Abstract
Globally, ultramafic outcrops are renowned for hosting floras with high levels of endemism, including plants with specialised adaptations such as nickel or manganese hyperaccumulation. Soils derived from ultramafic regoliths are generally nutrient-deficient, have major cation imbalances, and have concomitant high concentrations of potentially phytotoxic trace elements, especially nickel. The South and Southeast Asian region has the largest surface occurrences of ultramafic regoliths in the world, but the geoecology of these outcrops is still poorly studied despite severe conservation threats. Due to the paucity of systematic plant collections in many areas and the lack of georeferenced herbarium records and databased information, it is not possible to determine the distribution of species, levels of endemism, and the species most threatened. However, site-specific studies provide insights to the ultramafic geoecology of several locations in South and Southeast Asia. The geoecology of tropical ultramafic regions differs substantially from those in temperate regions in that the vegetation at lower elevations is generally tall forest with relatively low levels of endemism. On ultramafic mountaintops, where the combined forces of edaphic and climatic factors intersect, obligate ultramafic species and hyperendemics often occur. Forest clearing, agricultural development, mining, and climate change-related stressors have contributed to rapid and unprecedented loss of ultramafic-associated habitats in the region. The geoecology of the large ultramafic outcrops of Indonesia’s Sulawesi, Obi and Halmahera, and many other smaller outcrops in South and Southeast Asia, remains largely unexplored, and should be prioritised for study and conservation.
Keywords: Adaptations, Conservation, Edaphic endemism, Edaphic flora, Extreme environments, Geobotany, Plant–soil relations, Serpentine vegetation, Ultramafic plants, Metal hyperaccumulators
Background
Ultramafic soils are weathered products of lithologies, such as peridotite and serpentinite bedrock, consisting predominantly of ferromagnesian silicate minerals (Cardace et al. 2014; Moores 2011). Ultramafic soils are generally deficient in essential plant mineral nutrients (phosphorus, potassium), have major cation imbalances (low calcium-to-magnesium molar ratios), and have high concentrations of certain phytotoxic elements, including nickel, cobalt and manganese (Brady et al. 2005; Kazakou et al. 2008; O’Dell and Rajakaruna 2011). Tropical ultramafic soils, unlike those in temperate regions (Alexander 2009; Alexander and DuShey 2011), can be strongly weathered due to rainfall intensity and high temperature, and depending on elevation, can develop as laterites (e.g. Ferralsols) (Kruckeberg 2002; Mandal et al. 2015; van der Ent et al. 2013a; Vithanage et al. 2014).
Depauperate ultramafic soils may generate selective pressures promoting speciation and the evolution of ultramafic endemism (Anacker 2014; Kay et al. 2011; Rajakaruna 2004), often leading to distinctive plant communities worldwide (Anacker 2011; Brooks 1987). The biota of ultramafic soils has contributed greatly to the development of ecological and evolutionary theory (Harrison and Rajakaruna 2011; Strauss and Cacho 2013) and to the study of the genetics of adaptation and speciation (Brady et al. 2005; Palm and Van Volkenburgh 2014; von Wettberg and Wright 2011). Ultramafic floras are, however, threatened by deforestation, agricultural development, mining, and climate change-associated stressors (Boyd et al. 2009; Harrison et al. 2009; Rajakaruna and Boyd 2008; Vallano et al. 2012). These threats to ultramafic biota provide opportunities for conservation and restoration-oriented research (Elam et al. 1998; O’Dell and Claassen 2011; Weiss 1999; Whiting et al. 2004; Wolf 2001).
South and Southeast Asia contain several globally significant biodiversity hotspots (Mittermeier et al. 2005), including areas in Indo-Burma, Philippines, Sundaland (western half of the Indo-Malayan archipelago), and Western Ghats and Sri Lanka. The Borneo lowlands is the only ecoregion globally to surpass 10,000 plant species (Kier et al. 2005) and North Borneo is one of the top five biodiversity centres in the world (Barthlott et al. 2007). Despite South and Southeast Asia harboring several important biodiversity hotspots, the influence of edaphic factors on biodiversity is largely unknown (van der Ent et al. 2015a). Compared to research on ultramafic outcrops in temperate and Mediterranean regions (Alexander et al. 2007; Rajakaruna et al. 2009), ultramafic geoecology in this part of the world is also substantially understudied (Proctor 1992, 2003). In terms of tropical regions, most research related to ultramafic floras to date has focussed on New Caledonia (Isnard et al. 2016; Jaffré et al. 2010, 2013; Pillon et al. 2010; Pillon 2012). Although ultramafic outcrops of New Caledonia are of a similar latitude and general climate to South and Southeast Asia, the evolutionary histories of its flora and fauna are distinct. New Caledonia is on the east of the Lydekker’s Line, which separates the eastern edge of Wallacea from the Australian Region (which lies on the Sahul Shelf), marking a distinct change in floristic affinities. In this review, we also exclude New Guinea (Indonesian West Papua and Papua New Guinea) for the same reason, but note that despite the concomitant occurrence of ultramafic outcrops and exceptionally high biodiversity, virtually nothing is known about the ultramafic geoecology of this island. Research on the floristics and ecology of the understudied ultramafics of South and Southeast Asia is critical to provide a comprehensive assessment of the ultramafic geoecology of tropical Asia.
This review examines the literature on the geoecology of ultramafic areas in South and Southeast Asia, covering India, Pakistan, and Sri Lanka to the west, Myanmar and Cambodia to the north, and Malaysia, Indonesia (excluding West Papua), and the Philippines to the east (Fig. 1; Table 1); all of which lie on the western side of Lydekker’s line and share a similar climate. We focus on (i) soil–plant relations, including studies on floristic diversity, soil–plant elemental relations, and soil microbes; (ii) ecological aspects, including studies on vegetation structure and composition and plant endemism; (iii) cross-kingdom interactions, including studies on herbivory, mycorrhizal associations, and invertebrate diversity; (iv) evolutionary aspects; (v) physiology and genetics; (vi) phytotechnologies; and finally, (vii) threats and conservation. We conclude the review by highlighting countries within South and Southeast Asia requiring further study, drawing attention to major gaps in knowledge.
Fig. 1.
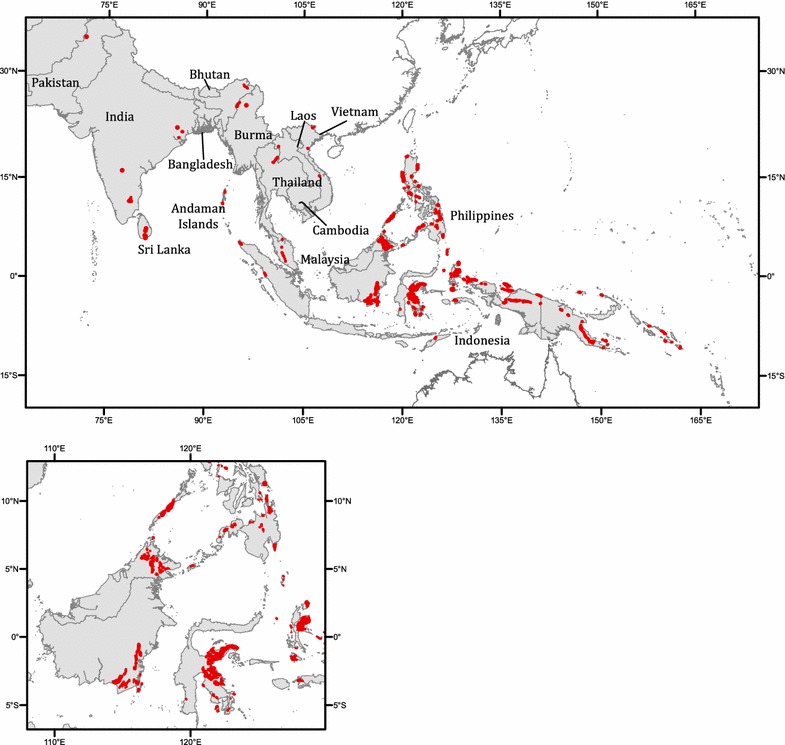
Map of South and Southeast Asia showing the distribution of ultramafic outcrops in the region. Bottom inset is a more detailed outline of ultramafic outcrops in Borneo, Palawan, Mindanao, Sulawesi, and Halmahera. Not all regions of India have complete geologic surveys, and we were unable to locate precise information about ultramafic outcrops in Burma and Laos. The ultramafic outcrop location in Northern Thailand is approximate. The extent of each outcrop shown is not to scale
[Figure compiled with data from Central Energy Resources Team (1999), Datta et al. (2015), Kfayatullah et al. (2001), Shi et al. (2012), Baker et al. (1992), Van der Ent et al. (2013a, 2015a), Tan and Khoo (1993), MacDonald and Barr (1984), Geological Survey of India, Geological and Mineral Maps of States and Regions (http://www.portal.gsi.gov.in/portal/page?_pageid=127,603606&_dad=portal&_schema=PORTAL), and OneGeology Portal (http://portal.onegeology.org/OnegeologyGlobal/)]
Table 1.
A summary of geoecological studies conducted on ultramafic outcrops in South and Southeast Asia
| Country | Area of study | References |
|---|---|---|
| India | Bioremediation of chromite mines and nickel recovery by fungi | Acharya et al. (1998), Biswas et al. (2013), Bohidar et al. (2009), Ghosh and Paul (2015), Mishra et al. (2009) |
| Discovery of nickel hyperaccumulators | Datta et al. (2015) | |
| Forest vegetation structure | Prasad et al. (2007) | |
| Heavy metal leaching into groundwater | Dhakate and Singh (2008) | |
| Heavy metal tolerance in ultramafic soil-associated microbes | Pal et al. (2004, 2005, 2006, 2007), Pal and Paul (2012) | |
| Origin and serpentinization of ultramafic rocks in the Indo-Myanmar subduction zone | Ningthoujam et al. (2012), Soibam et al. (2015) | |
| Phytoremediation of and bioaccumulation of metals from chromite mines | Mohanty et al. (2011, 2012) | |
| Plant–soil elemental relations on a chromite mine | Samantaray et al. (2001) | |
| Remote sensing for detecting and mapping ultramafic vegetation | Chaudhury et al. (2015) | |
| Ultramafic geology, geochemisty, mineral prospecting | Banerjee (1972), Chakraborty and Chakraborty (1984), Bhatta and Ghosh (2014), Mandal et al. (2015), Mitra (1973) | |
| Indonesia | Acidification of serpentinite-derived soils | Fujii et al. (2011) |
| Floristics and plant community structure | Proctor et al. (1994), van Balgooy and Tantra (1986) | |
| Geochemistry, petrography and thermobarometry of the ultramafics | Linthout and Helmers (1994) | |
| Nickel hyperaccumulators and phytotechnologies | Netty et al. (2012), van der Ent et al. (2013a) | |
| Species discovery on ultramafic soils | Cheek (2015) | |
| Malaysia | Copper accumulation in ultramafic plants | van der Ent and Reeves (2015) |
| Discovery of nickel hyperaccumulators | Hoffmann et al. (2003), van der Ent and Mulligan (2015), van der Ent et al. (2013b, 2016b, c) | |
| Ecology of nickel hyperaccumulators: nickel insects | van der Ent et al. (2015f) | |
| Floristics, plant–soil relations, ultramafic endemism | Chen et al. (2014), Fowlie (1985), Peng et al. (2015), Proctor et al. (1988a, b, 1989), Sugau and van der Ent (2016), van der Ent and Wood (2013), Wood and van der Ent (2012), Wong and van der Ent (2014), van der Ent and Wong (2015), van der Ent and Vanijajiva (2014) | |
| Metal localization; nuclear microprobe imaging analyses | Mesjasz-Przybylowicz et al. (2015) | |
| Ultramafic forest vegetation structure, plant ecology, community ecology | Adam (2002), Aiba et al. (2015), Aiba and Kitayama (1999), Brearley (2005), Bruijnzeel et al. (1993), Kitayama (1992), Proctor et al. (1988a, b), Sawada et al. (2015), Tashakor et al. (2013), van der Ent et al. (2015a, b, f, 2016a) | |
| Ultramafic geochemistry | Tashakor et al. (2011, 2013) | |
| Ultramafic plant–other biota interactions | Wells et al. (2011) | |
| Ultramafic-associated insects and soil invertebrates | Chung et al. (2013), Hasegawa et al. (2006), Jones et al. (2010), Leakey and Proctor (1987) | |
| Myanmar | Mineralogy of jadeitite and related rocks, including serpentinites | Shi et al. (2012) |
| Pakistan | Ultramafic geochemistry and soil–plant metal relations | Kfayatullah et al. (2001), Naseem et al. (2009), Shah et al. (2010, 2014) |
| Philippines | Discovery of Ni hyperaccumulators | Baker et al. (1992), Fernando et al. (2014), Gotera et al. (2014), Hoffmann et al. (2003), Quimado et al. (2015) |
| Herbivory on ultramafic soils | Proctor et al. (2000a) | |
| Metal tolerance in mycorrhizal fungi of ultramafic soils | Aggangan et al. (1998) | |
| Phytomining considerations | Fernando et al. (2013) | |
| Species discovery on ultramafic soils | Argent et al. (2007), Fernando and Rodda (2013), Fleischmann et al. (2011) | |
| Ultramafic forest vegetation structure and soil–plant relations | Bruijnzeel (1990), Proctor et al. (1997, 1998, 1999, 2000b) | |
| Ultramafic soil and forest litter invertebrates | Thomas and Proctor (1997) | |
| Sri Lanka | Antimicrobial activities of ultramafic-associated plants | Rajakaruna et al. (2002) |
| Ecotypic differentiation of ultramafic taxa | Chathuranga et al. (2015) | |
| Phyto- and bio-remediation of ultramafic soils; soil remediation | Bandara et al. (2017), Herath et al. (2014), Kumarathilaka et al. (2016), Seneviratne et al. (2016a, b) | |
| Soil–plant relations including floristics, soil–plant elemental relations, discovery of nickel and copper hyperaccumulators | Brooks (1987), Rajakaruna and Baker (2004), Rajakaruna and Bohm (2002), Samithri (2015), Senevirathne et al. (2000), Weerasinghe and Iqbal (2011) | |
| Ultramafic geology and geochemistry | Dissanayaka (1982), Dissanayake and Van Riel (1978), Munasinghe and Dissanayake (1980), Hewawasam et al. (2014), Rajapaksha et al. (2012, 2013), Ranasinghe (1987), Tennakoon et al. (2007), Vithanage et al. (2014) | |
| Southeast Asia: Regional Overviews | Floristics, plant–soil elemental relations, metal accumulators, quantitative bedrock (including ultramafic) geology of Southeast Asia | Brooks (1987), Brooks et al. (1977a, b), Brooks and Wither (1977), Peucker-Ehrenbrink and Miller (2004), Proctor (1992, 2003), Reeves (2003), van der Ent et al. (2015c, d), Wither and Brooks (1977) |
| Thailand | Petrography and geochemistry of ultramafic rocks | Hisada et al. (2004), Macdonald and Barr (1984), Orberger et al. (1995) |
| Vietnam | Heavy metal (Cr, Ni, Co) leaching from chromite mine | Kien et al. (2010) |
| Ultramafic geology | Thanh et al. (2014) |
Information within columns organized in alphabetical order
Soil–plant relations
Ultramafic soils worldwide share a distinct suite of chemical and physical features (Rajakaruna et al. 2009); however, tropical ultramafic soils may differ in elemental content, moisture, organic matter content, and soil pedology (Kierczak et al. 2007; Vithanage et al. 2014), compared to those in temperate and Mediterranean regions (Alexander 2009; Alexander et al. 2007). Table 2 lists key soil properties of ultramafic soils from South and Southeast Asia, focusing on pH, Ca:Mg molar ratio, Ni, Cr, and the major nutrients, P and K. Plants growing on ultramafic soils have to contend with a suite of edaphic stressors, including low nutrient content, high levels of phytotoxic elements, and, at times, water stress (Brady et al. 2005). Plants and soil microbes of ultramafic soils tolerate these edaphic stressors via efficient uptake of essential nutrients, and exclusion of, or conversely accumulation and localization of high concentrations, of certain phytotoxic elements, among other adaptations (see Palm and Van Volkenburgh 2014 for a discussion).
Table 2.
Selected soil chemical properties of ultramafic outcrops in South and Southeast Asia
| Country | Altitude (masl) | pH | Ca:Mg | Ca (exch.) cmol (+) kg−1 | Mg (exch.) cmol (+) kg−1 | K (exch.) cmol (+) kg−1 | K (μg g−1) | P (μg g−1) | P (extract.) μg g−1 | Ni (μg g−1) | Ni (extract.) μg g−1 | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulawesi, Indonesia | – | 5.3–6.3 | 0.9–5.7 | 4.6–13.3 | 11.1–26.2 | 0.05–0.5 | – | – | – | 825–4050 | – | Parry (1985) |
| Sulawesi, Indonesia | 200–300 | 5.8–7.0 | 0.1–1.6 | 0.2–1.6 | 0.5–4.6 | 0.01–0.1 | 3281–6260 | 14.4–237 | 0.23–3.87 | 3730–10,524 | 2.1–30.2 | Van der Ent et al. (2013a) |
| Talaud Island, Indonesia | 60–500 | 6.1–6.4 | 1.6–32 | 0.9–16 | 13.9–27.3 | 0.19–0.38 | – | – | 0.94–6.8 | – | 8.5–37 | Proctor et al. (1994) |
| Sibuyan Island, Philippines | 325–1540 | 4.3–5.5 | 0.3–2.9 | 0.5–3.4 | 0.75–3.64 | 0.04–0.41 | – | – | 0.41–2.07 | – | 1–24 | Proctor et al. (1998) |
| Palawan, Philippines | 50 | 6.8 | 0.24 | 4.27 | 18.1 | 0.32 | – | – | 1.02 | 6900 | 360 | Proctor et al. (1999) |
| Sabah, Malaysia | 400–2900 | 3.8–9.7 | 0.1–136 | 0.003–35 | 0.02–76 | 0.002–0.79 | 0.1–1056 | 4.4–585 | 0.1–32 | 17–9308 | 0.17–442 | Van der Ent (unpublished) |
| Sabah, Malaysia | 180 | 5.3 | 0.62 | 0.86 | 1.38 | 0.17 | – | 201 | 0.34 | 2980 | 10.8 | Brearley (2005) |
| Sabah, Malaysia | 280 | 5.7 | 0.31 | 7.7 | 24.6 | 0.14 | – | 4.1 | – | – | Proctor et al. (1988a) | |
| Ussangoda, Sri Lanka | 15–20 | 5.3–6.2 (4.3–4.9) | 0.6–1.9 (1.4–2.4) | 187–905a (112–212) | 311–456a (60–122) | – | 140–321 (163–350) | – | – | – | 101–151 (29–65) | Weerasinghe and Iqbal (2011), Rajakaruna and Bohm (2002) |
| Ginigalpalessa, Sri Lanka | 70–80 | 5.7–7.4 | 0.1–0.6 | 180–1580a | 2400–3400a | – | 70–230 | – | – | – | 15–180 | Rajakaruna and Bohm (2002) |
| Indikolapalessa, Sri Lanka | 70–80 | 4.7–6.1 | 0.2–2.6 | 395–1863a | 613–2625a | – | 78–1563 | – | – | – | 4–148 | Rajakaruna and Bohm (2002) |
| Yodhagannawa, Sri Lanka | 90–100 | 5.1–5.7 | 0.1–0.2 | 123–138a | 838–1000a | – | 53–75 | – | – | – | 47–79 | Rajakaruna and Bohm (2002) |
| Andaman, India | 50–732 | 6.0–6.8 | – | – | 2300–3600a | – | – | – | – | 3370–9030 | 397–913 | Pal et al. (2007) |
| Andaman, India | 50–732 | 4.4–7.1 | – | – | 2.8–3.9b | – | – | – | – | 244–10,107 | 192–907 | Datta et al. (2015) |
Units are listed under each soil variable except for values with superscripts: a μg g−1; b %
Plant diversity and soil–plant elemental profiles
In Sukinda, India, chromite mine spoils composed of ultramafic substrates have Ni ranging from 187 to 215 µg g−1 and Ca:Mg molar ratios of 1.69–2.27; from which, in total, 113 plant species belonging to 51 families have been recorded (Samantaray et al. 2001). Some species which colonize the substrate exhibit traits typical of plants adapted to ultramafic soils, including sclerophyllous and microphyllous leaves (Brady et al. 2005), but individual plants also show chlorosis, leaf curling, and necrosis.
On the Andaman Islands, India, ultramafic soils with high Ni concentrations (2700–10,100 μg g−1) harbor eight Ni hyperaccumulator plant species belonging to eight different genera and seven different families (Datta et al. 2015). Of these, Dichapetalum gelonioides subsp. andamanicum (Dichapetalaceae) and Rinorea bengalensis (Violaceae) accumulated up to 30,000 μg g−1 Ni. There is substantial potential for using remote sensing tools to examine the vegetation communities on the ultramafics of the Andaman Islands, where the ultramafic outcrops are mostly inaccessible and the vegetation deserves more intensive exploration (Chaudhury et al. 2015).
In Northern Pakistan, the ultramafics of Mingora and Kabal in the Swat region include assemblages of serpentinite, green schist, talc-carbonate schist, and metabasalts in the Mingora–Shangla mélange zone (Shah et al. 2010). Relatively high accumulation of Ni and Cr has been recorded in the plant tissue of Indigofera gerardiana (Fabaceae), Saccharum griffithii (Poaceae), Lycopersicon esculentum (Solanaceae), and Chrysopogon zizanioides (Poaceae) growing in the Kot Parang Ghar mélange zone in the Bucha Area, Pakistan (Shah et al. 2010, 2014).
In Sri Lanka, ultramafic rocks occur along a Precambrian suture zone at the boundary of the Vijayan and Highland Series, metamorphic remnants of two ancient tectonic plates (Dissanayaka 1982; Munasinghe and Dissanayake 1980). The geochemistry of these outcrops, particularly of Ussangoda along the southern coast, has been well-documented (Hewawasam et al. 2014; Rajapaksha et al. 2012, 2013; Tennakoon et al. 2007; Vithanage et al. 2014). The floristics of the ultramafic outcrops of Sri Lanka, especially of Ussangoda, have also received considerable attention (Brooks 1987; Rajakaruna and Baker 2004; Rajakaruna and Bohm 2002; Rajakaruna et al. 2002; Samithri 2015; Senevirathne et al. 2000; Weerasinghe and Iqbal 2011).
Research suggests that Sri Lanka’s ultramafic flora is impoverished with respect to the total number of plant species and percent proportion of endemic species. To date, 67 plant species belonging to 61 genera and 30 families have been identified from Ussangoda (Samithri 2015). Combined with an additional 40 taxa reported from three other sites surveyed by Rajakaruna and Bohm (2002), the total ultramafic flora of Sri Lanka stands at a mere 107 species, compared to many-fold more documented from other sites in Southeast Asia (van der Ent et al. 2015a). Of the species documented from ultramafic soils, only Vernonia zeylanica (Asteraceae) is endemic to Sri Lanka (MOE 2012), although the taxon is not restricted to the substrate.
Soil microbes
Several recent studies, conducted in temperate and Mediterranean regions of the world, explore the roles microbes play in the ecology of ultramafic habitats as well as in the remediation of metal-contaminated soils (Batten et al. 2006; Ma et al. 2015; Schechter and Branco 2014). Although studies on microbial ecology of ultramafic soils in South and Southeast Asia are minimal, Pal et al. (2004, 2005, 2006, 2007) and Pal and Paul (2012) have carried out a series of studies on microbial diversity and ecology of ultramafic soils on the Andaman Islands, India. In one of these studies, Pal et al. (2005) compared physicochemical and microbial properties of ultramafic soils with those from adjacent non-ultramafic localities. The elemental profiles were characteristic of ultramafic soils, with high concentrations of Mg, Ni, Cr, and Co. Furthermore, the ultramafic soils showed low microbial density (6.2–11.3 × 106 colony forming unit/g soil) and activity (1.7–3.5 µg fluorescein/g dry soil/h) relative to non-ultramafic soils. The ultramafic-associated microbial population (including bacteria and fungi) was dominated by bacteria and was more resistant to metals than populations from non-ultramafic soils. Among the ultramafic isolates, 8 and 11 bacteria tolerated >12.0 mM Ni and >16.0 mM Cr, respectively, while six fungal isolates showed a minimum inhibitory concentration (MIC) value >8.0 mM Co. The ultramafic strains also showed co-resistance to Cu, Zn, and Mn. Pal et al. (2007) also examined the soil microflora associated with the rhizosphere of two known Ni hyperaccumulators from the Andaman Islands, R. bengalensis and D. gelonioides subsp. andamanicum. Of the total 123 microbes (99 bacteria and 24 fungi) that were isolated, bacteria were more tolerant of Ni than fungi, showing their greater potential for Ni tolerance.
In a study focusing on medicinal qualities of wild-harvested plants, 32 plant species collected from ultramafic outcrops of Sri Lanka were screened for antimicrobial properties (Rajakaruna et al. 2002). Of these, 29 species belonging to 12 families proved effective against at least one microorganism. Photoactivity was also observed from extracts of 10 species belonging to 10 families. There was no observed correlation between trace element hyperaccumulation (Rajakaruna and Bohm 2002) and antimicrobial activity.
Ecological aspects
Ultramafic outcrops have long-provided model settings for studies on the ecology of plant species and plant communities. Studies range from those investigating aspects of the ecology of edaphically specialized plant populations and plant–plant interactions to those exploring factors and mechanisms driving the assembly of plant communities (see Harrison and Rajakaruna 2011). Compared to other regions of the world, ecological studies on ultramafics of South and Southeast Asia are mostly limited to those examining floristics, plant community structure, and edaphic-floristic associations.
Vegetation structure and composition
Mount Silam in Sabah, Malaysia, has been extensively studied, including the general floristics, forest structure, hydrology and chemical analysis of tree foliage and leaf litter (Proctor et al. 1988a, b, 1989; Bruijnzeel et al. 1993). The study plots on Mount Silam range from 280 to 870 masl in elevation, documenting a broad spectrum of vegetation changes with altitude. The site is extremely species-rich in terms of its tree flora, ranging between 19 species in a 0.04-ha plot at 870 masl to 104 species in a 0.4-ha plot at 480 masl (Proctor 1992). Ultramafic-associated rainforests on Mount Guiting-Guiting, Sibuyan Island, Philippines (Proctor et al. 1998) and those of Mount Silam, Sabah (Proctor et al. 1988a, b) are similar in their soil features (Ni, Ca:Mg, and depth) and lack of stunted lowland forests. At these locations, small-statured forests are associated with higher elevations.
On Mount Bloomfield in the western Philippines (Palawan), Proctor et al. (1999) described a very different forest structure from those of Mount Silam and Mount Guiting-Guiting. The soil depths on Mount Bloomfield are much less compared to these other sites; Bruijnzeel (1990) suggested that drought in the shallow soils is a major cause of forest stunting on ultramafics, perhaps in association with fire (Proctor et al. 1997). Mount Bloomfield lacks tall forests and instead is characterised by trees less than 18 m tall. No statistical relationship could be established between tree height and soil chemistry, although Proctor et al. (1999) did find a direct proportional relationship between maximum tree height and soil water retention. The authors indirectly linked soil water to fire susceptibility in establishing the particular vegetation pattern on Mount Bloomfield, one that superficially resembles fire-dependent vegetation of New Caledonia.
Proctor et al. (2000a, b) compared vegetation on ultramafic soils to those on non-ultramafic (greywacke-derived) soils in Palawan and found that the species richness and diversity of ultramafic and greywacke sites were similar. However, the individual species and familial composition were rather different, with only members of the Saxifragaceae occurring on both ultramafic and greywacke soils. Trees on the serpentinized peridotite had a high proportion of microphyllous leaves, which is not a general feature of ultramafic forests in the region. Differences in water supply and fire frequencies, in combination with edaphic difference, may contribute to the distinct forests overlying these soils (Proctor et al. 1999, 2000a, b).
Sulawesi and Halmahera in Indonesia have 15,400 and 8000 km2 of ultramafic outcrops, respectively (van der Ent et al. 2013a). Lateritic soils overlaying the bedrock harbor both sclerophyllous ultramafic vegetation and more cryptic tropical rainforest, which are nonetheless inhabited by a high proportion of endemic flora. Proctor et al. (1994) examined the ultramafic soil–plant relations of Mount Piapi on Karakelong part of the Talaud Islands, North Sulawesi, Indonesia and reported that the short stature of the local vegetation is a result of low water-holding capacity of the soil, while the unusual species assemblage likely results from the soil chemistry typical of ultramafic soils. They also documented an undescribed Ni-hyperaccumulating species of Rinorea from their study site.
Kinabalu Park, Sabah, one of the world’s most species-rich hotspots with more than 5000 plant species recorded in an area of just 1200 km2, is also home to extensive ultramafic exposures (van der Ent et al. 2014). Plant diversity on ultramafics of the Park decreases with elevation, with a mid-elevation (circum 1500 masl) ‘hump’ occurring for some plant groups (Orchidaceae, Pteridophytes) resulting from the presence of cloud forests (van der Ent et al. 2016a). Six main vegetation classes with associated soil types are described by van der Ent et al. (2016a), including Sub-Alpine Scrub and Graminoid Scrub, both associated with Hypermagnesic Cambisols (‘hypermagnesian soils’), Montane Cloud Forest, associated with Cambisols often with accumulation of humus, Mixed Dipterocarp Forest, associated with deep Ferralsols (‘laterites’), and Pioneer Casuarina Scrub and Mature Mixed Casuarina Forest, both associated with Hypermagnesic Leptosols. The ‘adverse’ soil chemistry exacerbates vegetation stunting but no clear correlation between elevation, soil chemistry and plant diversity was found, as some of the most ‘adverse’ soils on the summit of the entirely ultramafic Mount Tambuyukon (2359–2534 masl) had up to 132 species per 250 m2 (van der Ent et al. 2016a).
Samithri (2015) examined the vegetation community composition and patterns at Ussangoda, Sri Lanka’s most intensively studied ultramafic outcrop. She found a higher diversity of plant species in ‘forest islands’ compared to the extensive ‘plains’ characterizing the site (Fig. 2c). Although the plains make up over 90% of the outcrop area, they only harbor 18 herbaceous species belonging to 17 genera and 11 families compared to 49 tree, shrub, herb and climber species belonging to 44 genera and 27 families found in the ‘forest islands.’ Although the soil chemical features did not differ significantly between sites on the ‘plains’ versus those in the ‘forest islands,’ soil features such as depth and resulting water holding capacity in ‘forest islands’ may favor the growth of a wide range of species than on the exposed and shallow soils of the ‘plains.’
Fig. 2.
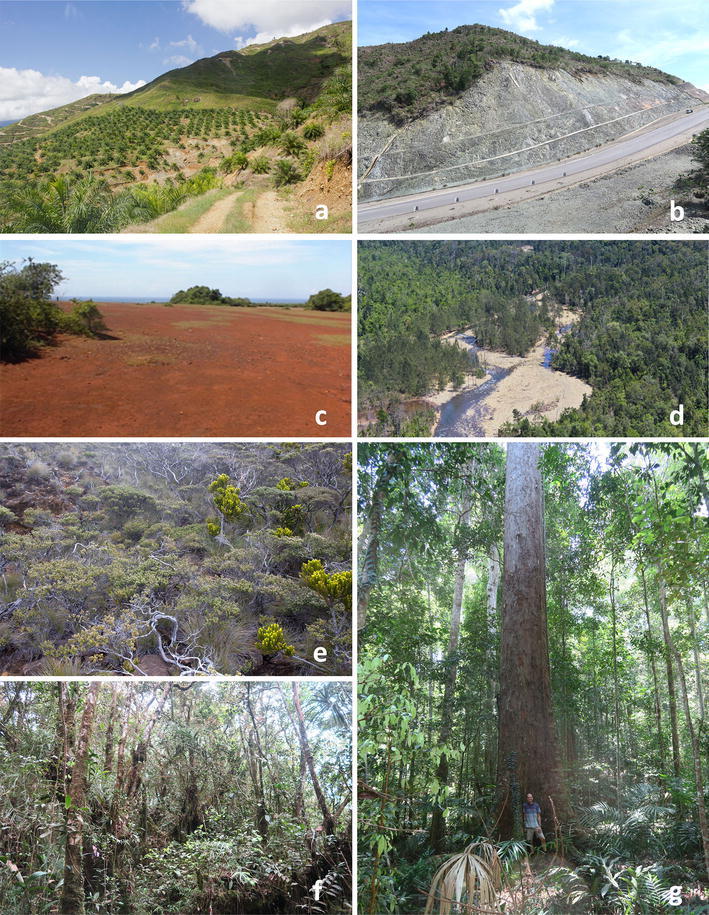
Ultramafic outcrops and vegetation in South and Southeast Asia: a Oil palm estate in Sabah, Malaysia on eroding ultramafic soils. b Road cut through strongly serpentinised bedrock in Sabah, Malaysia. c Bare red Ferralsols at Ussangoda in Sri Lanka. d River flowing through an ultramafic outcrop in Halmahera, Indonesia. e Extremely stunted sub-alpine vegetation on ultramafic soils in Kinabalu park, Malaysia. f Montane cloud forest on ultramafic soils on Mount Silam, Malaysia. g Exceptionally tall lowland mixed dipterocarp forest on ultramafic soils in Sabah, Malaysia
(all images are by A. van der Ent, except c by Y.A.S. Samithri and g by Isabella Zelano)
Studies on bryophytes, lichens, and epiphytes on ultramafic outcrops are sparse worldwide (but see Boyd et al. 2009; Briscoe et al. 2009; Favero-Longo et al. 2004; Rajakaruna et al. 2012). In South and Southeast Asia, such studies are mostly non-existent. However, one study from the Philippines (Proctor et al. 2000b) documents epiphytic plants on trees of ultramafic and adjacent greywacke soils. The trees on the greywacke had fewer lianas and much less bole bryophyte cover than those on the serpentinized peridotite. Forty-one percent of trees on peridotite had >10% bryophyte cover, while none of the trees on greywacke soils had >10% bryophyte cover. The greywacke soils also hosted significantly higher densities of ferns, Cyperaceae spp., rattans (Arecaceae: Calamoideae), and Pandanaceae spp. compared to ultramafic soils, while ultramafic soils harbored significantly more herbaceous and bamboo (Poaceae: Bambusoideae) species. Floristic differences between the sites were attributed to differences in geochemistry, hydrology, and fire-frequencies (Proctor et al. 1999, 2000b).
Plant endemism
Ultramafic soils, often with disproportionately high numbers of endemic species (Anacker 2011), are prime settings to explore the nature of edaphic endemism (Rajakaruna 2004). In New Caledonia, 2150 species occur on ultramafic soils of which 83% are restricted to these soils (Jaffré 1992; Jaffré and L’Huillier 2010), whereas in Cuba, 920 species (approximately one-third of the taxa endemic to Cuba) are found exclusively on ultramafic soils (Borhidi 1992). Similar restrictions and notable floristic associations are also found on ultramafic outcrops of Mediterranean climates (including California; Alexander et al. 2007; Safford et al. 2005), as well as in South Africa/Zimbabwe and Australia (Anacker 2011; Brooks 1987).
The restriction of habitat specialists to ultramafic soils is generally considered a consequence of their inherent slow growth rates that leads them to being outcompeted on more favorable soils (Anacker 2014; Anacker et al. 2011; Kay et al. 2011). Although some growth experiments have shown that habitat specialists can grow faster on more nutrient-rich soils (Kruckeberg 1954), species from the ultramafic maquis in New Caledonia have inherently slow growth, albeit becoming larger under more fertile conditions (Jaffré 1980). Table 3 lists the countries within the South and Southeast Asian region with ultramafic floras, including the number of ultramafic-associated species documented and the number of ultramafic endemics described in each country.
Table 3.
Surface area covered by ultramafic rocks, total number of species in the regional flora, number of ultramafic-associated species, and number of ultramafic endemic species along with percent ultramafic endemism in the region’s flora for a number of global hotspots for ultramafic endemism and for regions within South and Southeast Asia
| Region | Surface area of ultramafics (km2) | Total number of vascular plant species in the flora | Number of ultramafic-associated species | Number of ultramafic endemic species (% ultramafic endemism) | References |
|---|---|---|---|---|---|
| New Caledonia | 5470 | 3371 | 2150 | 1785 (83) | Jaffré (1992), van der Ent et al. (2015d), Isnard et al. (2016) |
| California, United States | ~6000 | 5271 | 492 | 246 (4.7) | Anacker et al. (2011), Burge et al. (2016), Jepson Flora Project (2016), Safford et al. (2005) |
| Queensland, Australia | 818 | 8500 | 553 | 18 (0.2) | Batianoff and Specht (1992), Batianoff and Singh (2001) |
| Western Australia, Australia | 5654 | ~12,000 | 1355 | 14 (0.12) | Van der Ent et al. (2015d) |
| New Zealand | ~310 | 2418 | ~800 | 15 (0.6) | Lee (1992), New Zealand Plant Conservation Network (2016), Van der Ent et al. (2015d) |
| Cuba | 5300 | 6375 | – | 920 (14) | Reeves et al. (1999) |
| Zimbabwe | ~3000 | 6385 | 322 | 322 (5) | Wild (1965), Proctor and Cole (1992) |
| Sabah | ~3500 | ~8000 | 4252 | 347 (4.3) | Van der Ent et al. (2015a) |
| Sulawesi, Indonesia | ~15,400 | ~5000 | na | na | Van der Ent et al. (2013a) |
| Palawan, Philippines | ~3000 | 1522 | na | na | Davis and Heywood (1995) |
| Sri Lanka | 7 | 3492 | 107 | 0 | MOE (2012), Rajakaruna and Bohm (2002), Samithri (2015) |
In Sabah, Malaysia, Borneodendron aenigmaticum (Euphorbiaceae) is one of the few large rainforest trees restricted to ultramafic soils (Proctor et al. 1988a). Van der Ent and Wood (2012, 2013) describing orchid species associated with ultramafics in Sabah, Malaysia, documented many endemic species (Orchidaceae) restricted to narrow valleys with steep slopes, dominated by Gymnostoma sumatranum (Casuarinaceae) and Ceuthostoma terminale (Casuarinaceae). Further, van der Ent et al. (2015b) show habitat partitioning among ultramafic endemic Nepenthes species (Nepenthaceae) of Mount Kinabalu and Mount Tambuyukon, with distinct habitats and elevation ranges for the different Nepenthes taxa. Eriobotrya balgooyi (Rosaceae) was described as a new species restricted to ultramafic soils on a hill near the eastern ridge of Mount Kinabalu and on the nearby Mount Tambuyukon in Sabah, Malaysia (Wong and van der Ent 2014). The importance of scientific exploration of the ultramafics of Southeast Asia cannot be stressed enough; a survey on the ultramafic Mount Guiting-Guiting, Philippines (Argent et al. 2007) also led to the discovery of a new species, Lobelia proctorii (Campanulaceae).
Sri Lanka’s ultramafic outcrops and their flora, compared with ultramafic floras of Southeast Asia and Australia-Pacific region (van der Ent et al. 2015c, d), have received relatively little attention partly because they do not harbor any endemic species nor many metal hyperaccumulators (Chathuranga et al. 2015). All species so far documented from the ultramafic outcrops of Sri Lanka also have non-ultramafic populations, and it is unclear whether the ultramafic populations are physiologically distinct (i.e. ecotypes).
Cross-kingdom interactions
Edaphically stressful substrates, like ultramafic soils, present plants with challenges that differ from more ‘benign’ substrates. Growing under such stress, ultramafic plants will likely encounter other organisms (herbivores, pathogens, beneficial insects and pathogens) that are also able to tolerate some of the same stressors affecting the plants (Strauss and Boyd 2011). There is evidence to suggest that pressures from enemies will be greater on edaphically stressful substrates than on normal soils (Strauss and Cacho 2013). Additionally, the enriched concentrations of certain trace elements, such as nickel, found in ultramafic soils may provide plants with opportunities for elemental defence (Boyd 2014). A significant body of research exists on plant–other biota interactions on ultramafic soils from temperate and Mediterranean climes, including studies on elemental defence (Boyd 2009), defence against pathogens (Hörger et al. 2013; Springer 2009), herbivory (Lau et al. 2008), mycorrhizal associations (Southworth et al. 2014), plant–pollinator interactions (Meindl et al. 2013; Wolf and Thorp 2011), and seed dispersal (Spasojevic et al. 2014). However, such studies are minimal in tropical Asia.
Herbivory
In the only known published study on herbivory in ultramafic ecosystems in the region, Proctor et al. (2000a) found that the percentage of leaf area consumed was similar for plants found on and off of ultramafic soils on Mount Bloomfield, Palawan (Philippines), although the actual leaf area consumed was greater for the ultramafic forest as it had plants with larger leaves. There was no relationship between herbivory and leaf elemental chemistry; even the metal-accumulating taxa were attacked by herbivores. Proctor et al. (2000a) speculate that the gall-forming and leaf-mining insects must be tolerant of nickel as they spend their entire juvenile stage in the leaf tissue.
Recent work by van der Ent and Mulligan (2015) show Ni accumulation in various parts of Ni hyperaccumulator plants occurring in Sabah, Malaysia, with the highest Ni concentration recorded in the phloem tissue (up to 7.9% in R. bengalensis) and phloem sap (up to 16.9% in Phyllanthus balgooyi); Ni localization in phloem tissue is visible by the bright green coloration in field-collected samples (Fig. 3b, f). The discovery of toxic levels of Ni in the phloem tissue suggests that the increased Ni in the phloem provides a defence against phloem-sap feeding insects, pathogens, and other herbivores (Boyd 2014; Hanson et al. 2004). However, Geometric moth larvae (Erebidae: Erebinae:Poaphilini) were found feeding on the leaves of the Ni hyperaccumulator P. balgooyi, furthermore aphids were found feeding on Phyllanthus cf. securinegioides (van der Ent et al. 2015f).
Fig. 3.

Nickel hyperaccumulator plants in South and Southeast Asia: a Phyllanthus balgooyi (Phyllanthaceae) in Sabah, Malaysia is a small understorey tree. b Phloem sap exuding from Phyllanthus balgooyi contains up to 20 wt% Ni. c Knema matanensis (Myristicaceae) in Sulawesi, Indonesia; d Rinorea bengalensis (Violaceae) can be locally dominant in lowland forest, in Sabah, Malaysia. e Dichapetalum gelonioides subsp. tuberculatum (Dichapetalaceae) from Mount Silam, Malaysia. f Main stem of Dichapetalum gelonioides subsp. tuberculatum showing its Ni-rich phloem tissue with colorimetric response in dimethylglyoxime test-paper. g Sarcotheca celebica (Oxalidaceae) from Sulawesi, Indonesia. h Psychotria sarmentosa (Rubiaceae) is the only known Ni hyperaccumulator in South and Southeast Asia that is a climber
(all images are by A. van der Ent, except c, g are by A. Tjoa, Tadulako University, Indonesia)
Mycorrhizal associations
Pisolithus tinctorius (Sclerodermataceae), an ectomycorrhizal fungus, is found in the rhizosphere of Eucalyptus urophylla (Myrtaceae) from ultramafic soils in the Philippines, New Caledonia, and Western Australia (Aggangan et al. 1998). Pisolithus tinctorius was cultured with E. urophylla to determine the effects of Cr and Ni on the fungal growth rate. The fungus concentrates metals in the extramatrical hyphae and extra-hyphal slime and is particularly tolerant of high concentrations of Ni and Cr. There was geographic variation in terms of metal tolerance in the fungus, with the New Caledonian isolate outperforming both the Australian and the Philippines isolates. The Philippines isolate grew well on agar in the presence of Cr up to 2000 µmol L−1 and Ni up to 200 µmol L−1, but formed fewer mycorrhizae in vitro and in vivo than its counterparts from New Caledonia and Western Australia.
Soil invertebrates
A study comparing termite assemblages on ultramafic-derived forest soils to those on non-ultramafic soils in Borneo, Malaysia shows that ultramafic sites have low species density (<35%), low relative abundance (<30%), a virtual absence of soil-feeders, significantly fewer wood-feeders, and a near-absence of species of Rhinotermitidae, Amitermes-group, Termes-group, Pericapritermes-group and Oriensubulitermes-group (Jones et al. 2010). The authors suggest that metal toxicity, high pH disrupting gut physiology, metal poisoning of essential microbiota in the termite gut, and metal bioaccumulation by gut microbes with subsequent poisoning of the termite host, as possible reasons for the depauperate termite communities on ultramafic soils.
A study on the patterns of Oribatid mite communities in relation to elevation and geology on the slopes of Mount Kinabalu, Sabah, Malaysia, shows that the density and morphospecies richness of Oribatid mites are greater in non-ultramafic soils than in the ultramafic soils at each of the same elevations (Hasegawa et al. 2006). The density and richness of Oribatid mites decreased with elevation on both substrates, but the effects of elevation on their density in non-ultramafic soil were less significant than in the ultramafic substrate.
An investigation of the invertebrate communities in forest litter and soil on Mount Guiting-Guiting in the Philippines, shows that ultramafic soils, even at higher elevations, were not poor in soil invertebrates, including Oligochaeta (Thomas and Proctor 1997), similar to earlier findings on Mount Silam, Sabah (Leakey and Proctor 1987).
Physiology and genetics
There is considerable interest in understanding the physiology and the underlying genetic basis for traits conferring adaptation to ultramafic soils (Bratteler et al. 2006; Palm and Van Volkenburgh 2014; von Wettberg and Wright 2011; Wu et al. 2008). The advent of novel molecular methods has provided unique approaches to exploring stress tolerance (Selby et al. 2014; Visioli and Marmiroli 2013) and ultramafic-associated plants will continue to provide model systems for such investigations (Arnold et al. 2016; von Wettberg et al. 2014). While these advances have not yet been made in tropical Asia, the region provides numerous opportunities for investigating the physiological and genetic aspects of adaptation to ultramafic soils. To date, much of the research in South and Southeast Asia has focused on discovering new hyperaccumulating plant species from ultramafic soils in the region.
Trace element hyperaccumulation
Plants found on ultramafic soils have long-been recognized as model systems to explore trace element hyperaccumulation (Gall and Rajakaruna 2013). There are well over 450 Ni hyperaccumulator plant species globally, all occurring on ultramafic soils (van der Ent et al. 2013c). Ultramafic associated plants are known to hyperaccumulate cobalt (Co) and Cu (>300 μg g−1 in their dry leaf tissue), and Ni (>1000 μg g−1 in their dry leaf tissue). For recent reviews of trace element hyperaccumulation, see Reeves (2003), Krämer (2010), van der Ent et al. (2013c, 2015e) and Pollard et al. (2014). Table 4 lists documented hyperaccumulator plants from the South and Southeast Asia region, listing the element hyperaccumulated, country of discovery, and relevant references. Figure 3 documents some of the nickel hyperaccumulator plants discovered from ultramafic soils in parts of South and Southeast Asia.
Table 4.
Unusual foliar elemental accumulation (Ni, Co, Cu, Mn or Zn—maximum recorded values in μg g−1) in plants from South and Southeast Asia
| Family | Species | Life-form | Locality | Ni | Cu | Co | Mn | Zn | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Acanthaceae | Daedalacanthus suffruticosus | Shrub | India | 1235–1862 | – | – | – | – | Datta et al. (2015) |
| Acanthaceae | Ptyssiglottis cf. fusca | Herb | Sabah, Malaysia | 1160 | – | – | – | Van der Ent et al. (2015f) | |
| Amaranthaceae | Aerva scandens | Herb | Sulawesi, Indonesia | – | 395 | – | – | – | Brooks et al. (1978) |
| Amaranthaceae | Cyathula prostrata | Herb | Sulawesi, Indonesia | – | 553 | – | – | – | Brooks et al. (1978) |
| Apocynaceae | Calotropis gigantea | Climber | Sri Lanka | – | 583 | – | – | – | Rajakaruna and Bohm (2002) |
| Apocynaceae | Carissa spinarum | Climber | Sri Lanka | – | 702 | – | – | – | Rajakaruna and Bohm (2002) |
| Asteraceae | Vernonia actaea | Herb | Sulawesi, Indonesia | – | 300 | – | – | – | Brooks et al. (1978) |
| Asteraceae | Vernonia cinerea | Herb | Sri Lanka | 1026 | – | – | – | – | Samithri (2015) |
| Chrysobalanaceae | Licania splendens | Shrub | Zambales, Philippines | 2728 | – | – | – | – | Fernando et al. (2013) |
| Convolvulaceae | Evolvulus alsinoides | Herb | Sri Lanka | 1478 | – | – | – | Rajakaruna and Bohm (2002) | |
| Dichapetalaceae | Dichapetalum gelonioides subsp. pilosum | Climber/shrub | Sabah, Malaysia | – | – | – | – | 7000 | Baker et al. (1992) |
| Dichapetalaceae | Dichapetalum gelonioides subsp. sumatranum | Shrub | SE Asia | – | – | – | – | 30,000 | Baker et al. (1992) |
| Dichapetalaceae | Dichapetalum geloniodes subsp. tuberculatum | Shrub | Malaysia and Philippines | 26,600 | – | – | – | – | Baker et al. (1992) |
| Dichapetalaceae | Dichapetalum gelonioides subsp. andamanicum | Shrub | Andaman Islands, India | 3160; 9740–36,100 | – | – | – | – | Brooks (1987), Datta et al. (2015) |
| Dipterocarpaceae | Shorea tenuiramulosa | Tree | Sabah, Malaysia | 1790 | – | – | – | – | Proctor et al. (1988a, b), Van der Ent et al. (2015a, b, c, d, e, f, g) |
| Euphorbiaceae | Croton bonplandianus | Tree | Sri Lanka | – | 2163 | – | – | – | Rajakaruna and Bohm (2002) |
| Euphorbiaceae | Euphorbia thymifolia | Shrub | Sri Lanka | 1074 | – | – | – | – | Samithri (2015) |
| Fabaceae | Cassia auriculata | Shrub | Sri Lanka | – | 885 | – | – | – | Rajakaruna and Bohm (2002) |
| Fabaceae | Dalbergia beccarii | Shrub | Sabah, Malaysia | 2623 | – | – | – | – | Van der Ent and Reeves (2015) |
| Fabaceae | Tephrosia villosa | Herb | Sri Lanka | – | 1858 | – | – | – | Rajakaruna and Bohm (2002) |
| Lamiaceae | Clerodendrum infortunatum | Herb | Sri Lanka | – | 2278 | – | – | – | Rajakaruna and Bohm (2002) |
| Lamiaceae | Coleus scutellarioides | Herb | Sri Lanka | – | 500 | – | – | – | Brooks et al. (1978) |
| Lamiaceae | Ocimum tenuiflorum | Herb | Sri Lanka | – | 2266 | – | – | – | Rajakaruna and Bohm (2002) |
| Loganiaceae | Strychnos andamanensis | Climber | India | 2606–6893 | – | – | – | – | Datta et al. (2015) |
| Loganiaceae | Strychnos minor | Climber | India | 3220–10,214 | – | – | – | – | Datta et al. (2015) |
| Loganiaceae | Strychnos wallichiana | Climber | India | 2924–15,630 | – | – | – | – | Datta et al. (2015) |
| Malvaceae | Abutilon indicum | Shrub | Sri Lanka | – | 915 | – | – | – | Rajakaruna and Bohm (2002) |
| Malvaceae | Waltheria indica | Shrub | Sri Lanka | – | 1504 | – | – | – | Rajakaruna and Bohm (2002) |
| Meliaceae | Walsura monophylla | Tree | Malaysia and Philippines | 7090 | – | – | – | – | Baker et al. (1992) |
| Meliaceae | Walsura pinnata | Tree | SE Asia | 4580 | – | – | – | – | Van der Ent et al. (2015f) |
| Monimiaceae | Kibara coriacea | Tree | SE Asia | 5840 | – | – | – | – | Van der Ent et al. (2015f) |
| Moraceae | Ficus brevicuspis | Tree | India | 28,322–30,564 | – | – | – | – | Datta et al. (2015) |
| Myristicaceae | Knema matanensis | Tree | Indonesia | 5000 | – | – | – | – | Van der Ent et al. (2013a) |
| Myristicaceae | Myristica laurifolia var. bifurcata | Tree | Indonesia | 1100 | – | – | – | Wither and Brooks (1977) | |
| Myrtaceae | Decaspermum blancoi | Shrub | Zambales, Philippines | 1996 | – | – | – | Fernando et al. (2013) | |
| Ochnaceae | Brackenridgea palustris subsp. foxworthyi | Shrub | Philippines | 7600 | – | – | – | – | Baker et al. (1992) |
| Ochnaceae | Brackenridgea palustris subsp. kjellbergii | Tree | Sulawesi, Indonesia | 1440 | – | – | – | – | Reeves (2003) |
| Ochnaceae | Ochna integerrima | Tree | India | 2465–5210 | – | – | – | – | Datta et al. (2015) |
| Olacaceae | Olax imbricata | Tree | Sri Lanka | 1082 | – | – | – | – | Samithri (2015) |
| Oxalidaceae | Sarcotheca celebica | Tree | Indonesia | 1000 | – | – | – | – | Van der Ent et al. (2013a, b, c) |
| Papilionaceae | Cassia sophera | Shrub | Sulawesi, Indonesia | – | 333 | – | – | – | Brooks et al. (1978) |
| Phyllanthaceae | Actephila alanbakeri | Shrub | Sabah, Malaysia | 11,520 | – | – | – | – | Van der Ent et al. (2016c) |
| Phyllanthaceae | Aporosa chalarocarpa | Tree | SE Asia | 1560 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Baccaurea lanceolata | Tree | SE Asia | 1450 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Breynia cernua | Shrub | Zambales, Philippines | 3573 | – | – | – | – | Gotera et al. (2014) |
| Phyllanthaceae | Cleistanthus sp. 1 | Tree | Sabah, Malaysia | 2110 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Glochidion aff. acustylum | Tree | Sulawesi, Indonesia | 6060 | – | – | – | – | Reeves (2003) |
| Phyllanthaceae | Glochidion brunneum | Tree | SE Asia | 6200 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Glochidion cf. lanceisepalum | Tree | Sabah, Malaysia | 3270 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Glochidion cf. mindorense | Tree | SE Asia | 2280 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Glochidion cf. rubrum | Tree | SE Asia | 7000 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Glochidion cf. sericeum | Tree | Sabah, Malaysia | 2190 | – | 1310 | – | – | Van der Ent et al. (2015f); Van der Ent (unpublished) |
| Phyllanthaceae | Glochidion sp. ‘bambangan’ | Tree | Sabah, Malaysia | 16,700 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Glochidion sp. ‘nalumad’ | Tree | Sabah, Malaysia | 9000 | – | – | – | – | Van der Ent et al. (2015f) |
| Phyllanthaceae | Phyllanthus balgooyi | Tree | Malaysia and Philippines | 8610 | – | – | – | – | Hoffmann et al. (2003), Mesjasz-Przybylowicz et al. (2015) |
| Phyllanthaceae | Phyllanthus erythrotrichus | Shrub | Zambales, Philippines | 17,520 | – | – | – | – | Quimado et al. (2015) |
| Phyllanthaceae | Phyllanthus securinegioides | Shrub | Sabah, Malaysia | 23,300 | – | – | – | – | Baker et al. (1992), Van der Ent et al. (2015f) |
| Phyllanthaceae | Phyllanthus sp. undet. | Shrub | Sri Lanka | – | 821 | – | – | – | Rajakaruna and Bohm (2002) |
| Piperaceae | Peperomia pellucida | Shrub | Sulawesi, Indonesia | – | 300 | – | – | – | Brooks et al. (1978) |
| Rubiaceae | Psychotria cf. gracilis | – | Sabah, Malaysia | 10,590 | – | – | – | – | Reeves (2003) |
| Rubiaceae | Psychotria sarmentosa | Climber | Sabah, Malaysia | 24,200 | – | – | – | – | Van der Ent et al. (2015f) |
| Rubiaceae | Psychotria sp. undet. | – | Sulawesi, Indonesia | 1820 | – | – | – | – | Reeves (2003) |
| Rubiaceae | Urophyllum cf. macrophyllum | Herb | Sabah, Malaysia | – | – | – | 10,464 | – | Van der Ent and Reeves (2015) |
| Salicaceae | Flacourtia indica | Tree | Sri Lanka | 1165 | – | – | – | – | Samithri (2015) |
| Salicaceae | Flacourtia kinabaluensis | Tree | Sabah, Malaysia | 7280 | – | – | – | – | Van der Ent et al. (2015f) |
| Salicaceae | Xylosma luzonensis | Tree | SE Asia | 5360 | – | – | – | – | Van der Ent et al. (2015f) |
| Sapindaceae | Mischocarpus sundaicus | Tree | SE Asia | 4425 | – | – | – | Van der Ent et al. (2015f) | |
| Sapotaceae | Planchonella obovata | Tree | Zambales, Philippines | 1005 | – | – | – | – | Fernando et al. (2013) |
| Sapotaceae | Planchonella oxyedra | Tree | Obi Island, Indonesia | 19,600 | – | – | – | – | Wither and Brooks (1977) |
| Tiliaceae | Trichospermum kjellbergii | Tree | Indonesia | 3770 | – | – | – | – | Wither and Brooks (1977) |
| Urticaceae | Laportea ruderalis | Herb | Sulawesi, Indonesia | – | 600 | – | – | – | Brooks et al. (1978) |
| Verbenaceae | Callicarpa sp. undet. | Shrub | Zambales, Philippines | 1052 | – | – | – | – | Fernando et al. (2013) |
| Violaceae | Hybanthus enneaspermus | Shrub | Sri Lanka | 1862 | – | – | – | – | Rajakaruna and Bohm (2002) |
| Violaceae | Rinorea bengalensis | Tree | S & SE Asia and Australia | 2723–18,840 | – | – | – | – | Brooks and Wither (1977); Datta et al. (2015) |
| Violaceae | Rinorea javanica | Tree | SE Asia | 9680 | – | – | – | – | Brooks and Wither (1977) |
| Violaceae | Rinorea niccolifera | Shrub | Luzon Island, Philippines | 18,388 | – | – | – | – | Fernando et al. (2014) |
| Violaceae | Rinorea sp. nov. | Shrub | Talaud Island, Indonesia | 1830 | – | – | – | – | Proctor et al. (1994) |
In one of the earliest geoecological studies of the region, Wither and Brooks (1977) and Brooks et al. (1977b) analysed herbarium samples of plants originating from Obi Island (North Moluccas). They identified Myristica laurifolia var. bifurcata (Myristicaceae), Planchonella oxyhedra (Sapotaceae), and Trichospermum kjellbergii (Malvaceae) as hyperaccumulators of Ni. The authors then analysed Ni concentrations in herbarium specimens of T. kjellbergii and P. oxyhedra from throughout their range in Southeast Asia and Oceania. The findings confirmed previously known ultramafic areas in Sulawesi and Indonesian New Guinea, as well as one in Ambon (South Moluccas) which was not documented on geological maps. Their suspicions about the substrate were confirmed by a 1994 geological study that mapped peridotite and serpentinite outcrops in both Ambon and Seram (Linthout and Helmers 1994). A more recent study in Soroako, Sulawesi, examined leaf tissue from 23 plant species from former Ni mining sites in search of hyperaccumulator plants (Netty et al. 2012). As a result, Sarcotheca celebica (Oxalidaceae) was confirmed as a Ni hyperaccumulator, with 1039 µg g−1 Ni in dry leaf tissue.
In a study describing the general influence of the ultramafic geochemistry on growth patterns of plants overlying two Malaysian massifs, the Bukit Rokan and Petasih along the Bentong-Raub suture zone on the Peninsula, Tashakor et al. (2013) document that the serpentinite of the area is strongly weathered and gives rise to characteristic red lateritic soils (Ferralsols). They point out that the greatest physiological stress experienced by plants growing on ultramafic soils is due to the low Ca: Mg ratio and the generally low available nutrients, and not due to potentially phytotoxic elements present in the soil, which are, for the most part, not in a plant-available form.
In a study of the Bela Ophiolite in the Wadh area of Balochistan, Pakistan, Naseem et al. (2009) discovered Pteropyrum olivieri (Polygonaceae) in a localized population over ultramafic soils. Although the plant did not hyperaccumulate, it had moderate concentrations of Ni, Co, and Cr in its tissues, typical of most plants growing on ultramafic soils.
The ultramafics of Malaysia and Indonesia have received considerable attention with regard to taxa with high metal-accumulating behavior. A chemical analysis of leaf litter from trees growing on ultramafics in Sabah, Malaysia (Proctor et al. 1989) confirmed that trees grow at low foliar nutrient concentrations and can concentrate Ca in their leaf tissue. Leaf litter showed an average Ca:Mg ratio as well as a high level of Ni, suggesting that senescence may act as a way of excreting excess Ni. From analysis of leaf litter, they found that Shorea tenuiramulosa (Dipterocarpaceae) and an unidentified species of Syzygium (Myrtaceae) accumulated Ni and Mn, respectively, with 1000 µg g−1 Ni and 13,700 µg g−1 Mn dry leaf weight. Proctor et al. (1994) also reported a yet to be named Ni-hyperaccumulating species of Rinorea from Mt Piapi on Karakelong Island, northeast of Sulawesi in Indonesia with up to 1830 µg g−1 foliar Ni.
In an analysis of 51 herbarium specimens from both Malaysia and Indonesia, including from Mount Kinabalu (Sabah), Soroako and Malili (Sulawesi) and Yapen Island, Reeves (2003) found high Ni values in Phyllanthus insulae-japen (Phyllanthaceae), which had been collected once in 1961, and in R. bengalensis, Brackenridgea palustris subsp. kjellbergii (Ochnaceae), Glochidion spp. (Phyllanthaceae), and two species of Psychotria (Rubiaceae) which could not be identified to species level. One ultramafic subspecies of D. gelonioides was identified as a Ni hyperaccumulator (subsp. tuberculatum), whereas another subspecies was confirmed as a Zn hyperaccumulator on non-ultramafic soils (subsp. pilosum) (Baker et al. 1992).
In recent studies of Mt. Kinabalu, van der Ent et al. (2013b, 2015a, f) discovered nine species of Ni hyperaccumulators from the flora of Kinabalu Park in Sabah, Malaysia. Previously known hyperaccumulators from the region included R. bengalensis (Brooks and Wither 1977a, b), Rinorea javanica (Brooks et al. 1977a), P. balgooyi (Phyllanthaceae; Hoffmann et al. 2003), D. gelonioides (Baker et al. 1992), Psychotria cf. gracilis (Rubiaceae; Reeves 2003), and Shorea tenuiramulosa (Proctor et al. 1989). Van der Ent et al. (2013b, 2015f) added several more Ni hyperaccumulators, including Actephila alanbakeri (Cleistanthus sp. nov. in the original report) (Phyllanthaceae; 11,520 µg g−1), Flacourtia kinabaluensis (Salicaceae; 7280 µg g−1), Glochidion mindorense (Phyllanthaceae; 2280 µg g−1), Kibara coriacea (Monimiaceae; 5840 µg g−1), Mischocarpus sundaicus (Sapindaceae) (4425 µg g−1), Phyllanthus cf. securinegioides (Phyllanthaceae; 23,300 µg g−1), Psychotria sarmentosa (Rubiaceae; 24,200 µg g−1), Walsura pinnata (Meliaceae; 4580 µg g−1), and Xylosma luzoniensis (Salicaceae; 5360 µg g−1) to the list, thereby documenting the highest number of Ni hyperaccumulators (15) known from any region within South and Southeast Asia.
In an effort to understand the factors contributing to Ni hyperaccumulation in Sabah, Malaysia, van der Ent et al. (2016b) examined the soil chemistry associated with 18 Ni hyperaccumulator plant species, comparing the chemistry of ultramafic soils where Ni hyperaccumulators were absent. The results showed that Ni hyperaccumulators are restricted to circum-neutral soils with relatively high phytoavailable Ca, Mg, and Ni. They hypothesized that either hyperaccumulators excrete large amounts of root exudates, thereby increasing Ni phytoavailability through intense rhizosphere mineral weathering, or that they have extremely high Ni uptake efficiency, thereby severely depleting Ni and stimulating re-supply of Ni via diffusion from labile Ni pools. Their results, however, tend to favor the latter hypothesis.
Nuclear microprobe imaging (micro-PIXE) shows that in P. balgooyi collected from ultramafic soils in Sabah, Malaysia, Ni concentrations were very high in the phloem of the stems and petioles, while in the leaves Ni was enriched in the major vascular bundles (Mesjasz-Przybylowicz et al. 2015). The preferential accumulation of Ni in the vascular tracts suggests that Ni is present in a metabolically active form. This research is important as the elemental distribution of P. balgooyi differs from that of many other Ni hyperaccumulators from temperate and Mediterranean regions where Ni is preferentially accumulated in leaf epidermal cells (Bhatia et al. 2004; Broadhurst et al. 2004; Tylko et al. 2007; Baklanov 2011).
In the Philippines, much of the ultramafic vegetation remains underexplored (Fernando et al. 2008; but see Baker et al. 1992; Fernando et al. 2013; Proctor et al. 1998, 2000a, b). Studies to date have revealed new Ni hyperaccumulators (e.g. Fernando and Rodda 2013; Hoffmann et al. 2003), including Breynia cernua (Phyllanthaceae; Gotera et al. 2014) and P. balgooyi, P. erythrotrichus, and P. securinegioides (Phyllanthaceae; Hoffmann et al. 2003; Quimado et al. 2015). A recent study described Rinorea niccolifera (Violaceae) as a novel taxon and Ni hyperaccumulator from Luzon Island, Philippines (Fernando et al. 2014).
Although in Sri Lanka’s ultramafic outcrops are not associated with many Ni hyperaccumulator species, unlike those in Sabah, Malaysia (van der Ent et al. 2015a), several plant species currently found at Ussangoda hyperaccumulate Ni (see citations in Chathuranga et al. 2015; Samithri 2015). Notable in this regard are Evolvulus alsinoides (Convolvulaceae), Hybanthus enneaspermus (Violaceae), Flacourtia indica (Flacourtiaceae), Olax imbricata (Olacaceae), Toddalia asiatica (Rutaceae), Euphorbia heterophylla (Euphorbiaceae), Vernonia cinerea (Asteraceae) and Crotalaria sp. (Fabaceae). Senevirathne et al. (2000) also document Striga euphrasioides (Orobanchaceae), Cassia mimosoides (Fabaceae), and Blumea obliqua (Asteraceae) from Ussangoda as hyperaccumulating Ni, although subsequent studies have failed to confirm this earlier report. Five Cu hyperaccumulators [Geniosporum tenuiflorum (Lamiaceae; now Ocimum tenuiflorum), Clerodendrum infortunatum (Lamiaceae), Croton bonplandianus (Euphorbiaceae), Waltheria indica (Malvaceae), and Tephrosia villosa (Fabaceae)] are also found on ultramafic outcrops in Sri Lanka (Rajakaruna and Bohm 2002). Based on revised criteria for Cu hyperaccumulation (van der Ent et al. 2013c), Calotropis gigantea, Carissa spinarum, Cassia auriculata, Abutilon indicum, and Phyllanthus sp. undet., analysed by Rajakaruna and Bohm (2002), now also qualify as hyperaccumulators of Cu (Table 4). Although Cu hyperaccumulation is not a common phenomenon among ultramafic plants, a recent study has also documented unusual Cu uptake in a number of ultramafic plants in Malaysia and Brazil (van der Ent and Reeves 2015).
Evolutionary aspects
Ultramafic outcrops often harbor populations which are morphologically and physiologically distinct from those found on non-ultramafic soils. Such intraspecific variation, especially with respect to functionally important traits, is common in many ultramafic taxa worldwide (O’Dell and Rajakaruna 2011). Such variation can result from both local adaptation (i.e., ecotypic differentiation; Sambatti and Rice 2006; Turner et al. 2010) or phenotypic plasticity (Murren et al. 2006; Wu et al. 2010), and must be examined on a case-by-case basis. Suitable methods of examination include reciprocal or unilateral transplant experiments and common garden studies (Wright and Stanton 2011), as well as functional genomic and proteomic approaches (Selby et al. 2014; von Wettberg et al. 2014; von Wettberg and Wright 2011). Detecting intraspecific variation is the first step toward any investigation on the causes and consequences of adaptive evolution. Populations exhibiting intraspecific variation on ultramafic and non-ultramafic soils have led to numerous studies of speciation (Anacker 2014; Kay et al. 2011) and phylogenetic investigations (Anacker 2011; Anacker et al. 2011; Anacker and Harrison 2012), advancing our understanding of evolutionary and ecological theory (Harrison and Rajakaruna 2011). Molecular phylogenetic methods provide a unique protocol for testing and establishing species relationships, helping to shed light on how ultramafic endemics evolve (Baldwin 2005). The analysis of phylogenies for 23 genera from California shows that ultramafic endemics exhibit few transitions out of the endemic state (Anacker et al. 2011), suggesting that adaptation to ultramafics and subsequent diversification can lead to an evolutionary “dead end”. But ultramafic lineages may not always represent evolutionary “dead ends” and may have the potential to further diversify via independent polyploidization and hybridization, even providing a pathway to radiate off ultramafic soils (Kolář et al. 2012).
Compared to these studies from other regions of the world, there is little information on evolutionary aspects of plants associated with ultramafic soils in South and Southeast Asia. A recent study from Sri Lanka shows that the ultramafic and non-ultramafic populations of Fimbristylis ovata (Cyperaceae) may be locally adapted to their respective soils (Chathuranga et al. 2015). The ultramafic population translocated significantly more Ni from its roots to shoots (translocation factor 0.43) than the non-ultramafic population (translocation factor 0.29). However, additional studies are required to determine whether the populations of F. ovata, or other species, including those hyperaccumulating metals such as Ni and Cu, deserve ecotypic recognition. Several ultramafic-associated taxa in Sri Lanka might benefit from further observations and additional greenhouse studies to determine whether the ultramafic-associated populations are genetically distinct and are worthy of ecotypic recognition (Rajakaruna and Bohm 2002). These taxa include several Ni-accumulating and -hyperaccumulating species, particularly Hybanthus enneaspermus (Violaceae), Evolvulus alsinoides (Convolvulaceae), Crotalaria sp. (Fabaceae), Desmodium triflorum (Fabaceae) and Fimbristylis sp. (Cyperaceae), all of which show detectable phenotypic differences between ultramafic and non-ultramafic populations. Studies exploring causes and consequences of phenotypic differences between populations found on and off ultramafic soils can add much to our understanding of the origins of ultramafic specialists in the South and Southeast Asia region.
Phytotechnologies
The use of trace element hyperaccumulators to clean up polluted sites, i.e. phytoremediation, is gaining recognition as a viable green technology (Neilson and Rajakaruna 2014). Phytoremediation is based on the premise that plants which remove selected pollutants from the soil and translocate them to their above-ground biomass can then be harvested and disposed of through incineration or elemental recovery, a process known as phytomining (Chaney et al. 2014; van der Ent et al. 2015g). Ultramafic plants in the genera Alyssum (Brassicaceae), Streptanthus (Brassicaceae), Noccaea (Brassicaceae), and Berkheya (Asteraceae) have been used in phytoremediation and phytomining of Ni-enriched ultramafic sites in temperate and Mediterranean regions (Ho et al. 2013; Morel et al. 2006; Gall and Rajakaruna 2013; Sheoran et al. 2009; van der Ent et al. 2015g). Given the large number of hyperaccumulator species currently known from tropical Asia (Gall and Rajakaruna 2013; Reeves 2003), there should be considerable interest in using these unique plants in the remediation of regional sites contaminated with metal and metalloid pollutants.
Phytoremediation and phytomining
Bandara et al. (2017) investigated the effect of biochar and fungal-bacterial co-inoculation on soil enzymatic activity and immobilization of heavy metals in soil collected from an ultramafic outcrop in Sri Lanka. The addition of biochar to ultramafic soil immobilized heavy metals and decreased soil enzymatic activities while the addition of microbial inoculants improved plant growth by mitigating heavy metal toxicity and enhancing soil enzymatic activities. Additional studies from Sri Lanka confirm the importance of (i) bacterial-fungal inoculation as a soil-quality enhancer and a plant-growth promoter in the presence of heavy metals found in ultramafic soils (Seneviratne et al. 2016a, b), and, (ii) biochar as a soil amendment to immobilize Cr, Ni, and Mn in ultramafic soil, thereby reducing metal-induced plant toxicities (Herath et al. 2014).
The potential for microbial remediation (reduction) of Cr(VI) by indigenous microbial populations from the ultramafic soils of Sukinda mines in Jaipur, Orissa, India, was investigated by Mishra et al. (2009). The best reducer of Cr (V1) was Staphylococcus aureus, a gram-positive bacterium whose thick layer of peptidoglycan acts as a strong absorbent. The taxon tolerated a Cr concentration of 250 mg L−1 and was resistant to Ni up to 1000 mg L−1. The bacterium was recommended for the bioremediation of both Cr and Ni, showing complete Cr(VI) to Cr(III) degradation in 22 h, and Ni2+ degradation to 90% in 22 h. Similarly, Bohidar et al. (2009) explored the possibility of Ni recovery from chromite tailings at the Sukinda mines by using three fungal strains.
In another study, Mohanty et al. (2011) utilized phytoremediation in South Kaliapani, a chromite mining ultramafic area in Orissa, India. Chromium was extracted by growing Oryza sativa cv. Khandagiri (rice; Poaceae) in contaminated soil and irrigating with mine wastewater. Chromium levels were reduced (70–90%) after 100 days, with accumulation levels ranging from 125 to 498 µg g−1 in leaves, 25 to 400 µg g−1 in stems, and 5 to 23 µg g−1 in the grain. Absorption into roots was higher by two orders of magnitude than into any aerial part of the plant. Mohanty et al. (2012) also investigated the phytoremediation potential of O. sativa, Brachiaria mutica (Poaceae), and Eichhornia crassipes (Pontederiaceae) to reduce levels of Cr(VI) in mine waste-water. Eichhornia crassipes was most successful with 25–54% reduction while B. mutica contributed to an 18–33% reduction.
Kfayatullah et al. (2001), in a study of plants and soils of the Malakand chromite-rich ultramafic area and Mardan non-ultramafic areas of the North-West Frontier Province, Pakistan, focused on enzyme-bound metal accumulation in plant tissue. Verbascum thapsus (Scrophulariaceae), an edible plant, accumulated greater than 100 µg g−1 of several metals, including Ni and Cr, but was not recommended for phytoremediation efforts.
Indonesia (Sulawesi and Halmahera Islands) has some of the largest surface exposures of ultramafic bedrock in the world. Lateritic Ni-mining operations have continued in the region since the early twentieth century, setting the stage for exploring the use of native plants for phytoremediation and phytomining. Twelve native species known to hyperaccumulate Ni are recommended by van der Ent et al. (2013a) for use in phytotechnologies in Indonesia.
Threats and conservation
Ultramafic areas are a high priority for biodiversity conservation because of the relatively large numbers of endemic species, ecotypes, and rare species that they harbour (Boyd et al. 2009). The conservation and restoration of these naturally fragmented, edaphically unique, and biodiverse habitats require special attention (Baker et al. 2010; O’Dell 2014; Thorne et al. 2011; Whiting et al. 2004). It is unclear how stressors, such as atmospheric N deposition (Vallano et al. 2012), suppression of fire (Arabas 2000; Safford and Harrison 2004) and climate change (Damschen et al. 2012; Anacker and Harrison 2012) documented for temperate and Mediterranean ultramafics, impact tropical Asia’s ultramafic ecosystems.
The combined forces of forest clearing, agricultural development and mining contribute to unprecedented habitat loss in South and Southeast Asia (Duckworth et al. 2012; Hughes 2017; Sodhi et al. 2004). In fact, Southeast Asia has a higher annual rate of deforestation than Meso-America, South America, or sub-Saharan Africa, and that rate has continued to increase between 1990 and 2005 (Giam et al. 2010; Sodhi et al. 2010). This is especially of concern as Southeast Asia has a higher proportion of its vascular plant, reptile, bird, and mammal species categorised as globally threatened on the Red List compared to Meso- and South America and sub-Saharan Africa (Sodhi et al. 2010). With such limited study of ultramafics in South and Southeast Asia, it is unclear how increasing habitat loss is impacting biodiverse ultramafic outcrops in the region.
Malaysia has one of the most species-rich ultramafic floras in the world. The over 3500 km2 of ultramafic outcrops in Sabah (4.6% of the total landmass of the state) on the island of Borneo harbor a total of 4252 plant species (van der Ent et al. 2015a). Over 2542 plant species have been documented on ultramafic outcrops in Kinabalu Park alone, of which a large percentage is endemic to either Kinabalu Park or to Borneo (van der Ent et al. 2015a; Fig. 4). Despite the existence of this species-rich flora, the plant diversity and ecology of many ultramafic outcrops in Sabah remain largely unknown because of a lack of focused research. Furthermore, plant diversity in many areas of Sabah is severely threatened by land-use conversion and, because often plant species occur only at a single or a few ultramafic sites, and hence impacts on the ecosystems that support them could eventually result in their extinction. While it is necessary to identify stressors impacting ultramafic habitats of South and Southeast Asia for their proper management, it is even more critical that basic geoecological surveys of ultramafic outcrops, including the extensive exposures in Sulawesi and Halmahera, are prioritised for cataloguing plant diversity and other biota. This is especially critical as many of these outcrops likely harbor rare and endemic species in need of urgent conservation attention.
Fig. 4.

Ultramafic edaphic endemics from South and Southeast Asia: a The monotypic tree Borneodendron aenigmaticum (Euphorbiaceae) is endemic to Sabah (Malaysia) on ultramafic soils in the lowlands. b The world’s largest carnivorous pitcher plant, Nepenthes rajah (Nepenthaceae) is endemic to Kinabalu Park in Sabah where it occurs in the montane zone. c The epiphytic or lithophytic orchid Porpax borneensis (Orchidaceae) is restricted to ultramafic outcrops in Sabah, Malaysia. d The recently described Begonia moneta (Begoniaceae) occurs lithophytically in lowland ultramafic forest in Sabah, Malaysia. e Scaevola verticillata (Goodeniaceae) is endemic to the summit of the ultramafic Mount Tambukon in Sabah, Malaysia. f The carnivorous Drosera ultramafica (Droseraceae) is endemic to a limited number of mountainous ultramafic outcrops in Malaysia and the Philippines. g Rhododendron baconii (Ericaceae) is another hyper-endemic restricted to Kinabalu Park, Sabah, Malaysia. h The specific epithet of Pittosporum peridoticola (Pittosporaceae) indicates its habitat is on ultramafic soils in Sabah, Malaysia
(all images are by A. van der Ent)
Although Sri Lanka’s ultramafic flora appears to be impoverished with respect to endemic species or hyperaccumulator taxa, the ultramafic sites harbor several taxa worthy of conservation. For example, Ussangoda, the site that has received the most research attention, is home to: four near-threatened species, Striga angustifolia (Orobanchaceae), Maerua arenaria (Capparaceae), Salvadora percia (Salvadoraceae), and Olax imbricata (Olacaceae); two vulnerable species, Cyanotis adscendens (Commelinaceae), Pachygone ovata (Menispermaceae); and one data deficient species, Alysicarpus monilifer (Fabaceae; MOE 2012). Therefore, it is critical that Sri Lanka’s ultramafic outcrops receive regional and national recognition and are declared as ecologically sensitive sites (i.e. geoecological preserves) to be set aside for future investigations. In 2010, Ussangoda was declared as a National Park with approximately 350 hectares, including areas overlaying ultramafic rock, set aside for conservation purposes (Department of Wildlife Conservation 2015). Without such conservation, proper management, and research, these unique habitats and their physiologically distinct biota are extremely vulnerable. Rinorea bengalensis (Violaceae) offers an example of why such efforts are urgently needed. Brooks et al. (1977a, b) conducted a survey of herbarium specimens from the entire range of this species, encompassing Sri Lanka, the Malay Archipelago, New Guinea, the Solomon Islands and Queensland, Australia, and found that Ni hyperaccumulation is a constitutive trait in this species when growing on ultramafic soil. The herbarium specimen analysed from Sri Lanka contained 10,000 µg g−1 and the locality indicated on the map presented by Brooks et al. (1977a) suggests a collection in the central part of the island (see Fig. 1 in Rajakaruna and Baker 2004). However, it was not encountered in field exploration by Rajakaruna and Bohm (2002) and was presumed extinct in Sri Lanka (Ministry of Environment and Renewable Energy 2012). Interestingly, the taxon was recently recollected in southwestern Sri Lanka (Siril Wijesundara, National Institute of Fundamental Studies, Sri Lanka, pers. comm.), however, soil and plant tissue elemental concentrations have yet to be determined.
Conclusions
Information gaps and future directions
Ultramafic outcrops are natural laboratories for experimental and applied research in a wide range of disciplines. They provide numerous opportunities for collaborations among geologists, pedologists, botanists, zoologists, microbiologists, and land managers focusing on conservation and restoration research. However, research on the ultramafic outcrops in South and Southeast Asia has been limited, with most effort to date focused on Malaysia, the Philippines, the Andaman Islands (India), and Sri Lanka (Table 1). We were unable to find any published literature on ultramafic geoecology of other South (Afghanistan, Bhutan, Nepal) and Southeast Asian (Myanmar, Laos, Thailand, Vietnam) countries despite the known occurrences of ultramafic lithologies in these locales. The limited number of published studies we found for Myanmar, Thailand, and Vietnam (Table 1) focused on geological, mineralogical, or geochemical research.
Throughout South and Southeast Asia, detailed and systematic surveys will likely reveal numerous species new to science, including trace element hyperaccumulators. Recent research conducted in Sabah, Malaysia by van der Ent et al. (2014, 2015a, f) which led to the discovery of 24 new hyperaccumulator species, is a case in point. Detailed floristic surveys should be undertaken across the region and species showing unusual physiological behavior (such as trace element accumulation) or exhibiting distinct morphological traits relative to populations on non-ultramafic soils may be further studied under laboratory and greenhouse conditions. Additionally, species showing intraspecific variation between ultramafic and non-ultramafic populations may be evaluated via population genetic studies to determine whether ultramafic populations are genetically distinct from those found on non-ultramafic soils. For those species showing intraspecific variation with respect to morphological or physiological features, including flowering times between ultramafic and non-ultramafic populations, common garden and reciprocal transplant experiments can be undertaken to examine whether populations are locally adapted to their substrate. Such types of experimental studies are currently lacking entirely from the region.
In addition to detailed studies of vascular plants, it is important to pay close attention to non-vascular plants such as bryophytes, cryptogamic species such as lichens, soil algae and cyanoprokaryotes, and belowground microbes and soil invertebrates. Such investigations will likely reveal species that are endemic to the substrate or show a high affinity to ultramafic soils, as shown for such research conducted in South Africa (Venter et al. 2015) and California, USA (Rajakaruna et al. 2012).
Species documented as trace element hyperaccumulators may be investigated under controlled conditions for their suitability for phytoremediation or phytomining and tested under field conditions for their effectiveness in site reclamation and restoration. The resulting information can be added to the global database of metal hyperaccumulating species (Global Hyperaccumulator Database 2016: http://www.hyperaccumulators.org). Finally, it is critical that tropical Asia’s ultramafic outcrops receive regional, national, and global recognition and that key sites receive appropriate statutory protection so that future scientific research is possible.
One of the options for protection at a national level by the state is the inclusion of ultramafic sites in the Global Geopark Network (GGN). Conservation and protection of landscapes of geological significance at a national and international level is promoted by UNESCO under its Global Geoparks Scheme (UNESCO 2016). At a national level, relevant authorities should pursue this option as a long-term conservation strategy, which would provide a holistic approach to protection by incorporating a management strategy including education and sustainable development. The latter would mobilize the local population for economic benefits by participating in the conservation efforts through local and international ecotourism. This, however, also requires meeting the stringent guidelines laid out by UNESCO to be included in the GGN. Currently, ultramafic sites in South and Southeast Asia are not in the GGN but would meet the basic requirements laid out by UNESCO.
Authors’ contributions
Conceptualization, NR, AE, MCMI; writing original manuscript draft, MLG; writing and editing, NR, AE, MCMI; visualization, MLG, AE, NR. All authors read and approved the final manuscript.
Acknowledgements
A. van der Ent is the recipient of a Discovery Early Career Researcher Award (DE160100429) from the Australian Research Council. The French National Research Agency through the national “Investissements d’avenir” program (ANR-10-LABX-21, LABEX RESSOURCES21) and through the ANR-14-CE04-0005 Project “Agromine” is acknowledged for funding support to A. van der Ent. N. Rajakaruna is supported by a US Research Scholar Fulbright Award for 2016–2017 and the National Institute of Fundamental Studies, Kandy, Sri Lanka. We would like to thank Ian Medeiros for his constructive comments on an earlier draft of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
M. L. Galey, Email: galey004@umn.edu
A. van der Ent, Email: a.vanderent@uq.edu.au
M. C. M. Iqbal, Email: mcmif2003@yahoo.com
N. Rajakaruna, Email: nrajakaruna@gmail.com
References
- Acharya C, Kar R, Sukla L. Short communication: leaching of chromite overburden with various native bacterial strains. World J Microbiol Biotechnol. 1998;14:769–771. doi: 10.1023/A:1008888031842. [DOI] [Google Scholar]
- Adam JH. Demographic study of Nepenthes species (Nepenthaceae) recorded along the trail to the summit of Mount Kinabalu in Sabah, Malaysia. Pak J Biol Sci. 2002;5:419–426. doi: 10.3923/pjbs.2002.419.426. [DOI] [Google Scholar]
- Aggangan NS, Dell B, Malajczuk N. Effects of chromium and nickel on growth of the ectomycorrhizal fungus Pisolithus and formation of ectomycorrhizas on Eucalyptus urophylla S.T. Blake. Geoderma. 1998;84:15–27. doi: 10.1016/S0016-7061(97)00118-3. [DOI] [Google Scholar]
- Aiba S, Kitayama K. Structure, composition and species diversity in an altitude-substrate matrix of rain forest tree communities on Mount Kinabalu, Borneo. Plant Ecol. 1999;140:139–157. doi: 10.1023/A:1009710618040. [DOI] [Google Scholar]
- Aiba S-I, Sawada Y, Takyu M, Seino T, Kitayama K, Repin R. Structure, floristics and diversity of tropical montane rain forests over ultramafic soils on Mount Kinabalu (Borneo) compared with those on non-ultramafic soils. Aust J Bot. 2015;63(4):191–203. doi: 10.1071/BT14238. [DOI] [Google Scholar]
- Alexander EB. Soil and vegetation differences from peridotite to serpentinite. Northeast Nat. 2009;16(5):178–192. doi: 10.1656/045.016.0515. [DOI] [Google Scholar]
- Alexander EB, DuShey J. Topographic and soil differences from peridotite to serpentinite. Geomorphology. 2011;135(3–4):271–276. doi: 10.1016/j.geomorph.2011.02.007. [DOI] [Google Scholar]
- Alexander EB, Coleman RG, Keeler-Wolf T, Harrison SP. Serpentine geoecology of Western North America: geology, soils, and vegetation. New York: Oxford University Press; 2007. [Google Scholar]
- Anacker BL. Phylogenetic patterns of endemism and diversity. In: Harrison SP, Rajakaruna N, editors. Serpentine: the evolution and ecology of a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Anacker BL. The nature of serpentine endemism. Am J Bot. 2014;101:219–224. doi: 10.3732/ajb.1300349. [DOI] [PubMed] [Google Scholar]
- Anacker BL, Harrison SP. Climate and the evolution of serpentine endemism in California. Evol Ecol. 2012;26:1011–1023. doi: 10.1007/s10682-011-9532-4. [DOI] [Google Scholar]
- Anacker BL, Whittall JB, Goldberg EE, Harrison SP. Origins and consequences of serpentine endemism in the California flora. Evolution. 2011;65:365–376. doi: 10.1111/j.1558-5646.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- Arabas KB. Spatial and temporal relationships among fire frequency, vegetation, and soil depth in an eastern North American serpentine barren. J Torrey Bot Soc. 2000;127:51–65. doi: 10.2307/3088747. [DOI] [Google Scholar]
- Argent G, Wilkie P, Maduli D. Lobelia proctorii sp. nov. (Lobelioideae, Campanulaceae/Lobeliaceae) from the Philippines. Plant Ecol. 2007;192:157–160. doi: 10.1007/s11258-007-9306-9. [DOI] [Google Scholar]
- Arnold BA, Lahner B, DaCosta J, Weisman C, Hollister J, Salt D, Bomblies K, Yant L. Borrowed alleles and convergence in serpentine adaptation. Proc Natl Acad Sci USA. 2016;113:8320–8325. doi: 10.1073/pnas.1600405113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AJM, Proctor J, Van Balgooy MMJ, Reeves RD (1992) Hyperaccumulation of nickel by the flora of the ultramafics of Palawan, Republic of the Philippines. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (Serpentine) soils: proceedings of the first international conference on serpentine ecology. Intercept Ltd., Andover
- Baker AJM, Ernst WHO, Van der Ent A, Malaisse F, Ginocchio R. Metallophytes: the unique biological resource, its ecology and conservational status in Europe, central Africa and Latin America. In: Batty LC, Hallberg KB, editors. Ecology of industrial pollution. Ecological reviews. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- Baklanov IA. Heterogeneity of epidermal cells in relation to nickel accumulation in hyperaccumulator plants belonging to the genus Alyssum L. Cell Tissue Biol. 2011;5:603–611. doi: 10.1134/S1990519X11060034. [DOI] [PubMed] [Google Scholar]
- Baldwin BG. Origin of the serpentine-endemic herb Layia discoidea from the widespread L. glandulosa (Compositae) Evolution. 2005;59:2473–2479. [PubMed] [Google Scholar]
- Bandara T, Herath I, Kumarathilaka P, Seneviratne M, Seneviratne G, Rajakaruna N, Vithanage M. Role of woody biochar and fungal-bacterial co-inoculation on soil enzyme activity and heavy metal immobilization in serpentine soil. J Soils Sediments. 2017;17:655–673. doi: 10.1007/s11368-015-1243-y. [DOI] [Google Scholar]
- Banerjee PK. Geology and geochemistry of the Sukinda ultramafic field, Cuttack district. Orissa Mem Geol Surv India. 1972;103:1–158. [Google Scholar]
- Barthlott W, Hostert A, Kier G, Küper W, Kreft H, Mutke J, Rafiqpoor MD, Sommer JH. Geographic patterns of vascular plant diversity at continental to global scales. Erdkunde. 2007;61(4):305–315. doi: 10.3112/erdkunde.2007.04.01. [DOI] [Google Scholar]
- Batianoff GN, Specht RL. Queensland (Australia) serpentinite vegetation. In: Proctor J, Baker AJM, Reeves RD, editors. The vegetation of ultramafic (serpentine) soils. Andover, UK: Intercept Ltd; 1992. pp. 109–128. [Google Scholar]
- Batianoff GN, Singh S. Central Queensland serpentine landforms, plant ecology and endemism. S Afr J Sci. 2001;97:495–500. [Google Scholar]
- Batten KM, Scow KM, Davies KF, Harrison SP. Two invasive plants alter soil microbial community composition in serpentine grasslands. Biol Invasions. 2006;8:217–230. doi: 10.1007/s10530-004-3856-8. [DOI] [Google Scholar]
- Bhatia NP, Walsh KB, Orlic I, Siegele R, Ashwath N, Baker AJM. Studies on spatial distribution of nickel in leaves and stems of the metal hyperaccumulator Stackhousia tryonii Bailey using nuclear microprobe (micro- PIXE) and EDXS techniques. Funct Plant Biol. 2004;31:1061–1074. doi: 10.1071/FP03192. [DOI] [PubMed] [Google Scholar]
- Bhatta K, Ghosh B. Chromian spinel-rich black sands from eastern shoreline of Andaman Island, India: implication for source characteristics. J Earth Syst Sci. 2014;123:1387–1397. doi: 10.1007/s12040-014-0474-4. [DOI] [Google Scholar]
- Biswas S, Saikat S, Dey R, Mukherjee S, Banerjee PC. Microbial leaching of chromite overburden from Sukinda mines, Orissa, India using Aspergillus niger. Int J Miner Metall Mater. 2013;20:705–712. doi: 10.1007/s12613-013-0787-3. [DOI] [Google Scholar]
- Bohidar S, Mohapatra S, Sukla LB. Nickel recovery from chromite overburden of Sukinda using fungal strains. Int J Integr Biol. 2009;5:103–108. [Google Scholar]
- Borhidi A (1992) The serpentine flora and vegetation of Cuba. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (Serpentine) soils. Proc. 1st international conference on serpentine ecology. Intercept Ltd., Andover
- Boyd RS. High-nickel insects and nickel hyperaccumulator plants: a review. Insect Sci. 2009;16(1):19–31. doi: 10.1111/j.1744-7917.2009.00250.x. [DOI] [Google Scholar]
- Boyd RS. Ecology and evolution of metal-hyperaccumulating plants. In: Rajakaruna N, Boyd RS, Harris TB, editors. Plant ecology and evolution in harsh environment. Hauppauge: Nova Science Publishers; 2014. [Google Scholar]
- Boyd RS, Kruckeberg AR, Rajakaruna N. Biology of ultramafic rocks and soils: research goals for the future. Northeast Nat. 2009;16(5):422–440. doi: 10.1656/045.016.0530. [DOI] [Google Scholar]
- Brady KU, Kruckeberg AR, Bradshaw HD. Evolutionary ecology of plant adaptation to ultramafic soils. Annu Rev Ecol Evol Syst. 2005;36:243–266. doi: 10.1146/annurev.ecolsys.35.021103.105730. [DOI] [Google Scholar]
- Bratteler M, Lexer C, Widmer A. Genetic architecture of traits associated with serpentine adaptation of Silene vulgaris. J Evol Biol. 2006;19:1149–1156. doi: 10.1111/j.1420-9101.2006.01090.x. [DOI] [PubMed] [Google Scholar]
- Brearley FQ. Nutrient limitation in a Malaysian ultramafic soil. J Trop For Sci. 2005;17:596–609. [Google Scholar]
- Briscoe LRE, Harris TB, Dannenberg E, Broussard W, Olday FC, Rajakaruna N. Bryophytes of adjacent serpentine and granite outcrops on the Deer Isles, Maine, USA. Rhodora. 2009;111:1–20. doi: 10.3119/07-31.1. [DOI] [Google Scholar]
- Broadhurst CL, Chaney RL, Angle JS, Erbe EF, Maugel TK. Nickel localization and response to increasing Ni soil levels in leaves of the Ni hyperaccumulator Alyssum murale. Plant Soil. 2004;265:225–242. doi: 10.1007/s11104-005-0974-8. [DOI] [Google Scholar]
- Brooks RR. Serpentine and its vegetation: a multidisciplinary approach. Portland: Dioscorides Press; 1987. [Google Scholar]
- Brooks RR, Wither ED. Nickel hyperaccumulation by Rinorea bengalensis (Wall.) O.K. J Geochem Explor. 1977;7:295–300. doi: 10.1016/0375-6742(77)90085-1. [DOI] [Google Scholar]
- Brooks RR, Wither ED, Zepernick B. Cobalt and nickel in Rinorea species. Plant Soil. 1977;47:707–712. doi: 10.1007/BF00011041. [DOI] [Google Scholar]
- Brooks RR, Lee J, Reeves RD, Jaffré T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J Geochem Explor. 1977;7:49–57. doi: 10.1016/0375-6742(77)90074-7. [DOI] [Google Scholar]
- Brooks RR, Wither ED, Westra LY. Biogeochemical copper anomalies on Salajar Island Indonesia. J Geochem Explor. 1978;10:181–188. doi: 10.1016/0375-6742(78)90017-1. [DOI] [Google Scholar]
- Bruijnzeel LA. Soil moisture regime as a major factor determining vegetation structure on ultramafic soils in Palawan, the Philippines, and Sabah, Malaysia. Act Bot Need. 1990;39:402. [Google Scholar]
- Bruijnzeel LA, Waterloo MJ, Proctor J, Kuiters AT, Kotterink B. Hydrological observations in montane rain forests on Gunung Silam, Sabah, Malaysia, with special reference to the ‘Massenerhebung’ effect. J Ecol. 1993;81:145–167. doi: 10.2307/2261231. [DOI] [Google Scholar]
- Burge DO, Thorne JH, Harrison SP, O’Brien BC, Shevock JR, Alverson ER, Hardison LK, Delgadillo J, Junak S, Oberbauer T, Rebman JP, Riemann H, Vanderplank SE, Barry T. Plant diversity and endemism in the California Floristic Province. Madroño. 2016;63(2):3–206. doi: 10.3120/madr-63-02-3-206.1. [DOI] [Google Scholar]
- Cardace D, Meyer-Dombard DR, Olsen AA, Parenteau MN. Bedrock and geochemical controls on extremophile habitats. In: Rajakaruna N, Boyd RS, Harris TB, editors. Plant ecology and evolution in harsh environments. Hauppauge: Nova Science Publishers; 2014. [Google Scholar]
- Central Energy Resources Team (1999) Generalized Geology of the Far East (geo3al), US Geological Survey. https://catalog.data.gov/dataset/generalized-geology-of-the-far-east-geo3al. Accessed 12 Oct 2016
- Chakraborty KL, Chakraborty TL. Geological features and origin of the chromite deposits of Sukinda Valley, Orissa, India. Miner Deposita. 1984;19:256–265. doi: 10.1007/BF00204378. [DOI] [Google Scholar]
- Chaney RL, Reeves RD, Baklanov IA, Centofanti T, Broadhurst CL, Baker AJM, Van der Ent A, Roseberg RJ. Phytoremediation and phytomining: using plants to remediate contaminated or mineralized environments. In: Rajakaruna N, Boyd RS, Harris TB, editors. Plant ecology and evolution in harsh environment. Hauppauge: Nova Science Publishers; 2014. [Google Scholar]
- Chathuranga PKD, Dharmasena SKAT, Rajakaruna N, Iqbal MCM. Growth and nickel uptake by serpentine and non-serpentine populations of Fimbristylis ovata (Cyperaceae) from Sri Lanka. Aust J Bot. 2015;63:128–133. [Google Scholar]
- Chaudhury K, Datta S, Mukherjee PK. Mapping the vegetation of the ultramafic outcrops of Saddle Hills (North Andaman Islands, India) using remote-sensing tools. Aust J Bot. 2015;63:234–242. doi: 10.1071/BT14243. [DOI] [Google Scholar]
- Cheek M. Nepenthes (Nepenthaceae) of Halmahera, Indonesia. Blumea. 2015;59:215–225. doi: 10.3767/000651915X689091. [DOI] [Google Scholar]
- Chen J, Wong KM, Van der Ent A, Tan HTW. Nine new species of Timonius (Rubiaceae) from Kinabalu Park, Borneo. Phytotaxa. 2014;181:138–150. doi: 10.11646/phytotaxa.181.3.2. [DOI] [Google Scholar]
- Chung AYC, Chew SKF, Majapun R, Nilus R. Insect diversity of Bukit Hampuan Forest Reserve, Sabah, Malaysia. J Threat Taxa. 2013;5:4461–4473. doi: 10.11609/JoTT.o3243.4461-73. [DOI] [Google Scholar]
- Damschen EI, Harrison SP, Ackerly DD, Fernandez-Going BM, Anacker BL. Endemic plant communities on special soils: early victims or hardy survivors of climate change? J Ecol. 2012;100:1122–1130. doi: 10.1111/j.1365-2745.2012.01986.x. [DOI] [Google Scholar]
- Datta S, Chaudhury K, Mukherjee PK. Hyperaccumulators from the serpentines of Andaman, India. Aust J Bot. 2015;63:243–251. doi: 10.1071/BT14244. [DOI] [Google Scholar]
- Davis S, Heywood VH (eds) (1995) Centres of plant diversity, volume 2: Asia, Australasia and the Pacific. International Union for the Conservation of Nature and Natural Resources, p 578
- Department of Wildlife Conservation (2015) National Parks. http://www.dwc.gov.lk/index.php/en/national-parks. Accessed 27 Oct 2016
- Dhakate R, Singh VS. Heavy metal contamination in groundwater due to mining activities in Sukinda Valley, Orissa—a case study. J Geogr Reg Plann. 2008;1:48–67. [Google Scholar]
- Dissanayaka CB. The geology and geochemistry of the Uda Walawe serpentinite. Sri Lanka. J Natn Sci Coun Sri Lanka. 1982;10:13–34. [Google Scholar]
- Dissanayake CB, Van Riel BJ. The petrology and geochemistry of a recently discovered nickeliferous serpentinite from Sri Lanka. J Geol Soc India. 1978;19:464–471. [Google Scholar]
- Duckworth JW, Batters G, Belant JL, Bennett EL, Brunner J, et al. (2012) Why South-east Asia should be the world’s priority for averting imminent species extinctions, and a call to join a developing cross-institutional programme to tackle this urgent issue. S.A.P.I.E.N.S 5(2). http://sapiens.revues.org/1327. Accessed 12 Oct 2016
- Elam DR, Wright DH, Goettle B (1998) Recovery plan for serpentine soil species of the San Francisco Bay Area. US Fish and Wildlife Service, Region 1, Portland, OR
- Favero-Longo SE, Isocrono D, Piervittori R. Lichens and ultramafic rocks: a review. Lichenologist. 2004;36:391–404. doi: 10.1017/S0024282904014215. [DOI] [Google Scholar]
- Fernando ES, Rodda M. Marsdenia purpurella (Apocynaceae, Asclepiadoideae), a new species from the Philippines. Gard Bull. 2013;65:143–148. [Google Scholar]
- Fernando ES, Suh MH, Lee J, Lee DK. Forest formations of the Philippines. Seoul: ASEAN-Korea Environmental Cooperation Unit, Seoul National University; 2008. pp. 1–232. [Google Scholar]
- Fernando ES, Quimado MO, Trinidad LC, Doronila AI. The potential use of indigenous nickel hyperaccumulators for small scale mining in the Philippines. J Degrad Min Land Manag. 2013;1:21–26. [Google Scholar]
- Fernando ES, Quimado MO, Doronila AI. Rinorea niccolifera (Violaceae), a new, nickel-hyperaccumulating species from Luzon Island, Philippines. Phytokeys. 2014;37:1–13. doi: 10.3897/phytokeys.37.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A, Robinson A, Mcpherson S, Heinrich V, Gironella E, Madulid DA. Drosera ultramafica (Droseraceae), a new sundew species of the ultramafic flora of the Malesian highlands. Blumea. 2011;56:10–15. doi: 10.3767/000651911X560907. [DOI] [Google Scholar]
- Fowlie JA. Malaya revisited XXIX, rediscovering the habitat of Paphiopedilum dayanum on serpentine cliffs on Mount Kinabalu in Eastern Malaysia (Formerly North Borneo) Orchid Digest. 1985;49:124–129. [Google Scholar]
- Fujii K, Hartono A, Funakawa S, Uemuraa M, Sukartiningsih Kosaki T. Acidification of tropical forest soils derived ultramafic and sedimentary rocks in East Kalimantan, Indonesia. Geoderma. 2011;160(3–4):311–323. doi: 10.1016/j.geoderma.2010.09.027. [DOI] [Google Scholar]
- Gall JE, Rajakaruna N. The physiology, functional genomics, and applied ecology of heavy metal-tolerant Brassicaceae. In: Lang M, editor. Brassicaceae: characterization, functional genomics and health benefits. Hauppauge: Nova Science Publishers Inc; 2013. [Google Scholar]
- Ghosh S, Paul AK (2015) Heterotrophic leaching of metals from Indian chromite mining overburden. Int J Min Reclam Environ 1–12. doi: 10.1080/17480930.2015.1118181
- Giam X, Ng TH, Yap VB, Tan HTW. The extent of undiscovered species in Southeast Asia. Biodivers Conserv. 2010;19:943–954. doi: 10.1007/s10531-010-9792-2. [DOI] [Google Scholar]
- Global Hyperaccumulator Database (2016) http://hyperaccumulators.smi.uq.edu.au/collection/. Accessed 27 Oct 2016
- Gotera KC, Doronila AI, Claveria RJR, Perez TR, et al. Breynia cernua (Poir.) Müll. Arg. (Phyllanthaceae) is a hyperaccumulator of nickel. Asia Life Sci. 2014;23:231–241. [Google Scholar]
- Hanson B, Lindblom SD, Loeffler ML, Pilon-Smits EAH. Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol. 2004;162:655–662. doi: 10.1111/j.1469-8137.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- Harrison SP, Rajakaruna N, editors. Serpentine: evolution and ecology in a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Harrison SP, Damschen E, Going BM. Climate gradients, climate change, and special edaphic floras. Northeast Nat. 2009;16(5):121–130. doi: 10.1656/045.016.0510. [DOI] [Google Scholar]
- Hasegawa M, Ito MT, Kitayama K. Community structure of oribatid mites in relation to elevation and geology on the slope of Mount Kinabalu, Sabah, Malaysia. Eur J Soil Biol. 2006;42:S191–S196. doi: 10.1016/j.ejsobi.2006.07.006. [DOI] [Google Scholar]
- Herath I, Kumarathilaka P, Navaratne A, Rajakaruna N, Vithanage M. Immobilization and phytotoxicity reduction of heavy metals in serpentine soil using biochar. J Soils Sediments. 2014;15:126–138. doi: 10.1007/s11368-014-0967-4. [DOI] [Google Scholar]
- Hewawasam T, Fernando GWAR, Priyashantha D. Geo-vegetation mapping and soil geochemical characteristics of the Indikolapelessa serpentinite outcrop, southern Sri Lanka. J Earth Sci. 2014;25:152–168. doi: 10.1007/s12583-014-0409-7. [DOI] [Google Scholar]
- Hisada K, Sugiyama M, Ueno K, Charusiri P, Arai S. Missing ophiolitic rocks along the Mae Yuam Fault as the Gondwana–Tethys divide in north-west Thailand. Island Arc. 2004;13:119–127. doi: 10.1111/j.1440-1738.2003.00412.x. [DOI] [Google Scholar]
- Ho C-P, Hseu Z-Y, Chen N-C, Tsai C-C. Evaluating heavy metal concentration of plants on a serpentine site for phytoremediation applications. Environ Earth Sci. 2013;70:191–199. doi: 10.1007/s12665-012-2115-z. [DOI] [Google Scholar]
- Hoffmann P, Baker AJM, Madulid DA, Proctor J. Phyllanthus balgooyi (Euphorbiaceae s.l.), a new nickel-hyperaccumulating species from Palawan and Sabah. Blumea. 2003;48:193–199. doi: 10.3767/000651903X686178. [DOI] [Google Scholar]
- Hörger AC, Fones HN, Preston GM. The current status of the elemental defense hypothesis in relation to pathogens. Front Plant Sci. 2013;4:395. doi: 10.3389/fpls.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AC. Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere. 2017;8(1):e01624. doi: 10.1002/ecs2.1624. [DOI] [Google Scholar]
- Isnard S, L’Huillier L, Rigault F, Jaffré T. How did the ultramafic soils shape the flora of the New Caledonian hotspot? Plant Soil. 2016;403(1):53–76. doi: 10.1007/s11104-016-2910-5. [DOI] [Google Scholar]
- Jaffré T (1980) Etude écologique du peuplement végétal des sols dérivés de roches ultrabasiques en Nouvelle Calédonie. Coll. Trau. et Doc. ORSTOM 124
- Jaffré T (1992) Floristic and ecological diversity of the vegetation on ultramafic rocks in New Caledonia. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils: proceedings of the first international conference on serpentine ecology. Intercept Ltd., Andover
- Jaffré T, L’Huillier L (2010) La vegetation des roches ultramafiques ou terrains miniers. In: L’Huillier L, Jaffré T, Wulf A (eds) Mines et environnement en Nouvelle-Calédonie: les milieux sur substrats ultramafiques et leur restauration. IAC Ed, Noumea
- Jaffré T, Munzinger J, Lowry PP., II Threats to the conifer species found on New Caledonia’s ultramafic massifs and proposals for urgently needed measures to improve their protection. Biodivers Conserv. 2010;19:1485–1502. doi: 10.1007/s10531-010-9780-6. [DOI] [Google Scholar]
- Jaffré T, Pillon Y, Thomine S, Merlot S. The metal hyperaccumulators from New Caledonia can broaden our understanding of nickel accumulation in plants. Front Plant Sci. 2013;4:279. doi: 10.3389/fpls.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson Flora Project (eds) (2016) Jepson eFlora, http://ucjeps.berkeley.edu/IJM.html. Accessed 14 Oct 2016
- Jones DT, Rahman H, Bignell DE, Prasetyo AH. Forests on ultramafic-derived soils in borneo have very depauperate termite assemblages. J Trop Ecol. 2010;26:103–114. doi: 10.1017/S0266467409990356. [DOI] [Google Scholar]
- Kay KM, Ward KL, Watt LR, Schemske DW. Plant speciation. In: Harrison SP, Rajakaruna N, editors. Serpentine: evolution and ecology in a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Kazakou E, Dimitrakopoulos PG, Baker AJM, Reeves RD, Troumbis AY. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to ultramafic soils: from species to ecosystem level. Biol Rev. 2008;83:495–508. doi: 10.1111/j.1469-185X.2008.00051.x. [DOI] [PubMed] [Google Scholar]
- Kfayatullah Q, Shah MT, Arfan M. Biogeochemical and environmental study of the chromite-rich ultramafic terrain of Malakand area, Pakistan. Environ Geol. 2001;40:1482–1486. doi: 10.1007/s002540100374. [DOI] [Google Scholar]
- Kien CN, Noi NV, Son LT, Ngoc HM, Tanaka S, Nishina T, Iwasaki K. Heavy metal contamination of agricultural soils around a chromite mine in Vietnam. Soil Sci Plant Nutr. 2010;56:344–356. doi: 10.1111/j.1747-0765.2010.00451.x. [DOI] [Google Scholar]
- Kier G, Mutke J, Dinerstein E, Ricketts TH, Küper W, Kreft H, Barthlott W. Global patterns of plant diversity and floristic knowledge. J Biogeogr. 2005;32:1107–1116. doi: 10.1111/j.1365-2699.2005.01272.x. [DOI] [Google Scholar]
- Kierczak J, Neel C, Bril H, Puziewicz J. Effect of mineralogy and pedoclimatic variations on Ni and Cr distribution in serpentine soils under temperate climate. Geoderma. 2007;142:165–177. doi: 10.1016/j.geoderma.2007.08.009. [DOI] [Google Scholar]
- Kitayama K. An altitudinal transect study of the vegetation on Mount Kinabalu, Borneo. Vegetatio. 1992;102:149–171. doi: 10.1007/BF00044731. [DOI] [Google Scholar]
- Kolář F, Fér T, Štech M, Trávníček P, Dušková E, Schönswetter P, Suda J. Bringing together evolution on serpentine and polyploidy: spatiotemporal history of the diploid-tetraploid complex of Knautia arvensis (Dipsacaceae) PLoS ONE. 2012;7(7):e39988. doi: 10.1371/journal.pone.0039988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U. Metal hyperaccumulation in plants. Annu Rev Plant Biol. 2010;61:517–534. doi: 10.1146/annurev-arplant-042809-112156. [DOI] [PubMed] [Google Scholar]
- Kruckeberg AR. The ecology of serpentine soils: a symposium. III. Plant species in relation to serpentine soils. Ecology. 1954;35:267–274. [Google Scholar]
- Kruckeberg AR. Geology and plant life: the effects of landforms and rock type on plants. Seattle: University of Washington Press; 2002. [Google Scholar]
- Kumarathilaka P, Oze C, Vithanage V. Perchlorate mobilization of metals in serpentine soils. Appl Geochem. 2016 [Google Scholar]
- Lau JA, McCall AC, Davies KF, McKay JF, Wright JW. Herbivores and edaphic factors constrain the realized niche of a native plant. Ecology. 2008;89:754–762. doi: 10.1890/07-0591.1. [DOI] [PubMed] [Google Scholar]
- Leakey RJG, Proctor J. Invertebrates in the litter and soil at a range of altitudes on Gunung Silam, a small ultrabasic mountain in Sabah. J Trop Ecol. 1987;3:119–129. doi: 10.1017/S026646740000184X. [DOI] [Google Scholar]
- Lee WG. The serpentinized areas of New Zealand, their structure and ecology. In: Roberts BA, Proctor J, editors. The ecology of areas with serpentinized rocks: a world view. Dordrecht: Kluwer; 1992. [Google Scholar]
- Linthout K, Helmers H. Pliocene obducted, rotated and migrated ultramafic rocks and obduction-induced anatectic granite, SW Seram and Ambon, Eastern Indonesia. J Southeast Asian Earth Sci. 1994;9:95–109. doi: 10.1016/0743-9547(94)90068-X. [DOI] [Google Scholar]
- Ma Y, Rajkumar M, Rocha I, Oliveira RS, Freitas H. Serpentine bacteria influence metal translocation and bioconcentration of Brassica juncea and Ricinus communis grown in multi-metal polluted soils. Front Plant Sci. 2015;5:757. doi: 10.3389/fpls.2014.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AS, Barr SM. The Nan River mafic-ultramafic belt, northern Thailand: geochemistry and tectonic significance. Bull Geol Soc Malays. 1984;17:209–224. [Google Scholar]
- Mandal A, Mohanty WK, Prakash S, Sharma SP, Gupta S. Laterite covered mafic-ultramafic rocks: potential target for chromite exploration—a case study from southern part of Tangarparha, Odisha. J Geol Soc India. 2015;86:519–529. doi: 10.1007/s12594-015-0342-0. [DOI] [Google Scholar]
- Meindl GA, Bain DJ, Ashman TL. Edaphic factors and plant–insect interactions: direct and indirect effects of serpentine soil on florivores and pollinators. Oecologia. 2013;173:1355–1366. doi: 10.1007/s00442-013-2711-y. [DOI] [PubMed] [Google Scholar]
- Mesjasz-Przybylowicz J, Przybylowicz W, Barnabas A, van der Ent A. Extreme nickel hyperaccumulation in the vascular tracts of the tree Phyllanthus balgooyi from Borneo. New Phytol. 2015;209:1513–1526. doi: 10.1111/nph.13712. [DOI] [PubMed] [Google Scholar]
- Ministry of Environment and Renewable Energy (2012) The National Red List 2012 of Sri Lanka; Conservation status of the fauna and flora. Ministry of Environment, Colombo. http://www.environmentmin.gov.lk/web/index.php?option=com_content&view=article&id=175&Itemid=291&lang=en. Accessed 24 Mar 2014
- Mishra S, Das AP, Seragadam P. Microbial remediation of hexavalent chromium from chromite contaminated mines of Sukinda Valley, Orissa (India) J Environ Res Dev. 2009;3:1122–1127. [Google Scholar]
- Mitra S. Olivines from Sukinda ultramafites and the nature of the parental magma. N Jb Miner Mh. 1973;2:177–189. [Google Scholar]
- Mittermeier RA, et al. Hotspots revisited. Earth’s biologically richest and most endangered terrestrial ecoregions. Chicago: University of Chicago Press; 2005. [Google Scholar]
- MOE (2012) The National Red List 2012 of Sri Lanka; Conservation status of the fauna and flora. Ministry of Environment, Colombo
- Mohanty M, Pattnaik MM, Mishra AK, Patra HK. Chromium bioaccumulation in rice grown in contaminated soil and irrigated mine wastewater—a case study at South Kaliapani chromite mine area, Orissa, India. Int J Phytoremediat. 2011;13:297–409. doi: 10.1080/15226511003753979. [DOI] [PubMed] [Google Scholar]
- Mohanty M, Pattnaik MM, Mishra AK, Patra HK. Bio-concentration—an in situ phytoremediation study at South Kaliapani chromite mining area of Orissa, India. Environ Monit Assess. 2012;184:1015–1024. doi: 10.1007/s10661-011-2017-7. [DOI] [PubMed] [Google Scholar]
- Moores EM. Serpentinites and other ultramafic rocks: why they are important for Earth’s history and possibly for its future. In: Harrison SP, Rajakaruna N, editors. Serpentine: evolution and ecology in a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Morel J-L, Echevarria G, Goncharova N, editors. Phytoremediation of metal-contaminated soils. Dordrecht: Springer; 2006. [Google Scholar]
- Munasinghe T, Dissanayake CB. Is the Highland eastern Vijayan boundary in Sri Lanka a possible mineralized belt? Econ Geol. 1980;75:775–777. doi: 10.2113/gsecongeo.75.5.775. [DOI] [Google Scholar]
- Murren CJ, Douglass L, Gibson A, Dudash MR (2006) Individual and combined effects of Ca/Mg ratio and water on trait expression in Mimulus guttatus. Ecology 87:2591–2602. doi:10.1890/0012-9658(2006)87[2591:IACEOM]2.0.CO;2 [DOI] [PubMed]
- Naseem S, Bashir E, Shireen K, Shafiq S. Soil–plant relationship of Pteropyrum olivieri, a ultramafic flora of Wadh, Balochistan, Pakistan and its use in mineral prospecting. Geologia. 2009;54:33–39. doi: 10.5038/1937-8602.54.2.7. [DOI] [Google Scholar]
- Neilson S, Rajakaruna N. Phytoremediation of agricultural soils: using plants to clean metal-contaminated arable lands. In: Ansari AA, Gill SS, Lanza GR, Newman L, editors. Phytoremediation: management of environmental contaminants. Switzerland: Springer International Publishing; 2014. [Google Scholar]
- Netty S, Wardiyati T, Handayanto E, Maghfoer MD. Nickel accumulating plants in the post-mining land of Sorowako, South Sulawesi, Indonesia. J Trop Agric. 2012;50:45–48. [Google Scholar]
- New Zealand Plant Conservation Network (2016) http://www.nzpcn.org.nz/. Accessed 13 Oct 2016
- Ningthoujama PS, Dubeya CS, Guillotb S, Fagionb A-S, Shuklaa DP. Origin and serpentinization of ultramafic rocks of Manipur Ophiolite Complex in the Indo-Myanmar subduction zone, Northeast India. J Asian Earth Sci. 2012;50:128–140. doi: 10.1016/j.jseaes.2012.01.004. [DOI] [Google Scholar]
- O’Dell RE. Conservation and restoration of chemically extreme edaphic endemic flora in the Western US. In: Rajakaruna N, Boyd RS, Harris TB, editors. Plant ecology and evolution in harsh environments. Hauppauge: Nova Science Publishers; 2014. [Google Scholar]
- O’Dell RE, Claassen VP. Restoration and revegetation of harsh soils. In: Harrison SP, Rajakaruna N, editors. Serpentine: the evolution and ecology of a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- O’Dell RE, Rajakaruna N. Intraspecific variation, adaptation, and evolution. In: Harrison SP, Rajakaruna N, editors. Serpentine: evolution and ecology in a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Orberger B, Lorand JP, Girardeau J, Mercier JCC, Pitragool S. Petrogenesis of ultramafic rocks and associated chromitites in the Nan Uttaradit ophiolite, Northern Thailand. Lithos. 1995;35:153–182. doi: 10.1016/0024-4937(94)00041-Y. [DOI] [Google Scholar]
- Pal A, Paul AK. Accumulation of polyhydroxyalkanoates by rhizobacteria underneath nickel-hyperaccumulators from ultramafic ecosystem. J Polym Environ. 2012;20:10–16. doi: 10.1007/s10924-011-0355-8. [DOI] [Google Scholar]
- Pal A, Choudhuri P, Dutta S, Mukherjee PK, Paul AK. Isolation and characterization of nickel-resistant microflora from ultramafic soils of Andaman. World J Microbiol Biotechnol. 2004;20:881–886. doi: 10.1007/s11274-004-2776-1. [DOI] [Google Scholar]
- Pal A, Dutta S, Mukherjee PK, Paul AK. Occurrence of heavy metal-resistance in microflora from ultramafic soil of Andaman. J Basic Microbiol. 2005;45:207–218. doi: 10.1002/jobm.200410499. [DOI] [PubMed] [Google Scholar]
- Pal A, Ghosh S, Paul AK. Biosorption of cobalt by fungi from ultramafic soil of Andaman. Bioresour Technol. 2006;97:1253–1258. doi: 10.1016/j.biortech.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Pal A, Wauters G, Paul AK. Nickel tolerance and accumulation by bacteria from rhizosphere of nickel hyperaccumulators in serpentine soil ecosystem of Andaman, India. Plant Soil. 2007;293:37–48. doi: 10.1007/s11104-007-9195-7. [DOI] [Google Scholar]
- Palm ER, Van Volkenburgh E. Physiological adaptations of plants to serpentine soils. In: Rajakaruna N, Boyd RS, Harris TB, editors. Plant ecology and evolution in harsh environments. Hauppauge: Nova Science Publishers; 2014. [Google Scholar]
- Parry DE (1985) Ultramafic soils in the humid tropics with particular reference to Indonesia. Unpublished report of Hunting Technical Services Ltd., Taupo
- Peng CI, Lin CW, Rimi R, Kono Y, Leong W, Chung KF. Two new species of Begonia, B. moneta and B. peridoticola (Begoniaceae) from Sabah, Malaysia. Bot Stud. 2015;56:7. doi: 10.1186/s40529-015-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peucker-Ehrenbrink B, Miller MW. Quantitative bedrock geology of east and Southeast Asia (Brunei, Cambodia, eastern and southeastern China, East Timor, Indonesia, Japan, Laos, Malaysia, Myanmar, North Korea, Papua New Guinea, Philippines, far-eastern Russia, Singapore, South Korea, Taiwan, Thailand, Vietnam) Geochem Geophys. 2004 [Google Scholar]
- Pillon Y. Time and tempo of diversification in the flora of New Caledonia. Bot J Linn Soc. 2012;170:288–298. doi: 10.1111/j.1095-8339.2012.01274.x. [DOI] [Google Scholar]
- Pillon Y, Munzinger J, Amir H, Lebrun M. Ultramafic soils and species sorting in the flora of New Caledonia. J Ecol. 2010;98:1108–1116. doi: 10.1111/j.1365-2745.2010.01689.x. [DOI] [Google Scholar]
- Pollard AJ, Reeves RD, Baker AJM. Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci. 2014;217–218:8–17. doi: 10.1016/j.plantsci.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Prasad PRC, Reddy CS, Dutt CBS. Phytodiversity assessment of tropical rainforest of North Andaman Islands, India. Res J For. 2007;1:27–39. [Google Scholar]
- Proctor J. The vegetation over ultramafic rocks in the tropical far east. In: Roberts BA, Proctor J, editors. The ecology of areas with serpentinised rocks. A world view. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Proctor J. Vegetation and soil and plant chemistry on ultramafic rocks in the tropical Far East. Perspect Plant Ecol Evol Syst. 2003;6:105–124. doi: 10.1078/1433-8319-00045. [DOI] [Google Scholar]
- Proctor J, Cole MM (1992) The ecology of ultramafic areas in Zimbabwe. In: The ecology of areas with serpentinized rocks. Springer Netherlands, Dordrecht, pp 313–331
- Proctor J, Lee YF, Langley AM, Munro WR, Nelson T. Ecological studies on Gunung Silam, a small ultrabasic mountain in Sabah, Malaysia. I. Environment, forest structure and floristics. J Ecol. 1988;76:320–340. doi: 10.2307/2260596. [DOI] [Google Scholar]
- Proctor J, Howson G, Munro WRC, Robertson FM (1988b) Use of the cotton strip assay at 3 altitudes on an ultrabasic mountain in Sabah, Malaysia. In: Harrison AF, Latter PM, Walton DWH (eds) Cotton strip assay: an index of decomposition in soils. Grange-over-Sands, NERC/ITE. (ITE Symposium, 24: 117–122)
- Proctor J, Phillipps C, Duff DK, Heaney A, Robertson FM. Ecological studies on Gunung Silam, a small ultrabasic mountain in Sabah, Malaysia. II. Some forest processes. J Ecol. 1989;77:317–331. doi: 10.2307/2260752. [DOI] [Google Scholar]
- Proctor J, van Balgooy MMJ, Fairweather FM, Nagy L, Reeves RD. A preliminary re-investigation of a plant geographical ‘El Dorado’. Trop Biodivers. 1994;2:303–316. [Google Scholar]
- Proctor J, Baker AJM, van Balgooy MMJ, Bruijnzeel LA, Jones SH, Madulid DA. Mount Bloomfield, Palawan, the Philippines: the scrub and Gymnostoma woodland. In: Jaffré T, Reeves RD, Bacquer T, editors. The ecology of ultramafic and metalliferous areas. New Caledonia: ORSTOM; 1997. [Google Scholar]
- Proctor J, Argent GC, Madulid DA. Forests of the ultramafic Mount Giting-Giting, Sibuyan Island, Philippines. Edinb J Bot. 1998;55:295–316. doi: 10.1017/S0960428600002201. [DOI] [Google Scholar]
- Proctor J, Bruijnzeel LA, Baker AJM. What causes the vegetation types on Mount Bloomfield, a coastal tropical mountain of the western Philippines? Global Ecol Biogeogr. 1999;8:347–354. doi: 10.1046/j.1365-2699.1999.00147.x. [DOI] [Google Scholar]
- Proctor J, Baker AJM, Bruijnzeel LA, Van MMJ, Fairweather GM, Madulid DA. Foliar chemistry and leaf herbivory on Mount Bloomfield, Palawan, Philippines. Bot J Scotland. 2000;52:79–89. doi: 10.1080/03746600008684946. [DOI] [Google Scholar]
- Proctor J, Baker AJM, van Balgooy MMJ, Bruijnzeel LA, Jones SH, Madulid DA. Mount Bloomfield, Palawan, Philippines: forests on greywacke and serpentinized peridotite. Edinb J Bot. 2000;57:121–139. doi: 10.1017/S0960428600000081. [DOI] [Google Scholar]
- Quimado MO, Fernando ES, Trinidad LC, Doronila A. Nickel hyperaccumulating species of Phyllanthus (Phyllanthaceae) from the Philippines. Aust J Bot. 2015;63:103–110. [Google Scholar]
- Rajakaruna N. The edaphic factor in the origin of species. Int Geol Rev. 2004;46:471–478. doi: 10.2747/0020-6814.46.5.471. [DOI] [Google Scholar]
- Rajakaruna N, Baker AJM. Ultramafic: a model habitat for botanical research in Sri Lanka. Ceylon J Sci Biol Sci. 2004;32:1–19. [Google Scholar]
- Rajakaruna N, Bohm BA. Ultramafic and its vegetation: a preliminary study from Sri Lanka. J Appl Bot Angew Bot. 2002;76:20–28. [Google Scholar]
- Rajakaruna N, Boyd RS. The edaphic factor. In: Jorgensen SE, Fath B, editors. The encyclopedia of ecology. Oxford: Elsevier; 2008. [Google Scholar]
- Rajakaruna N, Harris CS, Towers GHN. Antimicrobial activity of plants collected from ultramafic outcrops in Sri Lanka. Pharm Biol. 2002;40:235–244. doi: 10.1076/phbi.40.3.235.5825. [DOI] [Google Scholar]
- Rajakaruna N, Harris TB, Alexander EB. Serpentine geoecology of eastern North America: a review. Rhodora. 2009;111:21–108. doi: 10.3119/07-23.1. [DOI] [Google Scholar]
- Rajakaruna N, Knudsen K, Fryday A, O’Dell RE, Pope N, Olday FC, Woolhouse S. Investigation of the importance of rock chemistry for saxicolous lichen communities of the New Idria serpentinite mass, San Benito County, California, USA. Lichenologist. 2012;44:695–714. doi: 10.1017/S0024282912000205. [DOI] [Google Scholar]
- Rajapaksha AU, Vithanage M, Oze C, et al. Nickel and manganese release in ultramafic soil from the Ussangoda ultramafic complex, Sri Lanka. Geoderma. 2012;189–190:1–9. doi: 10.1016/j.geoderma.2012.04.019. [DOI] [Google Scholar]
- Rajapaksha AU, Vithanage M, Ok YS, Oze C. Cr(VI) formation related to Cr(III)-muscovite and birnessite interactions in ultramafic environments. Environ Sci Technol. 2013;47:9722–9729. doi: 10.1021/es4015025. [DOI] [PubMed] [Google Scholar]
- Ranasinghe NS (1987) Serpentinites associated with the Precambrian of Sri Lanka. Geological Society of Sri Lanka special publication No. 3. Geological Survey Department, Colombo
- Reeves RD. Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant Soil. 2003;249:57–65. doi: 10.1023/A:1022572517197. [DOI] [Google Scholar]
- Reeves R, Baker A, Borhidi A, Berazain R. Nickel hyperaccumulation in the serpentine flora of Cuba. Ann Bot. 1999;83:1–10. doi: 10.1006/anbo.1998.0786. [DOI] [Google Scholar]
- Safford HD, Harrison SP. Fire effects on plant diversity in serpentine versus sandstone chaparral. Ecology. 2004;85:539–548. doi: 10.1890/03-0039. [DOI] [Google Scholar]
- Safford HD, Viers JH, Harrison SP. Serpentine endemism in the California flora: a database of serpentine affinity. Madroño. 2005;52:222–257. doi: 10.3120/0024-9637(2005)52[222:SEITCF]2.0.CO;2. [DOI] [Google Scholar]
- Samantaray S, Rout GR, Das P. Heavy metal and nutrient concentration in soil and plants growing on a metalliferous chromite minespoil. Environ Technol. 2001;22:1147–1154. doi: 10.1080/09593332208618204. [DOI] [PubMed] [Google Scholar]
- Sambatti JBM, Rice KJ. Local adaptation, patterns of selection, and gene flow in the Californian serpentine sunflower (Helianthus exilis) Evolution. 2006;60:696–710. doi: 10.1111/j.0014-3820.2006.tb01149.x. [DOI] [PubMed] [Google Scholar]
- Samithri YAS (2015) Ecology of the serpentine vegetation at Ussangoda, Sri Lanka. M. Phil Thesis, University of Peradeniya, Sri Lanka
- Sawada Y, Aiba S, Takyu M, Repin R, Nais J, Kitayama K. Community dynamics over 14 years along gradients of geological substrate and topography in tropical montane forests on Mount Kinabalu, Borneo. J Trop Ecol. 2015;31:117–128. doi: 10.1017/S0266467414000777. [DOI] [Google Scholar]
- Schechter S, Branco S. The ecology and evolution of mycorrhizal fungi in extreme soils. In: Rajakaruna N, Boyd RS, Harris TB, editors. Plant ecology and evolution in harsh environment. Hauppauge: Nova Science Publishers; 2014. [Google Scholar]
- Selby JP, Jeong AL, Toll K, Wright KM, Lowry DB. Methods and discoveries in the pursuit of understanding the genetic basis of adaptation to harsh environments in Mimulus. In: Rajakaruna N, Boyd RS, Harris TB, editors. Plant ecology and evolution in harsh environments. Hauppauge: Nova Science Publishers; 2014. [Google Scholar]
- Senevirathne AS, Nandadasa HG, Fernando WS, Sanjeevani, HHVM, Rajapakshe RLHR (2000) The serpentine vegetation of Ussangoda (Hambantota District) and nickel accumulating plant species. In: Proceedings of the Sixth Annual Forestry and Environmental Symposium, Kandy. http://journals.sjp.ac.lk/index.php/fesympo/article/view/1430. Accessed 11 Oct 2016
- Seneviratne M, Seneviratne G, Madawala HMSP, Iqbal MCM, Rajakaruna N, Vithanage M. A preliminary study of the role of bacterial–fungal co-inoculation on heavy metal phytotoxicity in serpentine soil. Aust J Bot. 2016;63:261–268. doi: 10.1071/BT14270. [DOI] [Google Scholar]
- Seneviratne M, Gunaratne S, Bandara T, Weerasundara L, Rajakaruna N, Madawala HMSP, Seneviratne G, Vithanage M. Plant growth promotion by Bradyrhizobium japonicum under heavy metal stress. S Afr J Bot. 2016;105:19–24. doi: 10.1016/j.sajb.2016.02.206. [DOI] [Google Scholar]
- Shah MT, Begum S, Khan S. Pedo and biogeochemical studies of mafic and ultramafic rocks in the Mingora and Kabal areas, Swat, Pakistan. Environ Earth Sci. 2010;60:1091–1102. doi: 10.1007/s12665-009-0253-8. [DOI] [Google Scholar]
- Shah MT, Ara J, Muhammad S, Khan S, Asad SA, Ali L. Potential heavy metals accumulation of indigenous plant species along the mafic and ultramafic terrain in the Mohmand Agency, Pakistan. Clean Soil Air Water. 2014;42:339–346. doi: 10.1002/clen.201200632. [DOI] [Google Scholar]
- Sheoran V, Sheoran AS, Poonia P. Phytomining: a review. Miner Eng. 2009;22:1007–1019. doi: 10.1016/j.mineng.2009.04.001. [DOI] [Google Scholar]
- Shi G, Harlow GE, Wang J, Wang J, Enoch NG, Wang X, Cao SM, Enyuancui W. Mineralogy of jadeitite and related rocks from Myanmar: a review with new data. Eur J Mineral. 2012;24:345–370. doi: 10.1127/0935-1221/2012/0024-2190. [DOI] [Google Scholar]
- Sodhi NS, Koh LP, Brook BW, Ng PK. Southeast Asian biodiversity: an impending disaster. Trends Ecol Evol. 2004;19:654–660. doi: 10.1016/j.tree.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Sodhi NS, Posa MRC, Lee TM, Bickford D, Koh LP, Brook BW. The state and conservation of Southeast Asian biodiversity. Biodivers Conserv. 2010;19:317–328. doi: 10.1007/s10531-009-9607-5. [DOI] [Google Scholar]
- Soibam I, Khuman MCH, Subhamenon SS (2015) Ophiolitic rocks of the Indo-Myanmar Ranges, NE India: relicts of an inverted and tectonically imbricated hyper-extended continental margin basin? Geological Society, London, Special Publications, pp 413. doi: 10.1144/SP413.12
- Southworth D, Tackaberry LE, Massicotte HB. Mycorrhizal ecology on serpentine soils. Plant Ecol Divers. 2014;7:445–455. doi: 10.1080/17550874.2013.848950. [DOI] [Google Scholar]
- Spasojevic MJ, Damschen EI, Harrison SP. Patterns of seed dispersal syndromes on serpentine soils: examining the roles of habitat patchiness, soil infertility and correlated functional traits. Plant Ecol Divers. 2014;7:401–410. doi: 10.1080/17550874.2012.678506. [DOI] [Google Scholar]
- Springer YP. Edaphic quality and plant–pathogen interactions: effects of soil calcium on fungal infection of a serpentine flax. Ecology. 2009;90:1852–1862. doi: 10.1890/08-0740.1. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Boyd RS. Herbivory and other cross-kingdom interactions on harsh soils. In: Harrison SP, Rajakaruna N, editors. Serpentine: the evolution and ecology of a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Strauss SY, Cacho NI. Nowhere to run, nowhere to hide: the importance of enemies and apparency in adaptation to harsh soil environments. Am Nat. 2013;182(1):E1–E14. doi: 10.1086/670754. [DOI] [PubMed] [Google Scholar]
- Sugau JB, van der Ent A. Pittosporum peridoticola (Pittosporaceae), a new ultramafic obligate species restricted to Kinabalu Park (Sabah, Malaysia) Bot Stud. 2016;57:4. doi: 10.1186/s40529-016-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BK, Khoo TT. Clinopyroxene composition and tectonic setting of the Bentong-Raub belt, Peninsular Malaysia. J Southeast Asian Earth Sci. 1993;8:539–545. doi: 10.1016/0743-9547(93)90051-P. [DOI] [Google Scholar]
- Tashakor M, Yaacob WZW, Mohamad H. Speciation and availability of Cr, Ni and Co in ultramafic soils of Ranau, Sabah. Am J Geosci. 2011;2:4–9. doi: 10.3844/ajgsp.2011.4.9. [DOI] [Google Scholar]
- Tashakor M, Yaacob WZW, Mohamad H. Ultramafic soils, adverse habitat for plants: case study at Peninsular. Am J Environ Sci. 2013;9:82–87. doi: 10.3844/ajessp.2013.82.87. [DOI] [Google Scholar]
- Tennakoon K, Senevirathna MKI, Kehelpannala KVW. Extraction of pure metallic nickel from ores and plants at Ussangoda, Sri Lanka. J Natl Sci Found. 2007;35:245–250. [Google Scholar]
- Thanh NX, Hai TT, Hoang N, Lan VQ, et al. Backarc mafic–ultramafic magmatism in Northeastern Vietnam and its regional tectonic significance. J Asian Earth Sci. 2014;90:45–60. doi: 10.1016/j.jseaes.2014.04.001. [DOI] [Google Scholar]
- Thomas L, Proctor J. Invertebrates in the litter and soil on the ultramafic Mount Giting-Giting, Philippines. J Trop Ecol. 1997;13:125–131. doi: 10.1017/S0266467400010300. [DOI] [Google Scholar]
- Thorne JH, Huber PR, Harrison SP. Systematic conservation planning: Protecting rarity, representation, and connectivity in regional landscapes. In: Harrison SP, Rajakaruna N, editors. Serpentine: evolution and ecology in a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Turner TL, Bourne EC, Von Wettberg EJ, Hu TT, Nuzhdin SV. Population resequencing reveals local adaptation of Arabidopsis lyrata to serpentine soils. Nat Genet. 2010;42:260–263. doi: 10.1038/ng.515. [DOI] [PubMed] [Google Scholar]
- Tylko G, Mesjasz-Przybyłowicz J, Przybyłowicz WJ. X-ray microanalysis of biological material in the frozen-hydrated state by PIXE. Microsc Res Tech. 2007;70:55–68. doi: 10.1002/jemt.20387. [DOI] [PubMed] [Google Scholar]
- UNESCO 2016. UNESCO Global Geoparks. http://www.unesco.org/new/en/natural-sciences/environment/earth-sciences/unesco-global-geoparks/. Accessed 27 Oct 2016
- Vallano DM, Selmants PC, Zavaleta ES. Simulated nitrogen deposition enhances the performance of an exotic grass relative to native ultramafic grassland competitors. Plant Ecol. 2012;213:1015–1026. doi: 10.1007/s11258-012-0061-1. [DOI] [Google Scholar]
- Van Balgooy MMJ and Tantra IGM (1986) The vegetation in two areas in Sulawesi, Indonesia, pp 1–61. Buletin Penelitian Hutan Special edition
- Van der Ent A, Mulligan D. Multi-element concentrations in plant parts and fluids of malaysian nickel hyperaccumulator plants and some economic and ecological considerations. J Chem Ecol. 2015;41:396–408. doi: 10.1007/s10886-015-0573-y. [DOI] [PubMed] [Google Scholar]
- Van der Ent A, Reeves RD. Foliar metal accumulation in plants from copper-rich ultramafic outcrops: case studies from Malaysia and Brazil. Plant Soil. 2015;389:401–418. doi: 10.1007/s11104-015-2385-9. [DOI] [Google Scholar]
- Van der Ent A, Vanijajiva O. Gynura tambuyukonensis (Asteraceae), an obligate ultramafic species endemic to Mount Tambuyukon (Kinabalu Park, Sabah, Malaysia) Phytotaxa. 2014;158:291–296. [Google Scholar]
- Van der Ent A, Wong KM. Range extension of Christisonia scortechinii from mainland Southeast Asia into Borneo, and notes on the distinction between Aeginetia and Christisonia (Orobanchaceae) Bot Stud. 2015;56:28. doi: 10.1186/s40529-015-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ent A, Wood JJ. Mount Tambuyukon—an intriguing mountain and its orchids. Malesian Orchid J. 2012;10:102–122. [Google Scholar]
- Van der Ent A, Wood JJ. Orchids of extreme serpentinite (ultramafic) habitats in Kinabalu Park. Males Orchid J. 2013;12:39–54. [Google Scholar]
- Van der Ent A, Baker AJM, van Balgooy MMJ, Tjoa A. Ultramafic nickel laterites in Indonesia (Sulawesi, Halmahera): mining, nickel hyperaccumulators and opportunities for phytomining. J Geochem Explor. 2013;128:72–79. doi: 10.1016/j.gexplo.2013.01.009. [DOI] [Google Scholar]
- Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H. Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil. 2013;362:319–334. doi: 10.1007/s11104-012-1287-3. [DOI] [Google Scholar]
- Van der Ent A, Mulligan DR, Erskine P (2013b) Discovery of nickel hyperaccumulators from Kinabalu Park, Sabah (Malaysia) for potential utilization in phytomining. In: Paper presented at environment 2013: 3rd international seminar on environmental issues in mining. Proceedings. Santiago, Chile December 4–6
- Van der Ent A, Repin R, Sugau J, Wong KM (2014) The ultramafic flora of Sabah: an introduction to the plant diversity on ultramafic soils. Natural History Publications (Borneo), Kota Kinabalu
- Van der Ent A, Wong KM, Sugau J, Repin R. Plant diversity and ecology of ultramafic outcrops in Sabah (Malaysia) Aust J Bot. 2015;63:204–215. doi: 10.1071/BT14214. [DOI] [Google Scholar]
- Van der Ent A, Sumail S, Clarke C. Habitat differentiation of obligate ultramafic Nepenthes endemic to Mount Kinabalu and Mount Tambuyukon (Sabah, Malaysia) Plant Ecol. 2015;216:789–807. doi: 10.1007/s11258-015-0468-6. [DOI] [Google Scholar]
- Van der Ent A, Rajakaruna R, Boyd RS, Echevarria G, Repin R, Williams D. Global research on ultramafic (serpentine) ecosystems (8th international conference on serpentine ecology in Sabah, Malaysia): a summary and synthesis. Aust J Bot. 2015;63:1–16. [Google Scholar]
- Van der Ent A, Jaffré T, L’Huillier L, Gibson N, Reeves RR. The flora of ultramafic soils in the Australia-Pacific Region: state of knowledge and research priorities. Aust J Bot. 2015;63:173–190. doi: 10.1071/BT15038. [DOI] [Google Scholar]
- Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H. Commentary: toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front Plant Sci. 2015;6:554. doi: 10.3389/fpls.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ent A, Erskine PD, Sumail S. Ecology of nickel hyperaccumulator plants from ultramafic soils in Sabah (Malaysia) Chemoecology. 2015;25:243–259. doi: 10.1007/s00049-015-0192-7. [DOI] [Google Scholar]
- Van der Ent A, Baker AJM, Reeves RD, Chaney RL, Anderson C, Meech J, Erskine PD, Simonnot M-O, Vaughan J, Morel J-L, Echevarria G, Fogliani B, Mulligan D. ‘Agromining’: farming for metals in the future? Environ Sci Technol. 2015;49:4773–4780. doi: 10.1021/es506031u. [DOI] [PubMed] [Google Scholar]
- Van der Ent A, Erskine PD, Mulligan DR, Repin R, Karim R. Vegetation on ultramafic edaphic islands in Kinabalu Park (Sabah, Malaysia) in relation to soil chemistry and altitude. Plant Soil. 2016;403(1):77–101. [Google Scholar]
- Van der Ent A, Echevarria G, Tibbett M. Delimiting soil chemistry thresholds for nickel hyperaccumulator plants in Sabah (Malaysia) Chemoecology. 2016;26:67–82. doi: 10.1007/s00049-016-0209-x. [DOI] [Google Scholar]
- Van der Ent A, Van Balgooy MMJ, Van Welzen P. Actephila alanbakeri (Phyllanthaceae): a new nickel hyperaccumulating species from localised ultramafic soils in Sabah (Malaysia) Bot Stud. 2016;57(1):6. doi: 10.1186/s40529-016-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter A, Levanets A, Siebert S, Rajakaruna N. A preliminary survey of the diversity of soil algae and cyanoprokaryotes on mafic and ultramafic substrates in South Africa. Aust J Bot. 2015;63:341–352. doi: 10.1071/BT14207. [DOI] [Google Scholar]
- Visioli G, Marmiroli N. The proteomics of heavy metal hyperaccumulation by plants. J Proteom. 2013;79:133–145. doi: 10.1016/j.jprot.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Vithanage M, Rajapaksha AU, Oze C, Rajakaruna N, Dissanayake CB. Metal release from serpentine soils in Sri Lanka. Environ Monit Assess. 2014;186(6):3415–3429. doi: 10.1007/s10661-014-3626-8. [DOI] [PubMed] [Google Scholar]
- Von Wettberg EJ, Wright JW. Genomic approaches to understanding adaptation. In: Harrison SP, Rajakaruna N, editors. Serpentine: the evolution and ecology of a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Von Wettberg EJ, Ray-Mukherjee J, D’Adesky N, Nesbeth D, Sistla S. The evolutionary ecology and genetics of stress resistance syndrome (SRS) traits: revisiting Chapin, Autumn and Pugnaire (1993) In: Rajakaruna N, Boyd RS, Harris TB, editors. Plant ecology and evolution in harsh environments. Hauppauge: Nova Science Publishers; 2014. [Google Scholar]
- Weerasinghe HAS, Iqbal MCM. Plant diversity and soil characteristics of the Ussangoda ultramafic site. J Natl Sci Found. 2011;39:355–363. [Google Scholar]
- Weiss SB. Cars, cows, and checkerspot butterflies: nitrogen deposition and management of nutrient-poor grasslands for a threatened species. Conserv Biol. 1999;13:1476–1486. doi: 10.1046/j.1523-1739.1999.98468.x. [DOI] [Google Scholar]
- Wells K, Lakim MB, Schulz S, Ayasse M. Pitchers of Nepenthes rajah collect faecal droppings from both diurnal and nocturnal small mammals and emit fruity odour. J Trop Ecol. 2011;27:347–353. doi: 10.1017/S0266467411000162. [DOI] [Google Scholar]
- Whiting SN, Reeves RD, Richards D, Johnson MS, et al. Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor Ecol. 2004;12:106–116. doi: 10.1111/j.1061-2971.2004.00367.x. [DOI] [Google Scholar]
- Wild H. The flora of the Great Dyke of southern Rhodesia with special reference to the serpentine soils. Kirkia. 1965;5:49–86. [Google Scholar]
- Wither ED, Brooks RR. Hyperaccumulation of nickel by some plants of South-East Asia. J Geochem Explor. 1977;8:579–583. doi: 10.1016/0375-6742(77)90100-5. [DOI] [Google Scholar]
- Wolf A. Conservation of endemic plants in ultramafic landscapes. Biol Conserv. 2001;100:35–44. doi: 10.1016/S0006-3207(00)00205-6. [DOI] [Google Scholar]
- Wolf A, Thorp RW. Plant–pollinator interactions in naturally fragmented habitats. In: Harrison SP, Rajakaruna N, editors. Serpentine: evolution and ecology in a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Wong KM, van der Ent A. Eriobotrya balgooyi (Rosaceae), a new obligate ultramafic endemic from Kinabalu Park, Borneo. Plant Ecol Evol. 2014;147:134–140. doi: 10.5091/plecevo.2014.938. [DOI] [Google Scholar]
- Wood JJ, van der Ent A. Mount Tambuyukon - an intriguing mountain and its orchids. Malesian Orchid J. 2012;10:103–122. [Google Scholar]
- Wright JW, Stanton ML. Local adaptation in heterogeneous landscapes: reciprocal transplant experiments and beyond. In: Harrison SP, Rajakaruna N, editors. Serpentine: evolution and ecology in a model system. Berkeley: University of California Press; 2011. [Google Scholar]
- Wu CA, Lowry DB, Cooley AM, Wright KM, Lee YW, Willis JH. Mimulus is an emerging model system for the integration of ecological and genomic studies. Heredity. 2008;100(2):220–230. doi: 10.1038/sj.hdy.6801018. [DOI] [PubMed] [Google Scholar]
- Wu C, Lowry D, Nutter L, Willis J. Natural variation for drought-response traits in the Mimulus guttatus species complex. Oecologia. 2010;162:23–33. doi: 10.1007/s00442-009-1448-0. [DOI] [PubMed] [Google Scholar]


