ABSTRACT
Human respiratory syncytial virus (RSV) is a common cause of severe respiratory disease among infants, immunocompromised individuals, and the elderly. No licensed vaccine is currently available. In this study, we evaluated two parainfluenza virus 5 (PIV5)-vectored vaccines expressing RSV F (PIV5/F) or G (PIV5/G) protein in the cotton rat and African green monkey models for their replication, immunogenicity, and efficacy of protection against RSV challenge. Following a single intranasal inoculation, both animal species shed the vaccine viruses for a limited time but without noticeable clinical symptoms. In cotton rats, the vaccines elicited RSV F- or G-specific serum antibodies and conferred complete lung protection against RSV challenge at doses as low as 103 PFU. Neither vaccine produced the enhanced lung pathology observed in animals immunized with formalin-inactivated RSV. In African green monkeys, vaccine-induced serum and mucosal antibody responses were readily detected, as well. PIV5/F provided nearly complete protection against RSV infection in the upper and lower respiratory tract at a dose of 106 PFU of vaccine. At the same dose levels, PIV5/G was less efficacious. Both PIV5/F and PIV5/G were also able to boost neutralization titers in RSV-preexposed African green monkeys. Overall, our data indicated that PIV5/F is a promising RSV vaccine candidate.
IMPORTANCE A safe and efficacious respiratory syncytial virus (RSV) vaccine remains elusive. We tested the recombinant parainfluenza virus 5 (PIV5) vectors expressing RSV glycoproteins for their immunogenicity and protective efficacy in cotton rats and African green monkeys, which are among the best available animal models to study RSV infection. In both species, a single dose of intranasal immunization with PIV5-vectored vaccines was able to produce systemic and local immunity and to protect animals from RSV challenge. The vaccines could also boost RSV neutralization antibody titers in African green monkeys that had been infected previously. Our data suggest that PIV5-vectored vaccines could potentially protect both the pediatric and elderly populations and support continued development of the vector platform.
KEYWORDS: PIV5 vectors, respiratory syncytial virus, vaccines
INTRODUCTION
Respiratory syncytial virus (RSV) is a member of the paramyxovirus family that causes acute respiratory tract infection in humans of all ages (1, 2); however, severe RSV diseases, such as bronchiolitis and pneumonia, are more commonly found in infants, young children, immunocompromised individuals, and the elderly. RSV is a leading cause of lower respiratory tract infection worldwide in children under the age of 5 years and is responsible for more than 3 million hospitalizations and up to 200,000 deaths annually (3–5). Among healthy adults over the age of 65, an average of 5.5% develop RSV infection each winter season, with rates of pneumonia and death of 10 to 20% and 2 to 5%, respectively (6–8). At present, there is no licensed vaccine available, and the use of passive immunoprophylaxis is limited to high-risk infants (9).
Developing an RSV vaccine for young children and adult populations faces different sets of obstacles (10). In the early period of life, the neonate and newborn immune systems have yet to be fully developed. Passively acquired maternal antibodies, which provide a certain degree of protection against RSV infection, may interfere with the active immune responses to vaccines. Adverse clinical experiences with early vaccine candidates revealed the need for caution when testing RSV vaccines in seronegative infants and toddlers. A formalin-inactivated RSV (FI-RSV) candidate tested in the 1960s not only failed to protect against infection, but also increased the disease severity when the vaccinated infants were subsequently exposed to RSV (11). In contrast, most adults have experienced prior RSV infection, but natural RSV infections induce only short-lived and incomplete protection (1). The decreased immune functions in the elderly population also lead to a weaker response to vaccination. Therefore, different vaccine strategies may be needed for each target population (10). Among vaccine modalities developed to date are live attenuated vaccines (12), replication-competent or replication-defective viral vector vaccines (13), nucleic acid vaccines (14, 15), virosomes (16–19), nanoparticle vaccines (20), virus-like particles, and subunit vaccines (21–23). Each offers unique advantages and challenges.
Parainfluenza virus 5 (PIV5) has been recently developed as a platform for vector-based vaccines against influenza virus (24), RSV (25), rabies virus (26), and Mycobacterium tuberculosis (27). PIV5 belongs to the family Paramyxoviridae. The virus has been isolated from tissues or cell lines of a number of species, including humans (28, 29), monkeys (30, 31), dogs (32), and pigs (33), but is not known to be associated with any disease except canine kennel cough (34–36). Even though humans are not considered to be a natural host for PIV5, neutralizing antibodies against PIV5 can be detected in about 30% of human serum samples (37), which is likely a result of the wide use of kennel cough vaccine containing live PIV5. The vector is stable when foreign antigen-encoding sequences are inserted between the HN and L genes of the viral genome (25). PIV5-vectored vaccines can be administered intranasally or intramuscularly. Interestingly, preexisting immunity to the vector does not negatively impact the immunogenicity of vaccine antigen in dogs, suggesting the vector could be used to prime and boost immune responses to vaccine antigens (37).
Two PIV5-vector-based RSV vaccines were previously shown to be immunogenic and protective against RSV challenge in BALB/c mice (25). The vaccines were constructed by inserting RSV fusion protein (F) or attachment protein (G) expression cassettes in the intergenic region between PIV5 HN and L. Both vaccines are genetically stable and replicate to high titers in tissue culture. In this study, we investigated the immunogenicity and efficacy of protection of the two vaccines in cotton rat (Sigmodon hispidus) and African green monkey (Chlorocebus sabaeus) models. Our data confirmed that PIV5-vectored RSV F is immunogenic and efficacious in preclinical models and support continued development of the PIV5/F vaccine.
RESULTS
PIV5 replication in cotton rats.
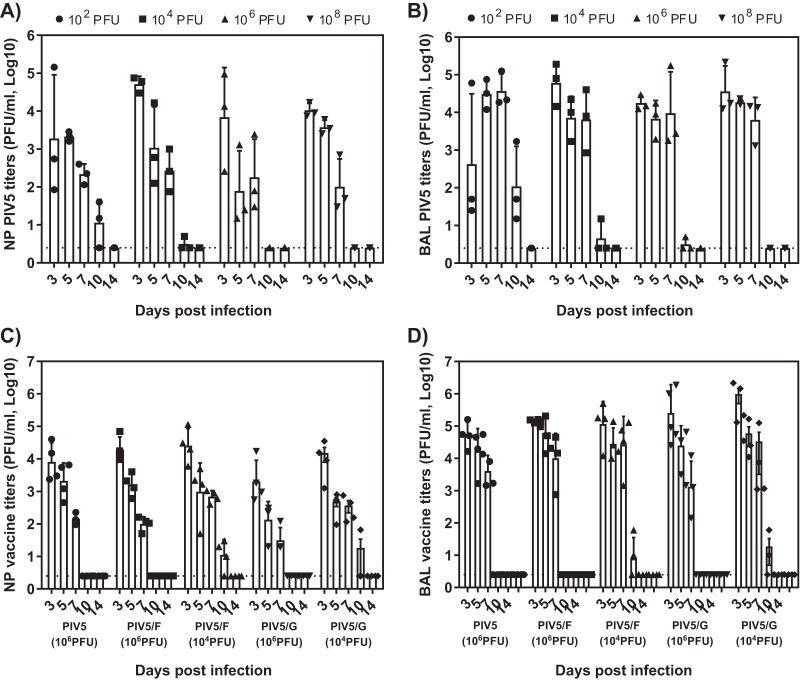
To our knowledge, the permissiveness of cotton rats to PIV5 infection has not been investigated previously. We first sought to assess the ability of PIV5 to replicate in the upper and lower respiratory tract in cotton rats (n = 4 per group), as well as the impact of the inoculation volume on the local tissue viral load. The animals were inoculated intranasally with 1 × 105 PFU of PIV5 at volumes of 10 μl or 100 μl. At 4 days postinfection (dpi), significant virus replication was detected in nose homogenates, and the viral titers reached 1 × 103 to 1 × 104 PFU/g (Fig. 1). Most of the nose virus was cleared by 6 dpi.
FIG 1.
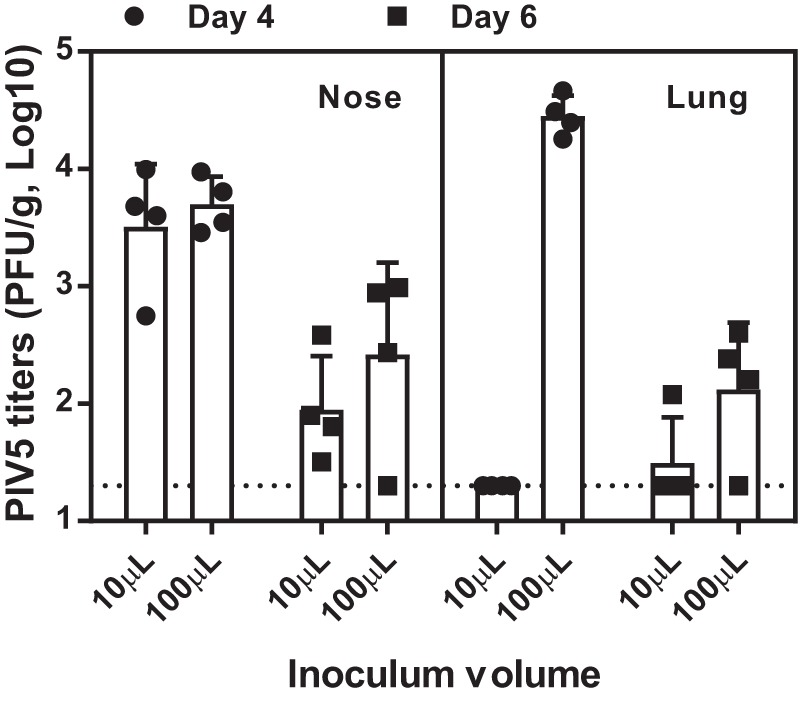
PIV5 replication in cotton rats. Cotton rats were infected intranasally with 1 × 105 PFU of PIV5 in 10-μl or 100-μl volumes. At 4 and 6 days postchallenge, noses and lungs were harvested, and viral loads were determined by plaque assay. Each group consisted of 4 cotton rats. The bars represent the GMT of each group. The dotted line represents the limit of detection. The error bars indicate standard deviations.
Virus replication in the lungs was dependent on the inoculum volume. When the animals were infected with 10 μl of the virus, PIV5 replication was largely confined to the nose. No virus was found in the lungs at 4 dpi, and only one animal showed a low level of virus at 6 dpi. In contrast, a geometric mean titer (GMT) of 3.1 × 105 PFU/g of PIV5 was found at 6 dpi in lungs of the animals in the 100-μl dose group. It is likely that a fraction of the inoculum descended to the lung. To prevent the vaccine virus from being delivered to the lungs in the subsequent cotton rat studies, an administration volume of 10 μl was used.
Immunogenicity of PIV5/F and PIV5/G in cotton rats.
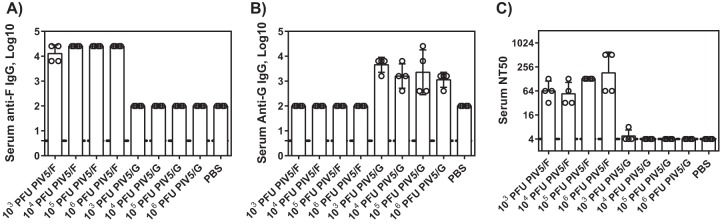
Single doses of PIV5/F or PIV5/G vaccine at 1 × 103, 1 × 104, 1 × 105, and 1 × 106 PFU were chosen to immunize cotton rats. Sera were collected 4 weeks postvaccination. F- and G-specific IgGs were detected by binding to recombinant F or G protein. As shown in Fig. 2A and B, immunization at all dose levels of PIV5/F or PIV5/G from 1 × 103 to 1 × 106 PFU was able to elicit specific antibodies against F or G. The titers were comparable among different dose groups. The sera obtained from PIV5/F-immunized animals neutralized the RSV A2 infection with a geometric mean 50% neutralization titer (NT50) between 64 and 256 (Fig. 2C). No neutralizing titer was detected in the animals immunized with PIV5/G.
FIG 2.
Serum antibody titers of cotton rats vaccinated with PIV5/F or PIV5/G. Cotton rats were immunized intranasally with 10 μl of vaccines containing 1 × 103, 1 × 104, 1 × 105, or 1 × 106 PFU of PIV5/F, PIV5/G, or PBS. Sera were collected 28 days postimmunization, and IgG endpoint titers were determined by ELISA. Functional antibody activity was measured by a microneutralization assay. Each group consisted of 4 cotton rats. The bars represent the GMT of each group. The dotted line represents the limit of detection. The error bars indicate standard deviations. Each circle represents an individual animal.
The mucosal antibody responses were measured by IgA levels in the lung homogenates (Fig. 3). Animals vaccinated with PIV5/F or PIV/G mounted significant levels of RSV F- or G-specific IgA. Consistent with the serum IgG responses (Fig. 2), vaccine dose titration from 1 × 106 to 1 × 103 PFU elicited similar titers of IgA.
FIG 3.
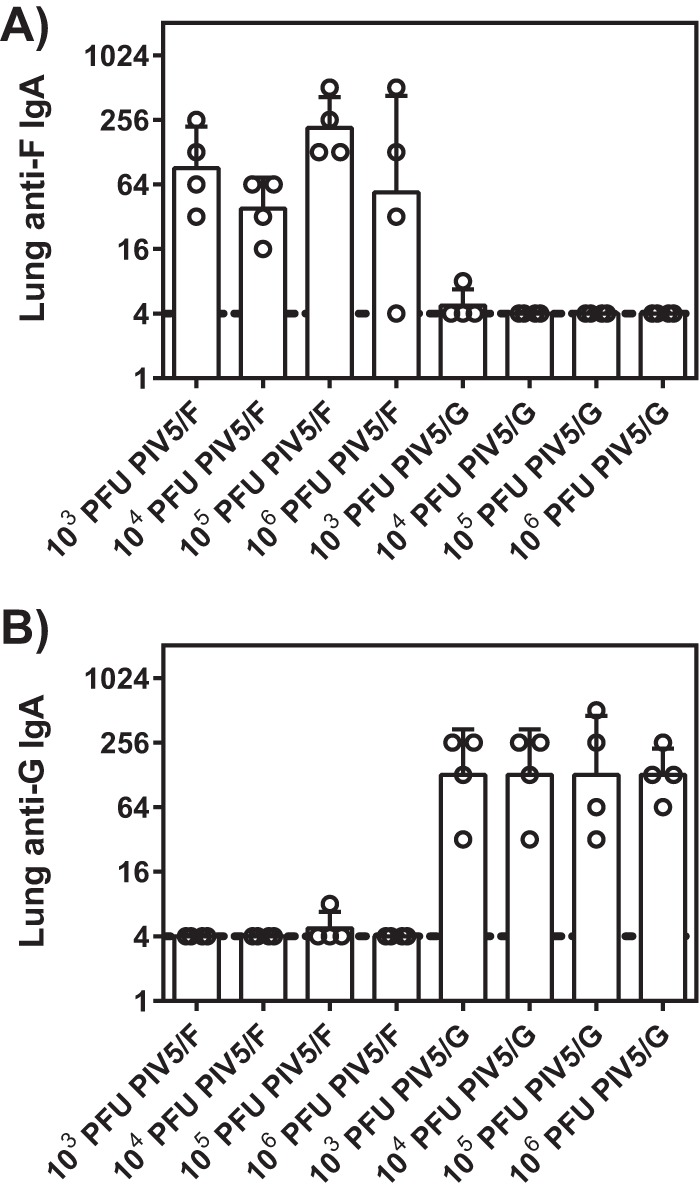
Mucosal IgA responses in cotton rats immunized with PIV5/F or PIV5/G. Cotton rats were immunized intranasally with 1 × 103, 1 × 104, 1 × 105, or 1 × 106 PFU of PIV5/F, PIV5/G, or PBS. Lungs were collected at 21 days postimmunization. Titers of IgA specific to RSV F (A) and RSV G (B) in the lung homogenates were measured by ELISA. Each group consisted of 4 animals. The dotted line represents the limit of detection. The error bars indicate standard deviations.
Protection of cotton rats from RSV challenge.
Cotton rats immunized with 1 × 103, 1 × 104, 1 × 105, and 1 × 106 PFU of PIV5/F and PIV5/G were challenged with RSV strain A2 at day 21 postimmunization (Fig. 4). Viral loads in the lung and nose tissues at 4 dpi were determined by plaque assay. The control group, which received no vaccine, showed high levels of RSV replication in the upper and lower respiratory tract, resulting in 2.2 × 105 PFU/g in the nose or 2.7 × 105 PFU/g in the lung. Viral loads in the nose or lung tissues of PIV/F- or PIV/G-vaccinated animals were significantly lower, mostly in a dose-dependent manner, except for the PIV5/F 1 × 106-PFU group. A single dose of 1 × 105 PFU of PIV5/F was able to protect the lung completely and reduced titers of virus in the nose by over 3 orders of magnitude. Interestingly, even though no NT50 was detected in PIV5/G-immunized cotton rats, PIV5/G reduced RSV titers in nose and in lungs by as much as 1,000-fold. These data suggested that both PIV5/F and PIV5/G induced protective anti-RSV immunity in cotton rats. In a separate study, PIV5 vector alone did not elicit detectable levels of RSV-specific antibodies or protect the animals from RSV challenge (data not shown).
FIG 4.
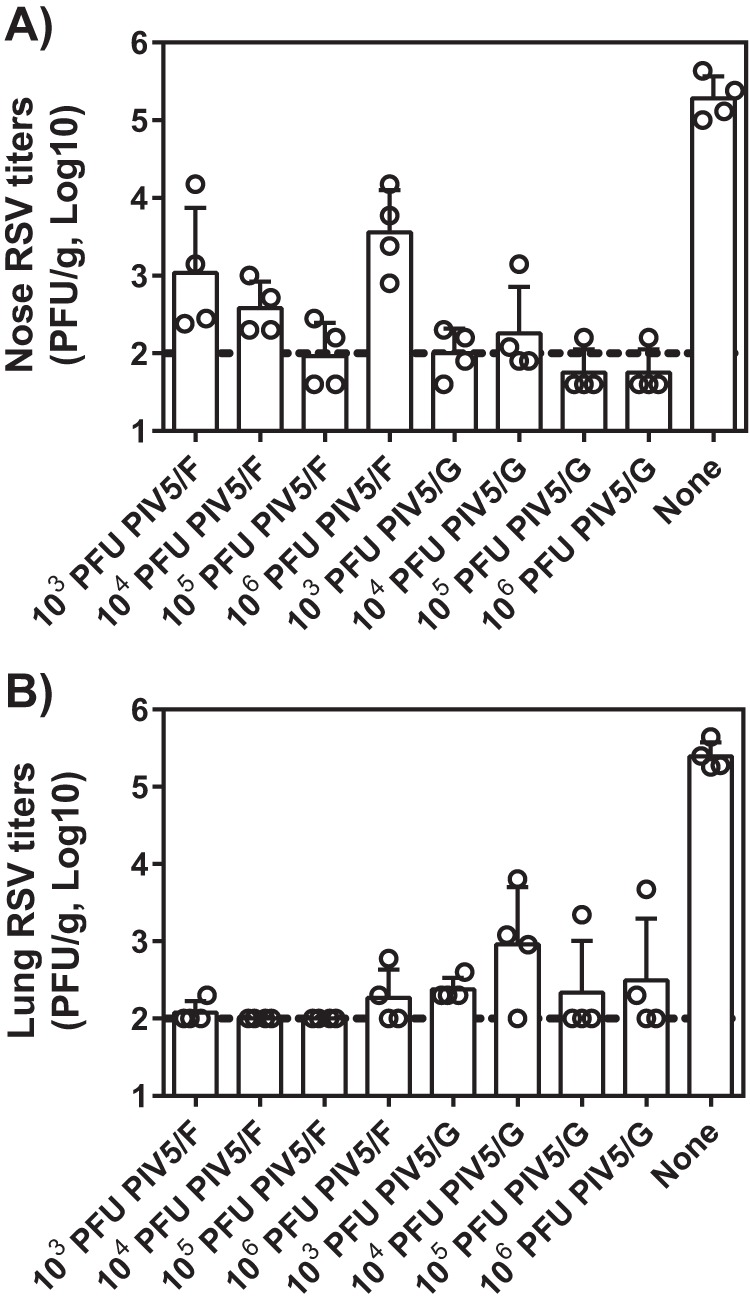
Immunization with PIV5/F or PIV5/G protected cotton rats against RSV challenge. Cotton rats were immunized intranasally with 1 × 103, 1 × 104, 1 × 105, or 1 × 106 PFU of PIV5/F, PIV5/G, or PBS. At 21 days postimmunization, the animals were challenged with 105.5 PFU of RSV A2. Four days postchallenge, noses (A) and lungs (B) were harvested, and viral loads were determined by plaque assay. Each group consisted of 4 cotton rats. The bars represent the GMT of each group. The dotted line represents the limit of detection. The error bars indicate standard deviations.
PIV5 replication in African green monkeys.
Several PIV5-related paramyxoviruses, such as RSV, PIV3, and metapneumovirus, are known to be able to replicate in the respiratory tract in African green monkeys. To determine the permissiveness of the animal species to support PIV5 replication, we first screened over 60 monkeys for anti-PIV5 antibodies and found them all seronegative. This confirms previous reports that PIV5 is not a simian virus (31). PIV5-seronegative animals were infected intranasally with 0.25 ml vaccine containing 1 × 102, 1 × 104, 1 × 106, or 1 × 108 PFU of PIV5 (n = 3 per group). Virus shedding was tested at 3, 5, 7, 10, and 14 dpi in the nasal wash and bronchoalveolar lavage (BAL) fluid. As shown in Fig. 5A and B, African green monkeys supported PIV5 replication in the upper and lower respiratory tract. In the 1 × 102-PFU dose group, PIV5 was shed in the noses and lungs for 10 days, and peak replication occurred at day 5. Peak GMTs of 2.1 × 103 and 3.2 × 104 PFU were observed for the upper and lower respiratory tract, respectively. Increasing the dose from 1 × 102 to 1 × 104, 1 × 106, or 1 × 108 PFU, however, increased only peak titers in the nose, but not those in the lung. Higher infection doses shortened the time required to reach the peak. The data suggested that African green monkeys were semipermissive to PIV5 infection.
FIG 5.
PIV5, PIV5/F, and PIV5/G replication in African green monkeys. (A and B) African green monkeys were infected intranasally with 1 × 102, 1 × 104, 1 × 106, or 1 × 108 PFU of PIV5. BAL (A) and nasopharyngeal swab (B) samples were collected on days 3, 5, 7, 10, and 14 postimmunization. (C and D) African green monkeys were immunized intranasally with 1 × 104 or 1 × 106 PFU of PIV5/F or PIV5/G or 1 × 106 PFU of PIV5. BAL (C) and nasopharyngeal swab (D) samples were collected on days 3, 5, 7, 10, and 14 postimmunization. Viral loads from each animal at each time point were determined by plaque assay. Each group consisted of 4 monkeys. All the monkeys were PIV5 seronegative and RSV seronegative. Each bar represents the GMT of the group. The dotted line represents the limit of detection. The error bars indicate standard deviations.
Immunogenicity of PIV5/F and PIV5/G in RSV-naive African green monkeys.
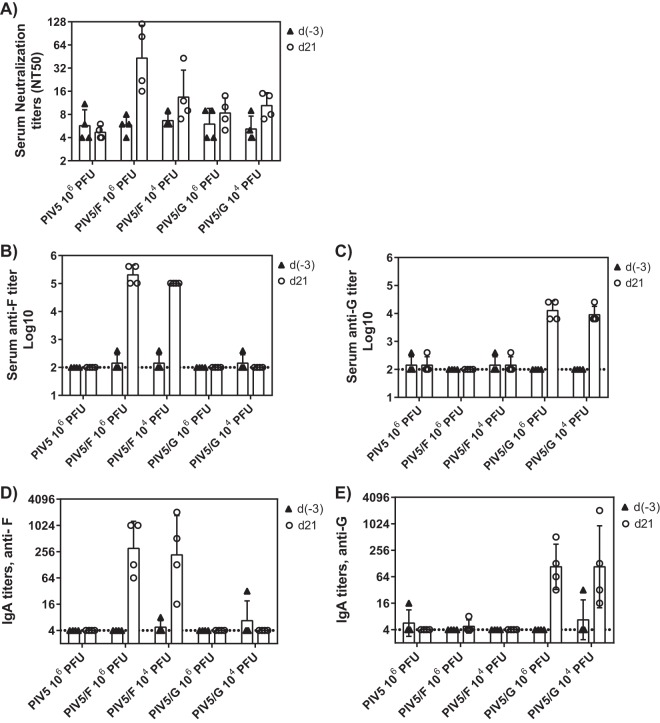
Based on the replication and growth kinetics of PIV5 in African green monkeys, single immunizations of 1 × 104 or 1 × 106 PFU of PIV5/F or PIV5/G were tested in PIV5- and RSV-seronegative animals (Fig. 5C and D). The replication kinetics of the vaccine viruses were comparable to those of the PIV5 control vector. Sera obtained before immunization at day −3 and after immunization at day 21 were tested for the presence of RSV F or G binding or neutralizing antibodies. RSV F-specific antibody titers were determined by binding to soluble recombinant F or G protein. All PIV5/F- or PIV5/G-vaccinated animals showed high titers of F- or G-specific antibodies (Fig. 6A to C). Neutralizing antibodies elicited by PIV5/F or PIV/G could also be detected at day 21 after single-dose immunization, although at low levels. The highest NT50 GMT, 52, was observed in the PIV5/F 1 × 106-PFU group.
FIG 6.
Serum and mucosal antibody responses in RSV-naive African green monkeys immunized with PIV5/F or PIV5/G. African green monkeys were immunized intranasally with 1 × 104 or 1 × 106 PFU of PIV5/F, PIV5/G, or 106 PFU of PIV5. (A to C) Sera were collected 3 days prior to immunization [d(−3)] and 21 days postimmunization (d21). The immunogenicity of the vaccine was determined by antibody titers against serum neutralization titers (A), RSV F protein (B), and RSV G protein (C). (D and E) Mucosal secretions were collected 3 days prior to immunization and 21 days postimmunization. IgA titers specific to RSV F (D) and RSV G (E) were measured by ELISA. Each group consisted of 4 PIV5- and RSV-seronegative monkeys. The dotted line represents the limit of detection. The error bars indicate standard deviations.
The mucosal antibody responses were evaluated by IgA production in nasal secretions (Fig. 6D and E). Prior to immunization, RSV F- or G-specific IgA was not detectable in any of the animals. Twenty-one days following immunization, animals vaccinated with PIV5/F or PIV/G, but not PIV5 vector, mounted significant levels of RSV F- or G-specific IgA.
The cell-mediated immune responses were assessed by gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assay. Peripheral blood mononuclear cells (PBMCs) were collected at 21 days postimmunization, about 2 weeks after clearance of the vaccine viruses. Low levels of F-specific T cells, but not G-specific T cells, were observed in animals vaccinated with 1 × 106 PFU of PIV5/F. The overall assay background of G-specific T cells is higher than that of F-specific T cells. No G-specific T cell responses were detected in animals vaccinated with 1 × 106 PFU of PIV5/G (Fig. 7).
FIG 7.
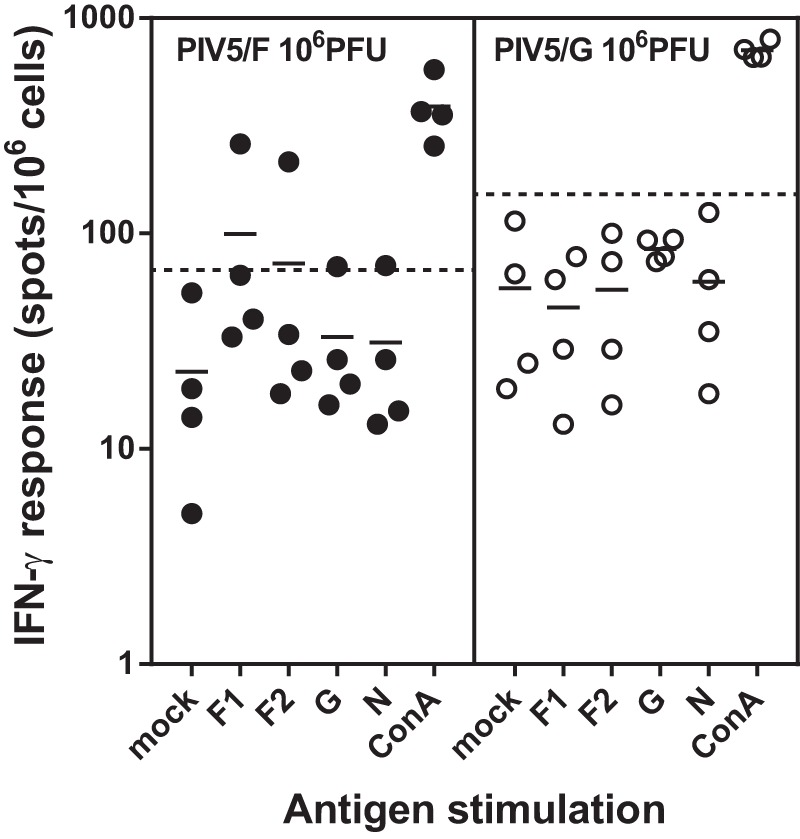
Cell-mediated immune responses in PIV5/F- or PIV5/G-vaccinated African green monkeys. PBMCs were collected from African green monkeys 21 days after intranasal immunization with 1 × 106 PFU of PIV5/F, PIV5/G, or PIV5. The cells were mock stimulated or stimulated with pools of synthetic peptides representing RSV proteins F, G, and N or concanavalin A (ConA). The F peptides were divided into two pools, F1 (aa 1 to 295) and F2 (aa 285 to 574). Each group consisted of 4 PIV5- and RSV-seronegative monkeys. The solid horizontal lines represent the geometric means within each group. Values above the dotted lines represent positive responses.
Protection of African green monkeys from RSV challenge.
The immunized animals were subsequently challenged with RSV at day 28 postimmunization (Fig. 8). Nasal wash and BAL samples were collected at 3, 5, 7, 10, and 14 days postchallenge, and the RSV loads were determined by plaque assay. In the nasopharynx, high titers of RSV (∼4 log10 PFU/ml) were detected in animals immunized with PIV5 parental vector virus. The shedding lasted for ≧7 days, and the virus was cleared by day 10. Immunization with PIV5/F or PIV/G did not shorten the duration of viral shedding. However, the vaccines reduced the peak viral loads by 10- to 100-fold in all the vaccine dose groups. The greatest peak titer reduction was seen in animals immunized with 1 × 106 PFU of PIV5/F.
FIG 8.
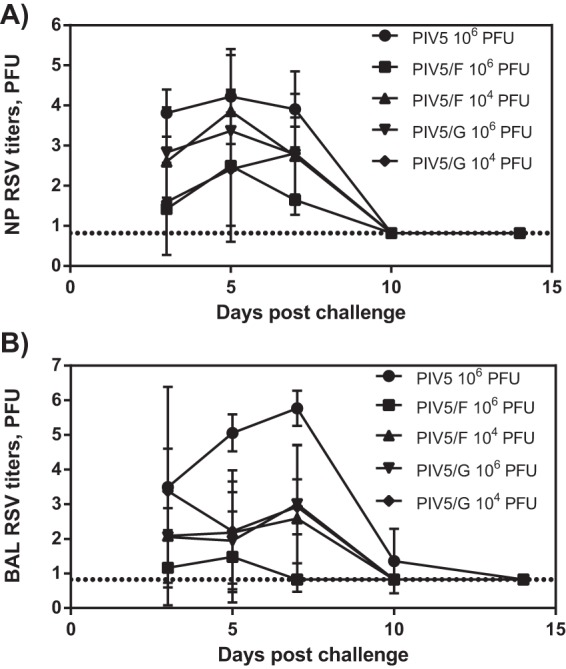
Protection against RSV challenge by PIV5/F or PIV5/G in vaccinated African green monkeys. African green monkeys were immunized intranasally with 104 or 106 PFU of PIV5/F or PIV5/G or 106 PFU of PIV5. At 28 days postimmunization, animals were challenged with 105.5 PFU of RSV A2 intranasally. Nasopharyngeal swabs and BAL samples were collected 3, 5, 7, 10, and 14 days postchallenge, and viral loads were determined by plaque assay. Each group consisted of 4 PIV5- and RSV-seronegative monkeys. The dotted line represents the limit of detection. The error bars indicate standard deviations.
In the BAL samples, RSV shedding among animals of the PIV5 control group could be detected for 10 days, with a peak GMT of 5.8 × 105 PFU at day 7. In contrast, animals immunized with PIV5/F or PIV5/G showed 2 to 5 orders of magnitude of peak titer reduction in the lung. PIV5/F at a 1 × 106-PFU dose produced the best protection in the lung, consistent with the results of nasal RSV shedding. In this group, two animals were completely protected throughout the study period, and the other animals shed low levels of RSV at day 3 and day 5.
Responses to PIV5/F and PIV5/G vaccination in RSV-exposed African green monkeys.
We also evaluated the antibody responses of RSV-seropositive animals to PIV5/F and PIV5/G vaccination (Fig. 9). The animals had been seroconverted by intranasal infection with RSV A2. At the time of PIV5/F or PIV5/G vaccination, the serum neutralization titers were low or below detection. Immunization with PIV5/F or PIV5/G dramatically increased the serum neutralization titers. At day 28, the GMT NT50 titer of the PIV5/F group was found to be 4,130, a 50-fold increase compared to the titers prior to vaccination.
FIG 9.
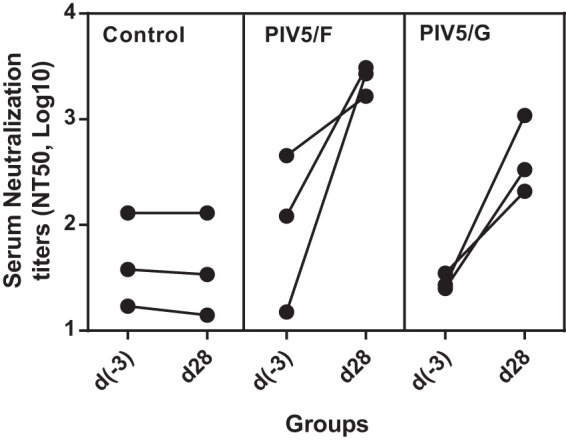
Serum neutralizing antibody responses in RSV-exposed African green monkeys immunized with PIV5/F or PIV5/G. RSV-seropositive African green monkeys were immunized intranasally with 1 × 106 PFU of PIV5/F, PIV5/G, or PIV5. Sera were collected 28 days postimmunization, and endpoint neutralizing antibody titers were determined. Each group consisted of 3 PIV5-seronegative, RSV-seropositive monkeys.
Lung pathology in PIV5/F- or PIV5/G-immunized cotton rats after RSV challenge.
It was previously reported that RSV challenge of PIV5/F- or PIV5/G-immunized mice did not result in lung pathology typical of that seen after RSV challenge of mice immunized with formalin-inactivated RSV (25). The risk of enhanced respiratory disease (ERD) following RSV challenge for the PIV5-vectored vaccines was further assessed in the cotton rat model, which is more permissive for RSV than BALB/c mice. Four groups of animals were immunized with a single dose of phosphate-buffered saline (PBS), 1 × 106 PFU of PIV5/F, 1 × 106 PFU of PIV5/G, or 2 doses of 100 μl of FI-RSV (lot 100 at a dilution of 1:100) (11, 38) at day 0 and day 21. The animals were challenged with RSV A2 at day 49. Five days following the challenge, lungs were harvested and examined for histological evaluation of inflammation. Representative images of the hematoxylin and eosin (H&E)-stained lung sections are shown in Fig. 10. The pathological changes representing alveolitis, interstitial pneumonitis, perivasculitis, and peribronchiolitis of the lung sections were scored blindly. The FI-RSV group showed the highest scores for all 4 individual H&E analyses. The histopathology scores for FI-RSV, but not PIV5/F or PIV5/G, were significantly higher than those observed for the PBS group challenged with RSV using one-way analysis of variance (ANOVA) of paired t test analyses (Table 1).
FIG 10.
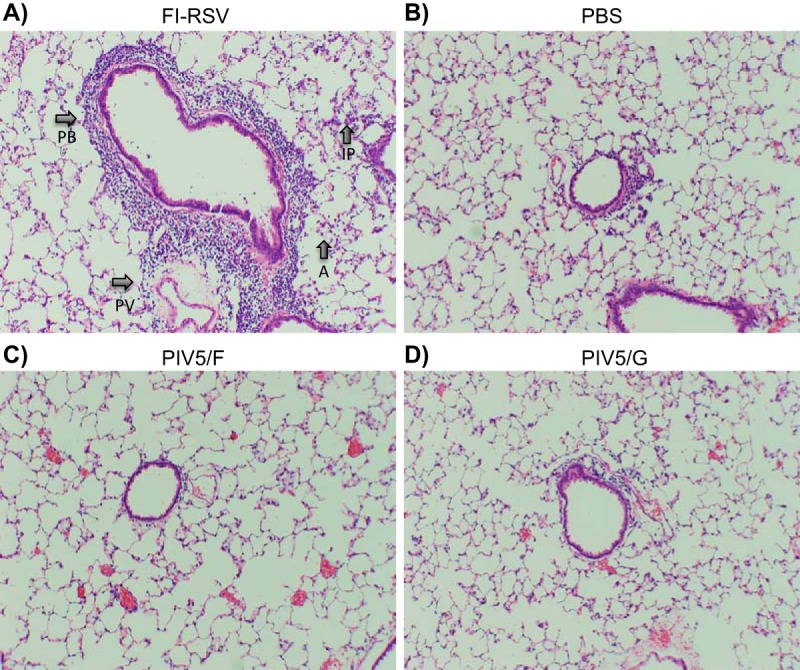
Cotton rat lung histopathology. Cotton rats were immunized intranasally with a single dose of 1 × 106 PFU of PIV5/F or PIV5/G or two doses of FI-RSV at day 0 and day 21. Control animals were immunized with PBS. The animals were then challenged with 105.5 PFU of RSV A2 at day 49. Lung tissues were harvested 5 days postchallenge, fixed, and stained with hematoxylin and eosin. Lung pathological changes—alveolitis (A), interstitial pneumonitis (IP), perivasculitis (PV), and peribronchiolitis (PB)—are indicated by arrows.
TABLE 1.
Lung pathology in PIV5/F- and PIV5/G-immunized animals after RSV challenge
| Vaccine | Challenge | Avg score |
||||
|---|---|---|---|---|---|---|
| Peribronchiolitis | Perivasculitis | Interstitial pneumonitis | Alveolitis | Mean | ||
| FI-RSV | RSV A2 | 2.67 | 3.00 | 2.00 | 2.00 | 2.42a |
| PIV5/F | RSV A2 | 2.33 | 2.00 | 0.67 | 1.00 | 1.50 |
| PIV5/G | RSV A2 | 2.33 | 1.67 | 1.00 | 1.00 | 1.50 |
| PBS | RSV A2 | 2.00 | 2.00 | 0.67 | 0.67 | 1.33 |
| PBS | PBS | 1.00 | 0.00 | 0.00 | 0.00 | 0.25 |
The histopathology score for FI-RSV was significantly higher than that observed for the PBS group challenged with RSV (P < 0.0001; t test).
DISCUSSION
In this study, we evaluated live PIV5-vectored RSV vaccines in cotton rats and African green monkeys, which are among the animals most commonly used to study RSV infection. These two species are also semipermissive to PIV5 infection and thus could serve as models to test PIV5-vectored vaccines (Fig. 1 and 5). In both models, a single intranasal immunization dose of PIV5/F or PIV5/G was able to produce systemic and local immunity and protect animals from RSV challenge. The vaccines could also boost RSV neutralization titers in RSV-exposed African green monkeys, indicating that the PIV5-vectored vaccine can potentially serve for both the pediatric (RSV-naive) and elderly (RSV-exposed) populations.
The intranasal route is the natural mode of transmission of RSV. An intranasal vaccine, such as PIV5/F, potentially has the advantage of inducing not only systemic immunity, but also mucosal immunity at the site of acquisition of the virus. Low nasal RSV-specific IgA has been identified as a risk factor for RSV infection in community-dwelling adults over 65 years of age (39). In an RSV human challenge study, the nasal IgA titer showed strong correlation with protection against infection (40). The study also suggested that natural RSV infection failed to induce robust IgA memory B cells, which may be essential to provide long-term protection. It is conceivable that both serum neutralizing antibodies and nasal IgA are needed to provide protection against RSV infection and disease. The presence of RSV-specific nasal IgA may block RSV at the site of virus entry, while serum neutralizing antibodies may protect the host from lower respiratory tract infection and disease. It is also interesting that a recombinant PIV5 expressing hemagglutinin of influenza A virus subtype 3 could generate H3 responses in dogs with prior PIV5 exposure (37). Therefore, preexisting immunity to the vector may not affect the efficacy of the vaccine.
The PIV5/G vaccine did not produce detectable levels of neutralizing antibody in cotton rats but still protected against RSV challenge (Fig. 2 and 4). The protection might be mediated by antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) (41–43). In addition, G protein has a CX3C chemokine motif that can bind CX3CR1, which may facilitate virus infection and modulate leukocyte chemotaxis (44). Mice vaccinated with polypeptides containing the CX3C motif generate antibodies that inhibit G protein CX3C-CX3CR1 binding and chemotaxis, reduce lung virus titers, and prevent body weight loss and pulmonary inflammation (45). Nanoparticle vaccines carrying the CX3C motif of G protein also induce higher levels of M2-specific CD8+ T cell responses following RSV challenge (46). In naive African green monkeys, PIV5/G produced low levels of neutralizing antibody (Fig. 6A) but was able to significantly boost neutralizing antibody titers in RSV-exposed monkeys (Fig. 9), suggesting that anti-G antibodies can be neutralizing and, as a result, protective. However, protection mechanisms for PIV5/G are likely to be different in cotton rats and African green monkeys (47, 48).
In this work, RSV F and G were introduced into the intergenic regions of HN and L. The position of the RSV antigen could be further optimized. Li et al. (49) demonstrated that insertion of the hemagglutinin (HA) of influenza virus A between the PIV5 SH and HN genes conferred the best balance between immunogenicity and stability. Deletion of PIV5 SH also led to an increased immune response to inserted genes in PIV5. Therefore, placing RSV F in front of HN genes in a PIV5 vector lacking SH may enhance the efficacy of the PIV5-based RSV vaccine. The immunogenicity of the current candidates could be further improved by replacing the wild-type F sequence used in this study with a stabilized prefusion form of F. A recent study showed that in the recombinant bovine/human PIV3 (b/hPIV3)-vectored vaccine, prefusion F conferred superior immunogenicity and higher efficacy of protection against RSV challenge in a hamster model (50) than wild-type F vaccine. The vaccine also induced higher levels of antibodies against site Ø (51, 52), a prefusion-specific epitope, as well as a major neutralizing epitope, in naturally acquired immunity (53). However, the correlate of protection against RSV infection or disease with site Ø antibodies in humans remains to be established. Alternatively, the F gene could be modified in a way that would inactivate the fusion activity and focus the immune response to site II, which is recognized by palivizumab, a monoclonal antibody recommended for RSV prophylaxis, as demonstrated by an F nanoparticle vaccine (20, 54). The recombinant F nanoparticle vaccine was shown to be capable of eliciting palivizumab-competing antibodies and showed protection against both RSV acquisition and disease severity in a phase II clinical trial (55). The combination of antigen modification, nasal delivery, and a novel PIV5 platform may allow the host to produce better responses than those elicited by natural RSV infection.
As replication-competent vectors, administered intranasally, PIV5 vaccines would be considered to have a low risk of causing ERD. So far, enhanced disease has been demonstrated in humans only for parenterally immunized formalin-inactivated RSV vaccines. A previous study showed that infection with PIV5/F in mice produced a similar but attenuated immune pathology to natural RSV infection. The lack of ERD was further confirmed in cotton rats infected with PIV5/F or PIV5/G (Table 1 and Fig. 10), which exhibited minimal to mild histopathological changes in the lung sections. A recent publication has suggested that the presence of non-RSV cell culture contaminants might also contribute to ERD (56) and obscure the RSV-specific response. The vaccines and challenge virus used in the current study were grown in the medium supplemented with FBS, and the preparations also contained significant amounts of host cell proteins. Using purified vaccines may therefore induce even better quality RSV responses with lower pulmonary inflammation.
MATERIALS AND METHODS
Cells and viruses.
The recombinant PIV5 vector expressing RSV F or G was constructed as previously described (25). The vaccine viruses were propagated in MDBK cells with Dulbecco's modified Eagle medium (DMEM) containing 2% fetal bovine serum (FBS) and 100 IU/ml penicillin–100 μg/ml streptomycin (1% P/S). Cell-free virus was harvested at 5 to 7 days postinfection, flash frozen on liquid nitrogen, and stored at −70°C. RSV strain A2 (ATCC VR-1540) and Long (ATCC VR-26) stocks were grown in Hep2 cells. The MDBK cells were maintained in DMEM supplemented with 5% FBS and 1% P/S. The Hep2 cells were cultured in Eagle minimum essential medium (EMEM) containing 10% FBS, 2 mM l-glutamine, 50 μg/ml Gentamicin, 25 μg/ml amphotericin B, and 1% P/S.
Serum IgG assay.
Immulon 2HB microtiter plates (Nunc) were coated with 2 μg/ml recombinant RSV F or G protein and incubated at 4°C overnight. The plates were then washed and blocked for 1 h with phosphate-buffered saline with Tween 20 (PBST) containing 3% milk (blocking buffer) at room temperature. Test samples were serially diluted 4-fold in blocking buffer starting at 1:100 dilution, transferred to the F- or G-coated plates, and incubated for 2 h at room temperature. Following three washes with PBST, horseradish peroxidase (HRP)-conjugated secondary antibody diluted in blocking buffer was added to the plates and incubated for an additional 1 h at room temperature. The plates were washed again and developed with SuperBlu Turbo TMB (Virolabs) in the dark. The reaction was stopped after 5 min, and absorbance was read at 450 nm on a VersaMax enzyme-linked immunosorbent assay (ELISA) microplate reader (Molecular Devices). Titers are reported as the reciprocal of the last dilution that was 2-fold greater than the background.
IgA assay.
The IgA titers in nasal samples from African green monkeys or lung homogenates from cotton rats were quantified by direct-binding ELISA on the Meso Scale platform (Meso Scale Discovery [MSD]). Briefly, the 96-well standard Meso Scale plates were coated with 0.2 μg/ml recombinant RSV F or G or ovalbumin protein at 4°C overnight. The plates were then washed and blocked for 1 h with PBST containing 3% milk at room temperature. Nasal samples from African green monkeys or lung homogenates from cotton rats were serially diluted 2-fold in Hispec buffer (Bio-Rad) starting at 1:4 dilution, transferred to the antigen-coated plates, and incubated for 1 h at room temperature. After washing, Sulfo-Tag (MSD)-conjugated secondary antibodies diluted 1:1,000 in Hispec buffer was added to the plates and incubated for 1 h at room temperature. The plates were washed again, and 1× Read Buffer T (MSD) was added to the plates and immediately read on a Sector S 600 plate reader (MSD). RSV F- or G-specific enhanced chemiluminescence (ECL) values were background adjusted by subtracting the ovalbumin values. Titers are expressed as the reciprocal of the last dilution that was greater than 3 times the background.
Neutralization assay.
All sera were treated at 56°C for 30 min to inactivate complement prior to neutralization assays (57). Twofold serial dilutions of the serum samples were prepared in EMEM containing 2% FBS, starting at 1:4 dilution. The diluted serum was added in duplicate to 96-well plates and mixed with RSV strain Long in a 100-μl total volume. The virus-antibody mixture was incubated for 1 h at 37°C and 5% CO2. Following incubation, Hep-2 cells at a concentration of 1.5 × 104 cells per well were added. The plates were incubated for 3 days at 37°C and 5% CO2. The cells were then washed and fixed with 80% acetone for 15 min. RSV-infected cells were then immunostained. Briefly, RSV F- and N-specific monoclonal antibodies were added to the test plates with fixed cells and incubated for 1 h at room temperature. After washing, biotinylated goat anti-mouse IgG was added and incubated for 1 h. The plates were washed again and developed with a dual-channel near-infrared detection (NID) system. Infrared dye-Streptavidin to detect RSV-specific signal and two cell stains for assay normalization were added to the 96-well plates and incubated for 1 h in the dark. The plates were washed and dried in the dark for 20 min and read on the Licor Aerius automated imaging system utilizing a 700-channel laser for cell normalization and an 800-channel laser for detection of RSV-specific signal. The 800/700 ratios were calculated, and serum neutralizing titers were determined by a four-parameter curve fit in GraphPad. The neutralization titers determined by the microneutralization (MNT) assay and the plaque reduction neutralization (PRNT) (58) assay were not statistically different.
IFN-γ ELISPOT assay.
PBMCs were isolated from whole blood by Ficoll gradient centrifugation and tested for IFN-γ ELISPOT responses to pools of synthetic peptides representing RSV proteins F, G, and N (15-mers overlapping by 11 amino acids). The F peptides were divided into two pools, F1 (amino acids [aa] 1 to 295) and F2 (aa 285 to 574). Ninety-six-well plates with polyvinylidene difluoride (PVDF) membranes (Millipore) were coated overnight at 4°C with anti-IFN-γ monoclonal antibody (U-Cytech). PBMCs were added to the blocked plates at 4 × 105/well. Peptide pools were added to the cells at approximately 2-μg/ml final concentration per peptide. After overnight incubation in a 37°C, 5% CO2 incubator, bound IFN-γ was detected with biotinylated anti-IFN-γ antibody (U-Cytech), followed by alkaline phosphatase-conjugated streptavidin (BD PharMingen) and NBT-BCIP (nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate) substrate (Pierce). Spots were enumerated using a digital imager and an automated counting system (AutoImmun Diagnostika [AID]), and responses were normalized to spot-forming cells (SFC) per 1 × 106 PBMCs. A positive response was defined as an antigen-specific response that was at least 55 spot-forming cells per 1 × 106 PBMCs and at least 3-fold over the corresponding unstimulated (mock) control.
Titration of PIV5 and RSV.
Tenfold serial dilutions of PIV5 in DMEM with 1% bovine serum albumin (BSA) were incubated with BHK-21 cells for 1 h at 37°C and 5% CO2. The inocula were removed, and the cells were overlaid with DMEM containing 2% FBS, 1% P/S, and 1% low-melting point agarose. After incubation for 5 to 7 days at 37°C and 5% CO2, the cells were then fixed with 2% formaldehyde and stained with crystal violet to visualize plaques.
To titrate RSV, Hep2 cells were incubated with serial dilutions of RSV prepared in Williams medium E with 2 mM l-glutamine and 50 μg/ml neomycin. After adsorption for 1 h at 37°C and 5% CO2, the cells were overlaid with Williams medium E (Thermo Fisher Scientific) containing 1.6% FBS, 2 mM l-glutamine, 50 μg/ml neomycin, and 0.8% methylcellulose. Five days later, the cells were fixed with glutaric dialdehyde and stained with crystal violet to visualize plaques.
Immunization and RSV challenge in cotton rats.
Female cotton rats 4 to 8 weeks old were purchased from SAGE (Boyertown, PA) and maintained at a Merck animal facility in West Point, PA. The animal studies were approved by the Merck Institutional Animal Care and Use Committee and conducted in accordance with animal care guidelines.
To determine the replication of PIV5 in cotton rats, animals were inoculated intranasally with 105 PFU of PIV5 in 10-μl or 100-μl volumes, equally divided between two nostrils. At day 4 or day 6, the animals were sacrificed by CO2 inhalation. The lung (left lobes) and nasal turbinates were removed and homogenized in Hanks balanced salt solution (HBSS) (Lonza) containing SPG buffer (0.2 M sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, and 5.4 mM monosodium glutamate) on wet ice. Samples were clarified by centrifugation at 2,000 rpm for 10 min, aliquoted, frozen on dry ice, and immediately stored frozen at −70°C. The PIV5-vectored vaccine titers were determined by plaque assay in BHK cells.
To determine the immunogenicity of PIV5/F or PIV5/G, cotton rats received one dose of 1 × 103, 1 × 104, 1 × 105, or 1 × 106 PFU of vaccine in a 10-μl volume by intranasal administration, equally divided between two nostrils. Serum samples at day 28 were collected to determine the neutralization titers. On day 28, all the cotton rats were inoculated intranasally, under isoflurane anesthesia (1 to 4%), with 100 μl of 105.5 PFU of RSV A2. Four days postchallenge, the animals were sacrificed by CO2 inhalation, and lung (left lobes) and nasal turbinates were removed and homogenized in Hanks balanced salt solution (Lonza) containing SPG buffer on wet ice. Samples were clarified by centrifugation at 2,000 rpm for 10 min, aliquoted, frozen on dry ice, and immediately stored frozen at −70°C. The RSV titers were determined by plaque assay in Hep2 cells.
Immunization and RSV challenge in African green monkeys.
African green monkeys were domestically bred, raised, and maintained at New Iberia Research Center (NIRC), New Iberia, LA. The animals were prescreened for the presence of RSV- and PIV5-specific antibodies. NT50 titers of <10 and anti-F endpoint ELISA titers of ≦400 were considered seronegative. The naive monkeys were 12 to 15 months of age, and the seropositive monkeys were 5- to 14-year-old adults. The animal studies were approved by the Merck Institutional Animal Care and Use Committee (IACUC) and conducted in accordance with animal care guidelines.
To determine the replication of PIV5 in African green monkeys, PIV5-seronegative animals (n = 3) were anesthetized with ketamine (10 mg/kg of body weight) and inoculated intranasally with 1 × 102, 1 × 104, 1 × 106, or 1 × 108 PFU of PIV5 in 0.25 ml split evenly between two nostrils. Nasopharyngeal swabs and BAL fluid were collected at days 3, 5, 7, 10, 14, and 21. The nasopharyngeal samples were collected by gently rubbing two areas of the oropharynx region using a Darcon swab and placing the tips in a solution containing HBSS with SPG buffer and 0.1% gelatin. For BAL, approximately 5 ml HBSS was infused directly into the lung and aspirated via a sterile French catheter and syringe. Recovered samples were supplemented with 0.1 volume of 10× SPG buffer and 0.1 volume of 1% gelatin, aliquoted, flash frozen, and stored at −70°C.
To determine the immunogenicity of PIV5-vectored vaccines, PIV5- and RSV-seronegative African green monkeys, identified by RSV F-specific IgG ELISA and serum neutralization titer, were immunized intranasally with 1 × 104 or 1 × 106 PFU of vaccines at day 0 in 0.25 ml evenly divided between two nostrils, using the same protocol described above, with PIV5 as the vector control. The BAL fluid and nasopharyngeal swabs were collected at days 3, 5, 7, and 14 postvaccination, as described above, to monitor vaccine virus replication. At day 21, sera and PBMCs were also collected. At day 28 postimmunization, all the monkeys were anesthetized and challenged with 2 × 105.5 PFU of RSV strain A2. The challenge virus was administered by intranasal and intratracheal inoculation, 1 ml by each route. Following challenge, nasopharyngeal swabs and BAL samples were collected at days 3, 5, 7, 10, and 14 postchallenge. The recovered samples were supplemented with 0.1 volume of 10× SPG buffer and 0.1 volume of 1% gelatin, aliquoted, flash frozen, and stored at −70°C.
Histopathology.
The original lot 100 FI-RSV that caused ERD in clinical trials (11) was used as a reference vaccine in our study. Five days after RSV challenge, the right lung lobes of RSV-infected cotton rats, bisected from the left lung lobes used for viral titration, were infused and fixed with 10% neutral buffered formalin. Paraffin-embedded tissue sections were stained with H&E. Four lung pathological changes–alveolitis, interstitial pneumonitis, perivasculitis, and peribronchiolitis–were examined. Each of these parameters was scored separately and given a score from 0 to 4, representing no to maximum pathological changes. The raw score can be translated into a pathological score as follows: 1 = 5, 2 = 25, 3 = 75, and 4 = 100. The entire process was performed by an independent pathologist in a random and blinded fashion.
Statistical analyses.
Statistical analysis was carried out with GraphPad Prism 7 software. To analyze the histopathology scores, one-way ANOVA of paired t tests was used to compare the mean scores of each group to those of the PBS-RSV challenge group.
ACKNOWLEDGMENTS
We thank Richard Peluso for critical readings of the manuscript. We are grateful for the excellent technical support provided by Merck Laboratory Animal Services at West Point, PA, and the animal care staff at New Iberia Research Center, New Iberia, LA. We thank Kevin Yim, Derrick Martin, and Jorge Blanco from Sigmovir Biosystems, Inc., for their assistance with the cotton rat ERD study.
This work was partially supported by an endowment from the Fred C. Davison Distinguished University Chair in Veterinary Medicine to B.H.
REFERENCES
- 1.Graham BS. 2011. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev 239:149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins PL, Karen RA. 2013. Respiratory syncytial virus and metapneumovirus, p 1086 In Knipe DM, Howley PM (ed), Fields virology, 6th ed Lippincott, Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. 1999. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 6.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 7.Han LL, Alexander JP, Anderson LJ. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis 179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 8.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 9.Meissner HC, Welliver RC, Chartrand SA, Law BJ, Weisman LE, Dorkin HL, Rodriguez WJ. 1999. Immunoprophylaxis with palivizumab, a humanized respiratory syncytial virus monoclonal antibody, for prevention of respiratory syncytial virus infection in high risk infants: a consensus opinion. Pediatr Infect Dis J 18:223–231. doi: 10.1097/00006454-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. 2013. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 31(Suppl 2):B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 12.Karron RA, Buchholz UJ, Collins PL. 2013. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol 372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loomis RJ, Johnson PR. 2013. Gene-based vaccine approaches for respiratory syncytial virus. Curr Top Microbiol Immunol 372:307–324. doi: 10.1007/978-3-642-38919-1_15. [DOI] [PubMed] [Google Scholar]
- 14.Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, O'Hagan DT, Singh M, Mason PW, Valiante NM, Dormitzer PR, Barnett SW, Rappuoli R, Ulmer JB, Mandl CW. 2012. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci U S A 109:14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ternette N, Tippler B, Uberla K, Grunwald T. 2007. Immunogenicity and efficacy of codon optimized DNA vaccines encoding the F-protein of respiratory syncytial virus. Vaccine 25:7271–7279. doi: 10.1016/j.vaccine.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Kamphuis T, Meijerhof T, Stegmann T, Lederhofer J, Wilschut J, de Haan A. 2012. Immunogenicity and protective capacity of a virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice. PLoS One 7:e36812. doi: 10.1371/journal.pone.0036812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamphuis T, Shafique M, Meijerhof T, Stegmann T, Wilschut J, de Haan A. 2013. Efficacy and safety of an intranasal virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice and cotton rats. Vaccine 31:2169–2176. doi: 10.1016/j.vaccine.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt MR, McGinnes LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. 2012. Long-term and memory immune responses in mice against Newcastle disease virus-like particles containing respiratory syncytial virus glycoprotein ectodomains. J Virol 86:11654–11662. doi: 10.1128/JVI.01510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walpita P, Johns LM, Tandon R, Moore ML. 2015. Mammalian cell-derived respiratory syncytial virus-like particles protect the lower as well as the upper respiratory tract. PLoS One 10:e0130755. doi: 10.1371/journal.pone.0130755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M, Zhou B, Lu H, Boddapati S, Li J, Flyer D, Glenn G. 2012. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One 7:e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Quan FS, Kwon Y, Sakamoto K, Kang SM, Compans RW, Moore ML. 2014. Additive protection induced by mixed virus-like particles presenting respiratory syncytial virus fusion or attachment glycoproteins. Antiviral Res 111:129–135. doi: 10.1016/j.antiviral.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YN, Hwang HS, Kim MC, Lee YT, Lee JS, Moore ML, Kang SM. 2015. Recombinant influenza virus expressing a fusion protein neutralizing epitope of respiratory syncytial virus (RSV) confers protection without vaccine-enhanced RSV disease. Antiviral Res 115:1–8. doi: 10.1016/j.antiviral.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW. 2011. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis 204:987–995. doi: 10.1093/infdis/jir474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tompkins SM, Lin Y, Leser GP, Kramer KA, Haas DL, Howerth EW, Xu J, Kennett MJ, Durbin RK, Durbin JE, Tripp R, Lamb RA, He B. 2007. Recombinant parainfluenza virus 5 (PIV5) expressing the influenza A virus hemagglutinin provides immunity in mice to influenza A virus challenge. Virology 362:139–150. doi: 10.1016/j.virol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phan SI, Chen Z, Xu P, Li Z, Gao X, Foster SL, Teng MN, Tripp RA, Sakamoto K, He B. 2014. A respiratory syncytial virus (RSV) vaccine based on parainfluenza virus 5 (PIV5). Vaccine 32:3050–3057. doi: 10.1016/j.vaccine.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Chen Z, Huang J, Fu Z, He B. 2015. Parainfluenza virus 5 expressing the G protein of rabies virus protects mice after rabies virus infection. J Virol 89:3427–3429. doi: 10.1128/JVI.03656-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Gupta T, Xu P, Phan S, Pickar A, Yau W, Karls RK, Quinn FD, Sakamoto K, He B. 2015. Efficacy of parainfluenza virus 5 (PIV5)-based tuberculosis vaccines in mice. Vaccine 33:7217–7224. doi: 10.1016/j.vaccine.2015.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goswami KK, Lange LS, Mitchell DN, Cameron KR, Russell WC. 1984. Does simian virus 5 infect humans? J Gen Virol 65:1295–1303. doi: 10.1099/0022-1317-65-8-1295. [DOI] [PubMed] [Google Scholar]
- 29.Hsiung GD. 1972. Parainfluenza-5 virus. Infection of man and animal. Prog Med Virol 14:241–274. [PubMed] [Google Scholar]
- 30.Hull RN, Minner JR, Smith JW. 1956. New viral agents recovered from tissue cultures of monkey kidney cells. I. Origin and properties of cytopathogenic agents S.V.1, S.V.2, S.V.4, S.V.5, S.V.6, S.V.11, S.V.12 and S.V.15. Am J Hyg 63:204–215. [DOI] [PubMed] [Google Scholar]
- 31.Tribe GW. 1966. An investigation of the incidence, epidemiology and control of simian virus 5. Br J Exp Pathol 47:472–479. [PMC free article] [PubMed] [Google Scholar]
- 32.Binn LN, Eddy GA, Lazar EC, Helms J, Murnane T. 1967. Viruses recovered from laboratory dogs with respiratory disease. Proc Soc Exp Biol Med 126:140–145. doi: 10.3181/00379727-126-32386. [DOI] [PubMed] [Google Scholar]
- 33.Heinen E, Herbst W, Schmeer N. 1998. Isolation of a cytopathogenic virus from a case of porcine reproductive and respiratory syndrome (PRRS) and its characterization as parainfluenza virus type 2. Arch Virol 143:2233–2239. doi: 10.1007/s007050050454. [DOI] [PubMed] [Google Scholar]
- 34.Cornwell HJ, McCandlish IA, Thompson H, Laird HM, Wright NG. 1976. Isolation of parainfluenza virus SV5 from dogs with respiratory disease. Vet Rec 98:301–302. doi: 10.1136/vr.98.15.301. [DOI] [PubMed] [Google Scholar]
- 35.McCandlish IA, Thompson H, Cornwell HJ, Wright NG. 1978. A study of dogs with kennel cough. Vet Rec 102:293–301. doi: 10.1136/vr.102.14.293. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg FJ, Lief FS, Todd JD, Reif JS. 1971. Studies of canine respiratory viruses. I. Experimental infection of dogs with an SV5-like canine parainfluenza agent. Am J Epidemiol 94:147–165. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, Xu P, Salyards GW, Harvey SB, Rada B, Fu ZF, He B. 2012. Evaluating a parainfluenza virus 5-based vaccine in a host with pre-existing immunity against parainfluenza virus 5. PLoS One 7:e50144. doi: 10.1371/journal.pone.0050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince GA, Curtis SJ, Yim KC, Porter DD. 2001. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with lot 100 or a newly prepared reference vaccine. J Gen Virol 82:2881–2888. doi: 10.1099/0022-1317-82-12-2881. [DOI] [PubMed] [Google Scholar]
- 39.Walsh EE, Falsey AR. 2004. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 40.Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, de Haan CA, Wrammert J, Openshaw PJ, Chiu C. 2015. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med 191:1040–1049. doi: 10.1164/rccm.201412-2256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyoglu-Barnum S, Todd SO, Chirkova T, Barnum TR, Gaston KA, Haynes LM, Tripp RA, Moore ML, Anderson LJ. 2015. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology 483:117–125. doi: 10.1016/j.virol.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miao C, Radu GU, Caidi H, Tripp RA, Anderson LJ, Haynes LM. 2009. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab′)2 components mediates reduced pulmonary inflammation in mice. J Gen Virol 90:1119–1123. doi: 10.1099/vir.0.009308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, Haynes LM. 2010. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J Virol 84:9632–9636. doi: 10.1128/JVI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2:732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, Tripp RA. 2010. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol 84:1148–1157. doi: 10.1128/JVI.01755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jorquera PA, Choi Y, Oakley KE, Powell TJ, Boyd JG, Palath N, Haynes LM, Anderson LJ, Tripp RA. 2013. Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease. PLoS One 8:e74905. doi: 10.1371/journal.pone.0074905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson SM, McNally BA, Ioannidis I, Flano E, Teng MN, Oomens AG, Walsh EE, Peeples ME. 2015. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog 11:e1005318. doi: 10.1371/journal.ppat.1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kauvar LM, Harcourt JL, Haynes LM, Tripp RA. 2010. Therapeutic targeting of respiratory syncytial virus G-protein. Immunotherapy 2:655–661. doi: 10.2217/imt.10.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Mooney AJ, Gabbard JD, Gao X, Xu P, Place RJ, Hogan RJ, Tompkins SM, He B. 2013. Recombinant parainfluenza virus 5 expressing hemagglutinin of influenza A virus H5N1 protected mice against lethal highly pathogenic avian influenza virus H5N1 challenge. J Virol 87:354–362. doi: 10.1128/JVI.02321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang B, Surman S, Amaro-Carambot E, Kabatova B, Mackow N, Lingemann M, Yang L, McLellan JS, Graham BS, Kwong PD, Schaap-Nutt A, Collins PL, Munir S. 2015. Enhanced neutralizing antibody response induced by respiratory syncytial virus prefusion F protein expressed by a vaccine candidate. J Virol 89:9499–9510. doi: 10.1128/JVI.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang GY, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko SY, Todd JP, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Yassine HM, Moin SM, Killikelly AM, Chuang GY, Druz A, Georgiev IS, Rundlet EJ, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Nason MC, Capella C, Peeples ME, Ledgerwood JE, McLellan JS, Kwong PD, Graham BS. 2015. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 7:309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glenn GM, Smith G, Fries L, Raghunandan R, Lu H, Zhou B, Thomas DN, Hickman SP, Kpamegan E, Boddapati S, Piedra PA. 2013. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine 31:524–532. doi: 10.1016/j.vaccine.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Belongia E. 2015. Randomized phase 2 trial of an RSV F nanoparticle vaccine in the elderly: epidemiology and efficacy. RSV Vaccines for the World (RSVVW), The Salk Institute, La Jolla, CA. [Google Scholar]
- 56.Shaw CA, Galarneau JR, Bowenkamp KE, Swanson KA, Palmer GA, Palladino G, Markovits JE, Valiante NM, Dormitzer PR, Otten GR. 2013. The role of non-viral antigens in the cotton rat model of respiratory syncytial virus vaccine-enhanced disease. Vaccine 31:306–312. doi: 10.1016/j.vaccine.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Yoder SM, Zhu Y, Ikizler MR, Wright PF. 2004. Role of complement in neutralization of respiratory syncytial virus. J Med Virol 72:688–694. doi: 10.1002/jmv.20046. [DOI] [PubMed] [Google Scholar]
- 58.Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. 2003. Correlates of immunity to respiratory syncytial virus (RSV) associated hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 21:3479–3482. doi: 10.1016/S0264-410X(03)00355-4. [DOI] [PubMed] [Google Scholar]