cell-mediated cytotoxicity. J Virol 89:10648–10655. doi: 10.1128/JVI.01911-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WS, Richard J, Lichtfuss M, Smith AB III, Park J, Courter JR, Melillo BN, Sodroski JG, Kaufmann DE, Finzi A, Parsons MS, Kent SJ. 2015. Antibody-dependent cellular cytotoxicity against reactivated HIV-1-infected cells. J Virol 90:2021–2030. doi: 10.1128/JVI.02717-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocka LJ, Bates P. 2012. Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog 8:e1002931. doi: 10.1371/journal.ppat.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinelt J, Neil SJ. 2014. Differential sensitivities of tetherin isoforms to counteraction by primate lentiviruses. J Virol 88:5845–5858. doi: 10.1128/JVI.03818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP Jr, Bjorkman PJ, Wilen CB, Doms RW, O'Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. 2013. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A 110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kmiec D, Iyer SS, Sturzel CM, Sauter D, Hahn BH, Kirchhoff F. 2016. Vpu-mediated counteraction of tetherin is a major determinant of HIV-1 interferon resistance. mBio 7:e00934–. doi: 10.1128/mBio.00934-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer SS, Bibollet-Ruche F, Sherrill-Mix S, Learn GH, Plenderleith L, Smith AG, Barbian HJ, Russell RM, Gondim MV, Bahari CY, Shaw CM, Li Y, Decker T, Haynes BF, Shaw GM, Sharp PM, Borrow P, Hahn BH. 2017. Resistance to type 1 interferons is a major determinant of HIV-1 transmission fitness. Proc Natl Acad Sci U S A 114:E590–E599. doi: 10.1073/pnas.1620144114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K, Misawa N, Fukuhara M, Iwami S, An DS, Ito M, Koyanagi Y. 2012. Vpu augments the initial burst phase of HIV-1 propagation and downregulates BST2 and CD4 in humanized mice. J Virol 86:5000–5013. doi: 10.1128/JVI.07062-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzzo C, Jung M, Graveline A, Banfield BW, Gee K. 2012. IL-27 increases BST-2 expression in human monocytes and T cells independently of type I IFN. Sci Rep 2:974. doi: 10.1038/srep00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. 2004. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol 172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 23.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. 2005. A role for IL-27 in early regulation of Th1 differentiation. J Immunol 175:2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 24.Imamichi T, Yang J, Huang DW, Brann TW, Fullmer BA, Adelsberger JW, Lempicki RA, Baseler MW, Lane HC. 2008. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS 22:39–45. doi: 10.1097/QAD.0b013e3282f3356c. [DOI] [PubMed] [Google Scholar]
- 25.Greenwell-Wild T, Vazquez N, Jin W, Rangel Z, Munson PJ, Wahl SM. 2009. Interleukin-27 inhibition of HIV-1 involves an intermediate induction of type I interferon. Blood 114:1864–1874. doi: 10.1182/blood-2009-03-211540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsahafi N, Ding S, Richard J, Markle T, Brassard N, Walker B, Lewis GK, Kaufmann DE, Brockman MA, Finzi A. 2015. Nef proteins from HIV-1 elite controllers are inefficient at preventing antibody-dependent cellular cytotoxicity. J Virol 90:2993–3002. doi: 10.1128/JVI.02973-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melillo B, Liang S, Park J, Schon A, Courter JR, LaLonde JM, Wendler DJ, Princiotto AM, Seaman MS, Freire E, Sodroski J, Madani N, Hendrickson WA, Smith AB III. 2016. Small-molecule CD4-mimics: structure-based optimization of HIV-1 entry inhibition. ACS Med Chem Lett 7:330–334. doi: 10.1021/acsmedchemlett.5b00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham TN, Lukhele S, Dallaire F, Perron G, Cohen EA. 2016. Enhancing virion tethering by BST2 sensitizes productively and latently HIV-infected T cells to ADCC mediated by broadly neutralizing antibodies. Sci Rep 6:37225. doi: 10.1038/srep37225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard J, Veillette M, Ding S, Zoubchenok D, Alsahafi N, Coutu M, Brassard N, Park J, Courter JR, Melillo B, Smith AB III, Shaw GM, Hahn BH, Sodroski J, Kaufmann DE, Finzi A. 2016. Small CD4 mimetics prevent HIV-1 uninfected bystander CD4+ T cell killing mediated by antibody-dependent cell-mediated cytotoxicity. EBioMedicine 3:122–134. doi: 10.1016/j.ebiom.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard J, Veillette M, Batraville LA, Coutu M, Chapleau JP, Bonsignori M, Bernard N, Tremblay C, Roger M, Kaufmann DE, Finzi A. 2014. Flow cytometry-based assay to study HIV-1 gp120 specific antibody-dependent cellular cytotoxicity responses. J Virol Methods 208:107–114. doi: 10.1016/j.jviromet.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M Jr, Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, Johnson RP, Evans DT. 2012. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog 8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banks ND, Kinsey N, Clements J, Hildreth JE. 2002. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses 18:1197–1205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 34.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol 157:2168–2173. [PubMed] [Google Scholar]
- 35.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. 2011. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A 108:7505–7510. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, Kaplan J. 1999. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis 180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 37.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog 8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Asmal M, Lane S, Permar SR, Schmidt SD, Mascola JR, Letvin NL. 2011. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J Virol 85:6906–6912. doi: 10.1128/JVI.00326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams KL, Cortez V, Dingens AS, Gach JS, Rainwater S, Weis JF, Chen X, Spearman P, Forthal DN, Overbaugh J. 2015. HIV-specific CD4-induced antibodies mediate broad and potent antibody-dependent cellular cytotoxicity activity and are commonly detected in plasma from HIV-infected humans. EBioMedicine 2:1464–1477. doi: 10.1016/j.ebiom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis GK, Pazgier M, Evans D, Ferrari G, Bournazos S, Parsons MS, Bernard NF, Finzi A. 13 January 2017. Beyond viral neutralization. AIDS Res Hum Retroviruses doi: 10.1089/AID.2016.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, Devico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 85:7029–7036. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mengistu M, Ray K, Lewis GK, DeVico AL. 2015. Antigenic properties of the human immunodeficiency virus envelope glycoprotein gp120 on virions bound to target cells. PLoS Pathog 11:e1004772. doi: 10.1371/journal.ppat.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, Lovo E, Wu X, Robinson JE, Seaman MS, Fouts TR, Gallo RC, DeVico AL, Lewis GK. 2013. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci U S A 110:E69–E78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruel T, Guivel-Benhassine F, Lorin V, Lortat-Jacob H, Baleux F, Bourdic K, Noel N, Lambotte O, Mouquet H, Schwartz O. 25 January 2017. Lack of ADCC breadth of human non-neutralizing anti-HIV-1 antibodies. J Virol doi: 10.1128/JVI.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavender KJ, Gibbert K, Peterson KE, Van Dis E, Francois S, Woods T, Messer RJ, Gawanbacht A, Muller JA, Munch J, Phillips K, Race B, Harper MS, Guo K, Lee EJ, Trilling M, Hengel H, Piehler J, Verheyen J, Wilson CC, Santiago ML, Hasenkrug KJ, Dittmer U. 2016. Interferon alpha subtype-specific suppression of HIV-1 infection in vivo. J Virol 90:6001–6013. doi: 10.1128/JVI.00451-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harper MS, Guo K, Gibbert K, Lee EJ, Dillon SM, Barrett BS, McCarter MD, Hasenkrug KJ, Dittmer U, Wilson CC, Santiago ML. 2015. Interferon-alpha subtypes in an ex vivo model of acute HIV-1 infection: expression, potency and effector mechanisms. PLoS Pathog 11:e1005254. doi: 10.1371/journal.ppat.1005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding S, Verly MM, Princiotto A, Melillo B, Moody AM, Bradley T, Easterhoff D, Roger M, Hahn BH, Madani N, Smith AB III, Haynes BF, Sodroski JMD, Finzi A. 19 December 2016. Small-molecule CD4 mimetics sensitize HIV-1-infected cells to antibody-dependent cellular cytotoxicity by antibodies elicited by multiple envelope glycoprotein immunogens in nonhuman primates. AIDS Res Hum Retroviruses doi: 10.1089/AID.2016.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veazey RS, Pilch-Cooper HA, Hope TJ, Alter G, Carias AM, Sips M, Wang X, Rodriguez B, Sieg SF, Reich A, Wilkinson P, Cameron MJ, Lederman MM. 2016. Prevention of SHIV transmission by topical IFN-beta treatment. Mucosal Immunol 9:1528–1536. doi: 10.1038/mi.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB Jr, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC. 2014. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. 2010. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood 115:1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fontaine J, Chagnon-Choquet J, Valcke HS, Poudrier J, Roger M, Montreal Primary HIV Infection and Long-Term Non-Progressor Study Groups. 2011. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood 117:145–155. doi: 10.1182/blood-2010-08-301887. [DOI] [PubMed] [Google Scholar]
- 54.Fontaine J, Coutlee F, Tremblay C, Routy JP, Poudrier J, Roger M, Montreal Primary HIV Infection and Long-Term Nonprogressor Study Groups. 2009. HIV infection affects blood myeloid dendritic cells after successful therapy and despite nonprogressing clinical disease. J Infect Dis 199:1007–1018. doi: 10.1086/597278. [DOI] [PubMed] [Google Scholar]
- 55.Peretz Y, Ndongala ML, Boulet S, Boulassel MR, Rouleau D, Cote P, Longpre D, Routy JP, Falutz J, Tremblay C, Tsoukas CM, Sekaly RP, Bernard NF. 2007. Functional T cell subsets contribute differentially to HIV peptide-specific responses within infected individuals: correlation of these functional T cell subsets with markers of disease progression. Clin Immunol 124:57–68. doi: 10.1016/j.clim.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Kamya P, Boulet S, Tsoukas CM, Routy JP, Thomas R, Cote P, Boulassel MR, Baril JG, Kovacs C, Migueles SA, Connors M, Suscovich TJ, Brander C, Tremblay CL, Bernard N, Canadian Cohort of HIV Infected Slow Progressors. 2011. Receptor-ligand requirements for increased NK cell polyfunctional potential in slow progressors infected with HIV-1 coexpressing KIR3DL1*h/*y and HLA-B*57. J Virol 85:5949–5960. doi: 10.1128/JVI.02652-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.International HIV Controllers Study, Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, Kadie CM, Carlson JM, Heckerman D, Graham RR, Plenge RM, Deeks SG, Gianniny L, Crawford G, Sullivan J, Gonzalez E, Davies L, Camargo A, Moore JM, Beattie N, Gupta S, Crenshaw A, Burtt NP, Guiducci C, Gupta N, Gao X, Qi Y, Yuki Y, Piechocka-Trocha A, Cutrell E, Rosenberg R, Moss KL, Lemay P, O'Leary J, Schaefer T, Verma P, Toth I, Block B, Baker B, Rothchild A, Lian J, Proudfoot J, Alvino DM, Vine S, Addo MM, Allen TM, et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. 2012. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bar KJ, Tsao CY, Iyer SS, Decker JM, Yang Y, Bonsignori M, Chen X, Hwang KK, Montefiori DC, Liao HX, Hraber P, Fischer W, Li H, Wang S, Sterrett S, Keele BF, Ganusov VV, Perelson AS, Korber BT, Georgiev I, McLellan JS, Pavlicek JW, Gao F, Haynes BF, Hahn BH, Kwong PD, Shaw GM. 2012. Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog 8:e1002721. doi: 10.1371/journal.ppat.1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fenton-May AE, Dibben O, Emmerich T, Ding H, Pfafferott K, Aasa-Chapman MM, Pellegrino P, Williams I, Cohen MS, Gao F, Shaw GM, Hahn BH, Ochsenbauer C, Kappes JC, Borrow P. 2013. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology 10:146. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]