Abstract
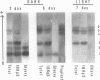
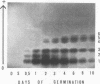
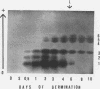
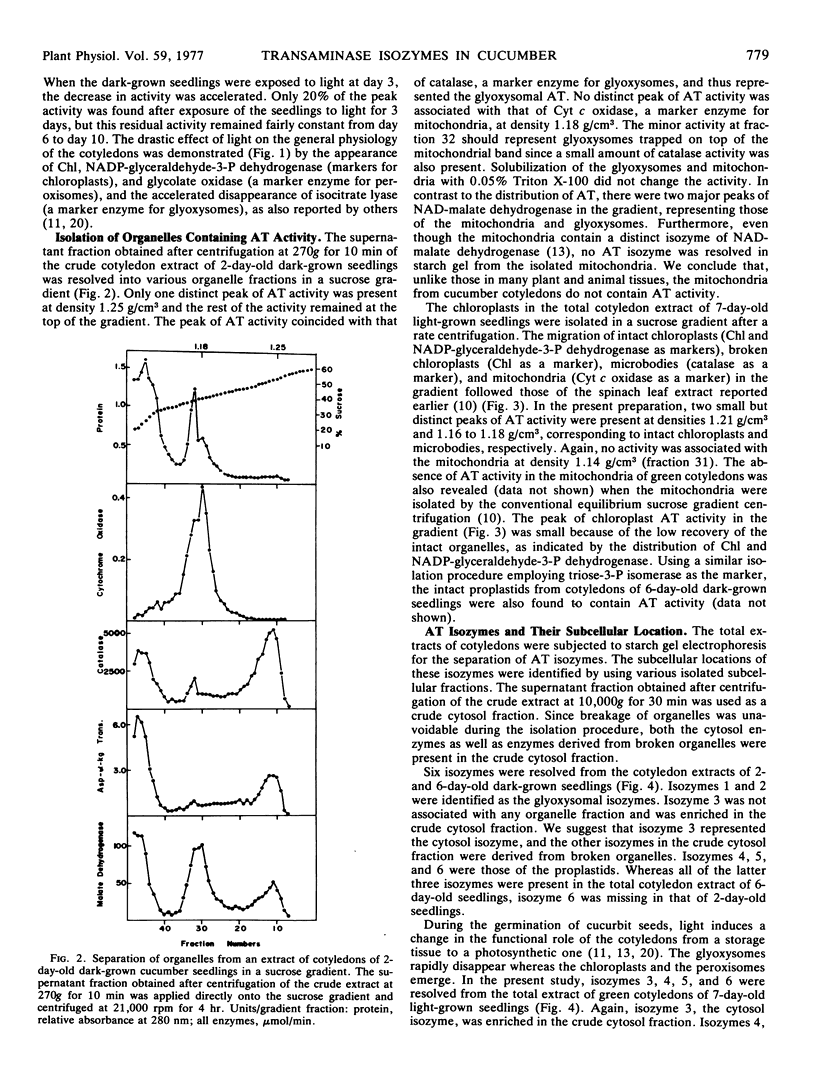
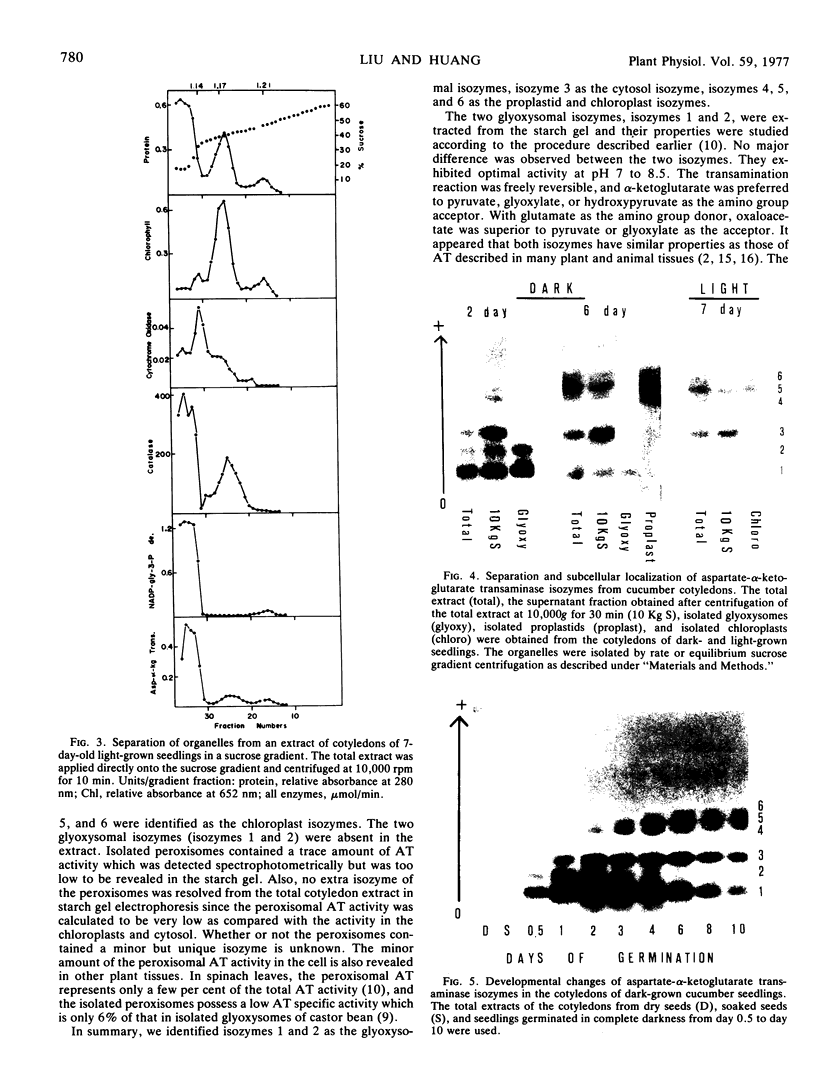
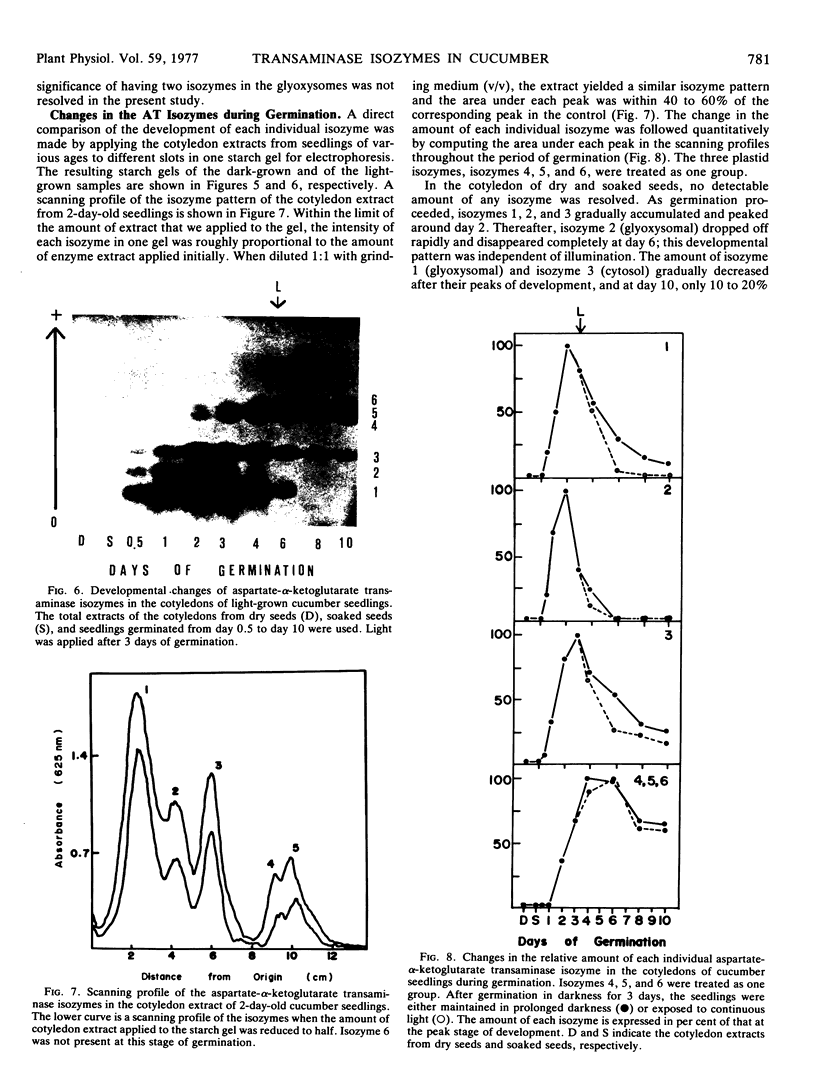
The total activity of aspartate-α-ketoglutarate transaminase in the cotyledons of cucumber (Cucumis sativus L.) seeds increased 17-fold during the first 2 days of germination in darkness and then declined gradually to 20% of the peak activity after 10 days. Exposure of the seedlings to light at day 3 accelerated the decline. The enzyme in the cotyledon extracts from seedlings at various ages was resolved into six distinct isozymes by starch gel electrophoresis. Isozymes 1 and 2 were glyoxysomal isozymes with different developmental patterns. Isozyme 1 followed the developmental pattern of the total enzyme activity in darkness, and was rapidly eliminated upon illumination. Isozyme 2 increased in activity to a peak at day 2 and declined rapidly thereafter, and disappeared completely at day 6; this developmental pattern was independent of light. No major difference in the optimal pH for activity, substrate specificity, and reversibility was observed between isozymes 1 and 2. The combined developmental pattern of isozymes 1 and 2 during germination correlated with that of the glyoxysomes. Isozyme 3 was located in the cytosol and its developmental pattern followed that of the total activity. Isozymes 4,5, and 6 were plastid isozymes and appeared only after 2 days of germination. Unlike many other chloroplast enzymes, the appearance of the chloroplast transaminase isozymes was under temporal control and was independent of illumination. No enzyme activity was detected in isolated mitochondria. The findings illustrate a complicated cellular control system for the appearance of various organelle-specific transaminase isozymes and thus the amino acid metabolism during germination.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter W. D., Beevers H. Distribution and Properties of Isocitritase in Plants. Plant Physiol. 1959 Jul;34(4):403–409. doi: 10.1104/pp.34.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- DeLorenzo R. J., Ruddle F. H. Glutamate oxalate transaminase (GOT) genetics in Mus musculus: linkage, polymorphism, and phenotypes of the Got-2 and Got-1 loci. Biochem Genet. 1970 Apr;4(2):259–273. doi: 10.1007/BF00485777. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H. Comparative studies of glyoxysomes from various Fatty seedlings. Plant Physiol. 1975 May;55(5):870–874. doi: 10.1104/pp.55.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Liu K. D., Youle R. J. Organelle-specific Isozymes of Aspartate-alpha-Ketoglutarate Transaminase in Spinach Leaves. Plant Physiol. 1976 Jul;58(1):110–113. doi: 10.1104/pp.58.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Beevers H. The development of microbodies (glyoxysomes and leaf peroxisomes) in cotyledons of germinating watermelon seedlings. Plant Physiol. 1975 Feb;55(2):258–264. doi: 10.1104/pp.55.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- Stewart C. R., Beevers H. Gluconeogenesis from amino acids in germinating castor bean endosperm and its role in transport to the embryo. Plant Physiol. 1967 Nov;42(11):1587–1595. doi: 10.1104/pp.42.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K. Leaf peroxisomes and their relation to photorespiration and photosynthesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):325–341. doi: 10.1111/j.1749-6632.1969.tb43119.x. [DOI] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]