Abstract
Although type-2 diabetes (T2D) has been reported to increase the risk of cognitive dysfunction and dementia, the underlying mechanisms remain unclear. Dementia-like pathology is attributed to the accumulation of cellular prion protein (PrPc) which plays a role in cognitive dysfunction. However, its involvement and regulation in diabetic dementia-like pathology is not well understood. Using T2D db/db (leptin receptor knockout) mice subjected to object recognition and Y-maze behavioral tests, we determined that short-term memory was compromised and that the mice displayed abrupt spontaneous behaviour compared to db/m control mice. MicroRNA analysis using qRT2-PCR array demonstrated a significant reduction in the transcript expression of microRNA-146a (miR-146a) in the brain of T2D db/db mice as compared to db/m controls. The sequence matching tools validated the binding of miR-146a to a conserved domain of the PrPc gene. Administration of mouse brain endothelial cell-derived exosomes (BECDEs) loaded with miR-146a into the brain’s ventricle of T2D db/db mice attenuated brain PrPc levels and restored short-term memory function though not significant. Also, we observed hyperphosphorylation of tau through decreased expression of glycogen synthase kinase-3 in T2D db/db brains that regulates microtubule organization and memory function. We conclude that underexpression of miR-146a upregulates PrPc production in T2D db/db mice and the delivery of BECDEs loaded with a miR-146a can down regulate PrPc levels and restore short term memory function.
Keywords: Cellular prion protein, diabetes, dementia, memory, microRNA
Introduction
Type-2 diabetes (T2D) describes a group of metabolic disorders that increases the risk factor for various cerebrovascular diseases and dementia (Haan et al., 2003; Pendlebury and Rothwell, 2009; Reitz et al., 2011). Dementia comprises several symptoms which include progressive loss of memory and disturbance in normal behaviour (Ritchie and Lovestone, 2002). The global prevalence of dementia is estimated to be 24 million people worldwide and the affected population is predicted to be doubled every 20 years, putting a costly burden of the disease globally (Ferri et al., 2005). Although different types of dementia exist, Alzheimer’s disease (AD) and vascular dementia (VaD) are the most prevalent types (Wiesmann et al., 2013). A meta-analysis performed on 28 studies suggests that diabetic patients face increased risk of AD by 56% and VaD by 127% (Gudala et al., 2013).
The key pathological events in AD brain involve deposition of amyloid beta (Aβ), a 36–43 amino acid peptide that accumulates extracellularly as plaques, and hyperphosphorylated tau (p-tau), a microtubule protein that intracellularly accumulates as neurofibrillary tangles (NFTs) (Takashima et al., 1998). Various mechanisms have been proposed previously whereby diabetes might influence the development of dementia by accelerating the pathological events. However, these studies are controversial. Some reports suggest that patients with diabetes have high Aβ plaques and NFT depositions (Janson et al., 2004; Peila et al., 2002), while others suggest no association or inverse relationship (Arvanitakis et al., 2006). Notably, some studies indicated a greater role of cellular prion protein (PrPc) over Aβ, which showed limited role in memory (Chung et al., 2010; Gimbel et al., 2010). Saedi et al have reviewed that there is strong evidence stating that diabetes mellitus increases the risk of cognitive impairment and dementia (Saedi et al., 2016). In a cohort study 1291 participants with type-2 diabetes from the Fremantle Diabetes Study and 5159 matched residents were documented (Davis et al., 2016). The study concluded that type-2 diabetes is associated with increased incidence of dementia.
MicroRNAs (miRNAs, miRs), a class of small non-coding RNAs, have been studied to play various functions in cerebrovascular complications as well as dementia (Kalani et al., 2014b; Maes et al., 2009). Recently, a study with deep sequencing of exosomes released from prion-infected neuronal cells revealed distinct miRNA signatures as compared to non-infected cells (Bellingham et al., 2012), suggesting the potential role of miRNAs over prion regulation. However, the role of miRNAs in memory loss related to prion in diabetes is not well studied. In this study we combined the therapeutic effects of exosomes and miR-146a to deliver in db/db mice T2D brains and evaluated the PrPc levels and cognitive function. Exosomes, a sub-population of micro-vesicles ranging 40–100 nm, have enormous potential to deliver therapeutic drugs, nucleic acids, and functional proteins (Haney et al., 2015; Kalani et al., 2014c). These nano-units can easily cross biological barriers, preserve mRNA and miRNAs, and deliver functional RNAs to the distal targets in the brain (Alvarez-Erviti et al., 2011; Kalani et al., 2014a; Valadi et al., 2007; Zomer et al., 2010). In this regard, exosomes can be a superior choice over other delivery vehicles for cerebral complications; though, more studies are still required to prove their suitability for the efficient molecular delivery and brain therapeutics.
In the current study, two aspects of T2D dementia-like factors were addressed: 1) We identified the miRNA which can regulate PrPc expression in dementia-like pathology; and 2) We explored the feasibility of using brain endothelial cell derived exosomes (BECDEs) to deliver miRNA to reduce PrPc and memory loss. Studies were performed using obese T2D db/db (leptin receptor knockout) mice that demonstrate hyperglycaemia, hyperinsulinemia, cerebro-vascular complications, AD and VaD pathology (Ramos-Rodriguez et al., 2013).
Materials and methods
Animals
All animal procedures were carefully reviewed and approved by the Institutional Animal Care and Use Committee, University of Louisville, in accordance with the animal care and guidelines of the National Institutes of Health. 12–14 weeks old mice were used for the experiments. The study groups consisted of: 1) obese male T2D db/db mice with background strain C57BL/KsJ (BKS.Cg-Dock7m +/+ Leprdb/J), and 2) lean age-matched non-diabetic (db/m) control mice. Mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and they were maintained on a 12 hour light/dark cycle with free access to food and water.
Novel object recognition test
The test was performed as published in our earlier report (Muradashvili et al., 2014), with some modifications. Briefly, the mice were acclimatized with familial objects two times a day for 3 days and an hour before the test. At the time of test, one of the old objects was changed with novel object and mouse tracking was performed for 5 min using a Top Scan behavioral analyzing system (Version 3.00 by CleverSys; Reston, VA, USA). The following formulas were used to calculate short-term memory: 1) Recognition Index= Novel object/novel object + old object; 2) Discrimination index= Novel object-old object/novel object + old object; 3) Preference Index= Novel object/novel object + old object x 100.
Y-maze test
Animals were acclimatized for 2 days, twice per day, for 10 min and then tested on Y-maze for determining spontaneous behaviour alterations. The test was performed for 8 min and the number of arm entries was counted when all four limbs of the mouse were within the arm. The number of triads (sequence of three consecutive visits to different arms) was recorded in order to calculate the percentage of spontaneous alternations according to the formula: Spontaneous alterations (%) =total number of triads/total number of arm entries-2 x 100.
BECDEs isolation, characterization, loading with miR-146a mimic and delivery
BECDEs were isolated from mouse brain endothelial cell line (bEnd.3) as described previously (Kalani et al., 2014a)(Kalani et al., 2016) with some modifications. Briefly, bEnd.3 cell culture conditioned media was ultracentrifuged at 1,40,000x g at 4°C for 3 h after following two centrifugations at 3,000x g and 10,000x g, respectively. The pellet obtained after ultracentrifugation was used as BECDEs source. BECDEs particle size and concentration was measured using the ZetaView PMX 110 nanoparticle tracking analysis (NTA) system (Particle Metrix, Meerbusch, Germany) and corresponding software ZetaView 8.02.31. The ZetaView system was calibrated using 100 nm polystyrene particles and the temperature was maintained ~25°C. BECDEs were loaded with miR-146a mimic or scramble mimic (Qiagen) using chemical transfection method (101 Bio, Palo Alto, CA, USA) as described by manufacture’s protocol. The excess of micelles, salts and short nucleotides were removed by spin columns (MW 3000, ThermoFisher Scientific, Grand Island, NY, USA). To visualize BECDEs in tissue sections they were labelled with lipophilic, carbocyanine fluorescent dye (Invitrogen), as described by manufacturer’s instructions. Alexa fluor 647-labelled miRNA was loaded in pre-labeled BECDEs and processed similarly as described above. The procedure of intracerebroventricular (i.c.v.) delivery was performed as described earlier (Kalani et al., 2014b). BECDEs loaded with miR-146a or scramble mimic were injected into the brain via. i.c.v. route (2–3μg, once a day for 3 successive days). As described previously (Kalani et al., 2014a; Sokolova et al., 2011), western blot using anti-tumor susceptibility gene101 (TSG101) antibody and activity assay for acetylcholinesterase (AchE) was used to characterize BECDEs integrity after miR-146a transfection.
Collection of brain samples
At the end of experiments mice were euthanized. Brain tissue samples were harvested, stored properly for Western blot, quantitative PCR (QPCR) and Immunohistochemistry analysis.
SDS-PAGE and Western Blotting
Equal quantities of mice brain extracts (25μg) were separated on polyacrylamide gel under reducing condition and transferred to polyvinyldifluoride membrane using an electrotransfer apparatus (Bio-Rad, Hercules, CA, USA). After blocking with 5 % non-fat dry milk for 1h, the membranes were probed with a primary antibody overnight at 4°C (PrPc, Sigma, 1:500; Glycogen synthase kinase-3 (GSK-3), Abcam, 1: 500; Microtubule-associated protein-2 (MAP-2), Abcam, 1:500; tau, Abcam, 1:1000; and, phospho-tau, Abcam, 1:1000). Next day, the membranes were probed with the appropriate secondary antibody (Santa cruz, 1:5000) for 1 h at room temperature and then developed using a Bio-Rad Molecular Imager (ChemiDoc XRS+, Hercules, CA, USA).
Quantitative gene expression analysis
Total RNA from the brain tissue was isolated using miRNeasy Kit (Qiagen, Valencia, CA, USA) and converted to cDNA using miScript II RT kit (Qiagen), as per manufacturer’s instructions. The cDNA samples were amplified for determining different miRNAs expressions in a 96 well format RT2-qPCR array (MIMM-107ZA-2, Mouse Neurologic Development and Disease, Qiagen) using miScript SYBR Green PCR kit (Qiagen). The cDNA samples were also analyzed for individual miRNAs expression (miR-146a, snoRD72 and RNU-6) (Qiagen). The amplification was performed in Stratagene Mx3000p (Agilent Technologies, Santa Clara, CA) and the cycle threshold (CT) values were determined after baseline and threshold adjustments. For the analysis, baseline and threshold adjustments were kept constant for all the experiments and the results were expressed as fold expression. The altered miRNAs (up-regulated or down-regulated) were checked for sequence similarity to PrPc by an online software targetscan provided by Whitehead institute for the targets of miRNAs (http://www.targetscan.org/).
Immunohistochemistry
At the completion of experiments, mice were deeply anesthetized with an overdose of anesthesia and infused with tetramethylrhodamine β-isothiocyanate (TRITC) - conjugated lycopersicon esculentum agglutinin (LEA) tomato lectin (Vector Laboratories, Burlingame, CA, USA) via a cannula in the carotid artery. Animals were euthanized via exsanguination by infusing transcardiacally a 50mM phosphate buffer saline (PBS) solution. The cranium was carefully opened and the brain was carefully removed. The brain was mounted in a protective (OCT) matrix (Polyscience, Inc., Warrington, PA, USA) and cryo-sectioned using a Leica CM 1850 cryostat (Bannockburn, IL, USA). Frozen brain blocks were cut into 25μm sections using a cryostat (Leica CM, USA) and fixed in ice-cold methanol for 10 min. The tissues were blocked with blocking solution [0.1% Triton X-100 TBS (TBS-T), 0.5% BSA, and 10% normal donkey serum] for 1 h at room temperature. The sections were incubated with primary antibody for PrPc (Sigma, 1:150), and phospho-tau (Abcam, 1:200) overnight at 4°C. After incubation with appropriate fluorescence secondary antibodies (1:300) (alexa fluor, Invitrogen, Grand Island, NY) for 60 min at room temperature, the sections were stained with 4’, 6-diamidino-2-phenylindole (DAPI, 1:10,000), and mounted with anti-fade mounting media. Images were acquired using a laser scanning confocal microscope (FluoView 1000; Olympus, PA, USA) and the data was analyzed with image analysis software (Image-Pro Plus).
Statistical Analysis
Statistical comparisons between two groups were performed using unpaired Student’s t-test. One way ANOVA was used to compare effects of miRNA treatments between groups and post-hoc pairwise comparisons were performed using Tukey’s test. P value of less than 0.05 was considered to be statistically significant. All values are expressed as mean ± standard error of the mean (SEM), unless otherwise stated.
Results
T2D db/db mice have impaired memory
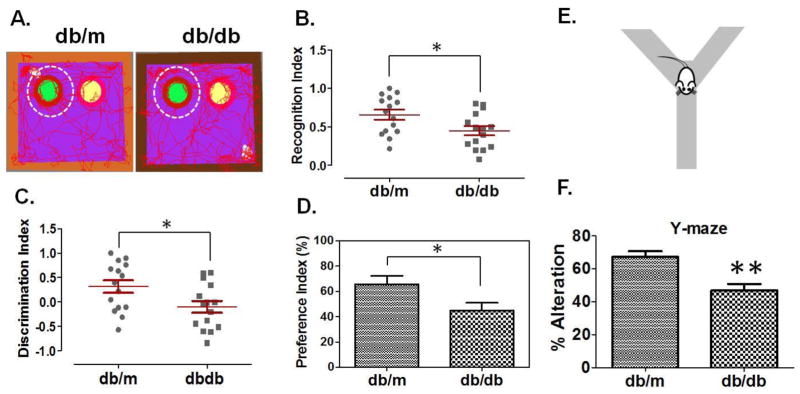
Figure-1A shows representative images of tracked mice (db/m control and T2D db/db) for their interest in a novel object to evaluate short-term memory. T2D db/db mice showed loss of short-term memory as determined by the recognition index (Figure-1B), the discrimination index (Figure-1C), and the preference index (Figure-1D), as compared to db/m controls. Y-maze test (Figure-1E) was used to evaluate alterations in spontaneous behaviour by analyzing the exploratory behaviour of mice. The analysis demonstrated that T2D db/db mice had significantly more alterations in spontaneous behaviour as compared to db/m controls (Figure-1F).
Figure 1. Memory impairment in T2D db/db mice.
(A) Representative tracking images of db/m control and T2D db/db mice for their interests in novel or old objects. Green circle, inside white dotted circle, represents novel object (left) and yellow circle represents old object (right). Figures representing recognition index (B), discrimination index (C), and preference index (D), in db/m control and T2D db/db mice. Recognition and discrimination indices were expressed in scatter plot and each dot represents the performance of each mouse in the test. Error bars are expressed in standard errors of the index values calculated through the formulas (Recognition Index= Novel object/novel object + old object; Discrimination index= Novel object-old object/novel object + old object;) (n=15) (E) Representation of Y-maze test and (F), bar graph representing analysis for spontaneous alterations in db/m control and T2D db/db mice. Error bars are expressed in errors of % spontaneous alteration values calculated through the formula (Spontaneous alterations (%) =total number of triads/total number of arm entries-2 x 100). (n=8), *p <0.05, **p <0.01–vs. db/m control.
T2D db/db mice brains exhibited disrupted microtubule associated proteins
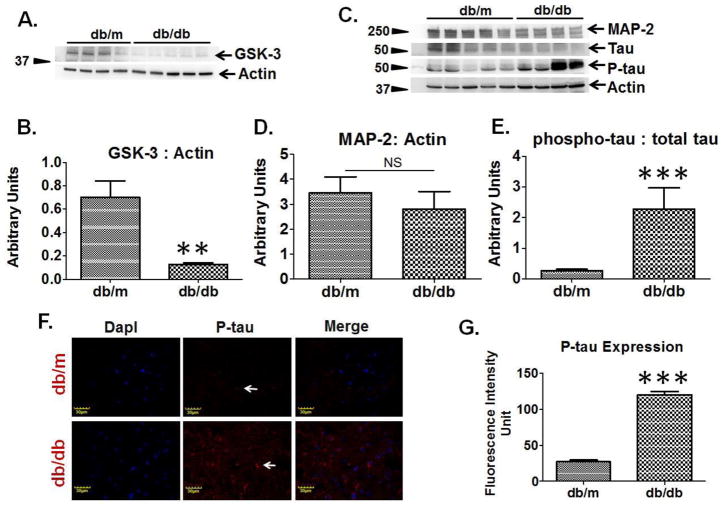
Western blot analysis exemplified decreased protein expression of GSK-3 in the brain of T2D db/db mice as compared to db/m controls (Figure-2A, 2B). Western blot analysis also showed reduced expression of microtubule associated proteins (MAP-2 and tau) and increased expression of phosphorylated tau (P-tau) in T2D db/db mice, as compared to db/m controls (Figure-2C, 2D). The brain ratio of phosphorylated tau to total tau was found to be highly increased in T2D db/db mice as compared to db/m controls (Figure-2E). Immunohistochemistry analysis of brain coronal sections also exhibited increased tau phosphorylation in T2D db/db mice as compared to db/m controls (Figure-2F, 2G).
Figure 2. Regulation of microtubule associated proteins in T2D db/db mice brain.
(A) Representative western blot images for GSK-3 and beta-actin in T2D db/db mice and db/m control. (B) Bar graph represents normalized band density of GSK-3 with actin in T2D db/db (n=5) and db/m brains (n=4). (C) Representative western blots images showing microtubule associated protein-2 (MAP-2), tau, phosphorylated-tau, and actin protein expressions in T2D db/db and db/m control brains (n=5). (D) Bar graph representing normalized band density of MAP-2 with actin in T2D db/db and db/m brains, NS= non-significant. (E) Bar graph presenting ratio of phospho-tau to total tau in T2D db/db and db/m control brains. (F) Immunohistochemistry images showing expression of phospho-tau (middle lane, red color, indicating with white arrows) in T2D db/db and db/m control brain coronal sections. Left most lane representing DapI (blue) stained nuclei, and right most lane showing merge images. (G) Bar graph showing fluorescence intensity of phospho-tau in T2D db/db and db/m control brains (n=4), **p <0.01, ***p <0.001–vs. db/m control.
T2D db/db mice showed altered regulations of miRNAs
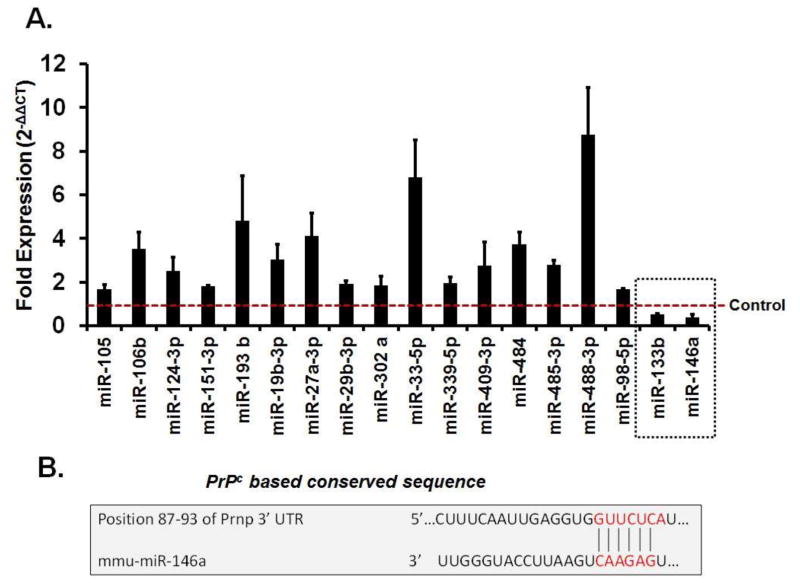
Using qRT2-PCR array, we determined that in brains of T2D db/db mice 16 miRNAs were upregulated and 2 were downregulated (miR-146a, miR-133b), as compared to db/m mice (Figure-3A). Bioinformatics analysis using targetscan revealed that only miR-146a sequence recognized the 87–93bp of the conserved sequence of PrPc (Figure-3B).
Figure 3. MiRNAs regulation in diabetic brains.
(A) Bar graph showing altered miRNAs in T2D db/db brains using qRT2-PCR analysis. Control is represented by red dashed line. Two miRNAs (miR-133b and miR-146a), indicated in dashed square box, were downregulated as compared to control. (B) Figure showing sequence homology of miR-146a with conserved sequence of PrPc (prion) protein.
T2D db/db mice showed induced expression of prion
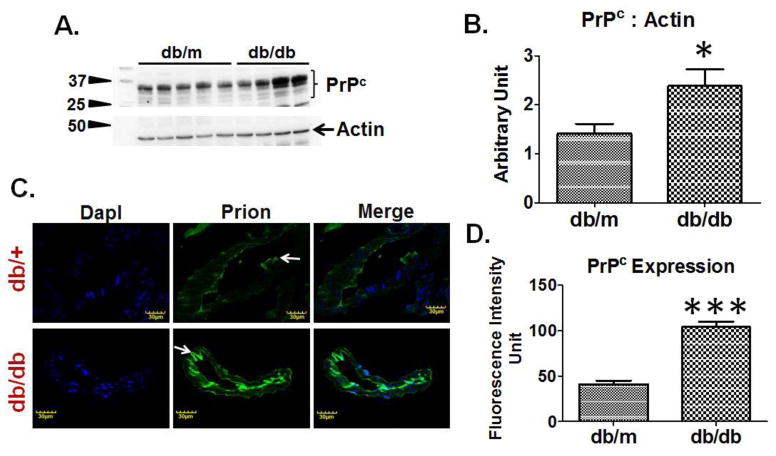
Western blot analysis determined that brains of T2D db/db mice had increased PrPc expression as compared to db/m controls (Figure-4A, 4B). Furthermore, IHC analysis demonstrated a significant increase in fluorescence intensity of PrPc around brain vessels of T2D db/db mice as compared to db/m controls (Figure-4C, 4D).
Figure 4. PrPc regulation in T2D db/db mice.
(A) Western blot images showing PrPc expression and, (B) bar graph showing normalized PrPc band density with actin in T2D db/db and db/m control brains (n=6). (C) Immunohistochemistry images and, (D) bar graph showing fluorescence intensity of PrPc protein in T2D db/db and db/m control brain vessels (n=3). *p <0.05, ***p <0.001–vs. db/m control.
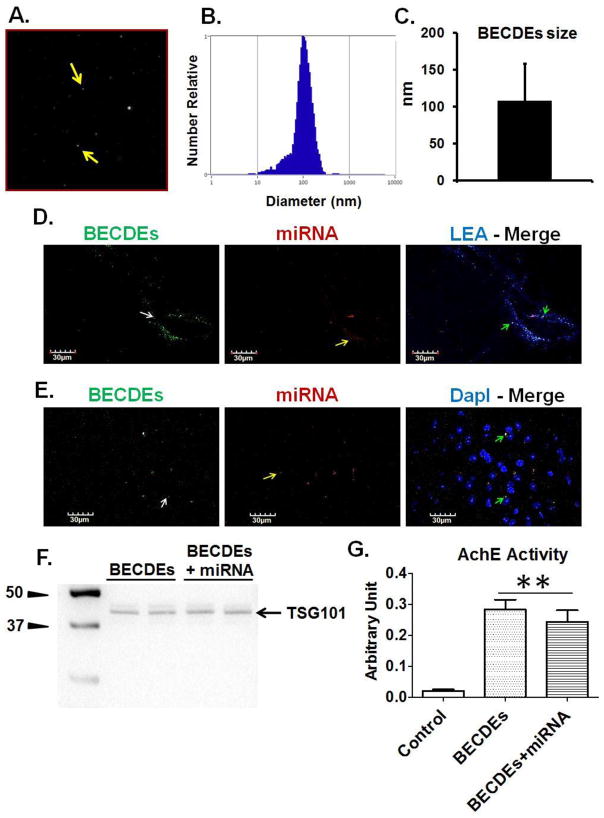
BECDEs characterization, transfection with miRNA and intracerebroventricular delivery
NTA analysis determined the size of BECDEs to be 108.4± 49.8 nm (Figure-5A, 5B, 5C). Brain coronal sections of T2D db/db mice showed the presence of BECDEs-loaded with alexa fluor-647 around brain blood vessels and cortical areas (Figure-5D, 5E), confirming successful i.c.v. delivery of miRNA through BECDEs. Further, BECDEs transfected with miR-146a showed specific band of TSG101 on Western blot and AchE activity similar to un-transfected BECDEs, confirming the integrity of BECDEs after transfection (Figure-5F, 5G).
Figure 5. Characterization of BECDEs and intracerebroventricular delivery of BECDEs loaded with miRNAs.
(A) Nano-tracking analysis (NTA) image showing BECDEs (yellow arrow). (B) NTA derived peak, confirming the size distribution of BECDEs nano-vesicles. (C) Bar diagram showing BECDEs size that appeared to be 108.4± 49.8 (mean ± SD). (D) Immunohistochemistry images showing presence of BECDEs (left lane, green color, white arrow) and miRNA (middle lane, red color, yellow arrow) around brain pial vessel and (E), cortical area. The brain vessel was infused with lycopersicon esculentum agglutinin (top right lane, blue color) and nuclei were stained with DapI (bottom right lane, blue color). (F) Western blot image showing TSG101 band and, (G) bar graph showing acetylcholinesterase activity in miR-146a loaded and non-loaded BECDEs along with control. Control is supernatant deprived of BECDEs (n=3). **p <0.001–vs. control
Effect of miR146a-loaded BECDEs administration on PrPc expression and memory restoration in T2D db/db mice
QPCR analysis further confirmed an increase in miR-146a in T2D db/db mice treated with miR146a-loaded BECDEs as compared to db/m mice treated with scramble (scr) miRNA-loaded BECDEs (Figure-6A). Furthermore, protein expression analysis using western blots determined that brains of T2D db/db mice had reduced expression levels of PrPc when treated with miR146a-loaded BECDEs as compared to T2D db/db mice treated with scramble-loaded BECDEs (Figure-6B, 6C). Administration of T2D db/db mice with miR146a-loaded BECDEs compared to T2D db/db mice receiving scramble-loaded BECDEs did not show an improvement in short-term memory (Figure-6D). The recognition index was not statistically different (P= 0.0617), but it was trending toward significance.
Figure 6. Effect of miR-146a mimic, loaded with BECDEs, on PrPc expression and memory function in T2D db/db mice.
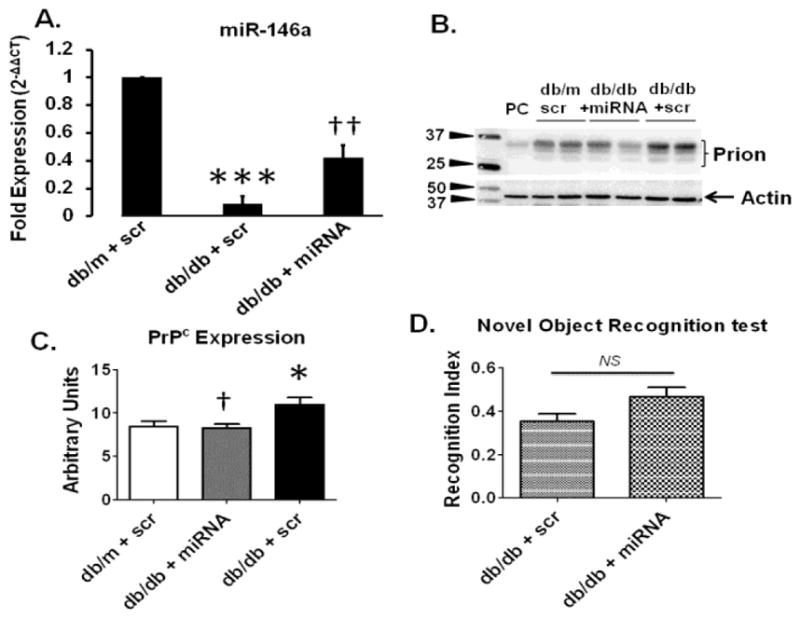
(A) QPCR analysis showing transcript level of miR-146a in T2D db/db mice administered with scramble (scr) miRNA-, and miR146a-loaded BECDEs (n=3). (B) Western blot images and, (C) analyzed band density of PrPc with actin in db/m and T2D db/db mice administered with scramble-loaded BECDEs, and T2D db/db mice miR146a-loaded BECDEs, PC, positive control, mouse heart tissue. Bar graph showing recognition index in T2D db/db mice administered with scramble-, and miR146a-loaded BECDEs (n=4). *p <0.05, ***p <0.001–vs. db/m + scramble, † p <0.05, †† p <0.01–vs. T2D db/db + scramble.
Discussion
Although the association between diabetes and dementia is controversial, many reports provide evidence that diabetes may influence underlying pathologies associated with dementia (Gispen and Biessels, 2000). However, clear mechanistic studies are still lacking. Our study using obese T2D db/db mice is an attempt to better understand the underlying mechanisms for diabetes-associated dementia since these mice have been documented to develop hyperglycemia, hyperinsulinemia, cerebro-vascular complications, AD-, and VaD-associated pathology (Ramos-Rodriguez et al., 2013). The obese db/db mice models resemble the pathology developed in type-2 diabetic humans and our study using different behavioural tests suggest that these mice have impaired short-term memory function related to the recognition of a novel object and related to their normal exploratory behaviour in a Y-maze test. Our study provided evidence that 12–14 weeks old T2D db/db mice exhibited abnormal exploratory behaviour and short-term memory loss as compared to non-diabetic db/m controls. PrPc expression was upregulated and GSK-3 expression was downregulated in the brains of T2D db/db diabetic mice as compared to db/m controls. GSK-3 is a key signalling molecule for memory function (Takashima et al., 1998). Also, brains from T2D mice exhibited disrupted microtubule associated proteins including reduced expression of microtubule associated proteins MAP-2 and tau. Tau phosphorylation along with Aβ tangles are important mediators of neurotoxicity and are associated with cognitive deficits (Nisbet et al., 2015). Interestingly, all the above protein anomalies are considered hall marks of dementia-associated pathology.
Development of therapies to treat diabetes-associated pathology in the brain requires a better understanding of the underlying mechanisms. One approach is based on the work developed by Poy et al (Poy et al., 2004) regarding the role of miRNAs in type-2 diabetes and its related complications (Kantharidis et al., 2011). Poy’s group identified miR-375 as playing a key role in insulin secretion, and this work spurred new miRNA associated research in type-2 diabetes including the present work. During our search for candidate miRNAs in the T2D db/db brain, we found that several miRNAs were upregulated and only two were downregulated using microRNA array. After matching miRNAs with genes through mirbase/targetscan, miR-146a was identified to match PrPc which is a known protein in dementia like pathology. In 12–14 weeks old T2D db/db brains we found that the PrPc is upregulated as compared to controls. Recent studies have determined that PrPc can play important role in Aβ deposition, and therefore, it could serve as a prime target for therapies treating cognitive impairment (Chung et al., 2010; Gimbel et al., 2010). Studies from our lab using a traumatic brain injury model in mice (Kalani et al., 2014b) demonstrates the importance of PrPc in memory loss. The high affinity of Aβ and tau for PrPc in the formation of oligomeric complexes underscores the apparent role of PrPc in cognitive decline that can eventually lead to severe dementia. Interestingly we found upregulated PrPc only in old mice (12–14 week) and not in young mice (8 weeks), which is in agreement with the fact that dementia-like pathology is most commonly age related.
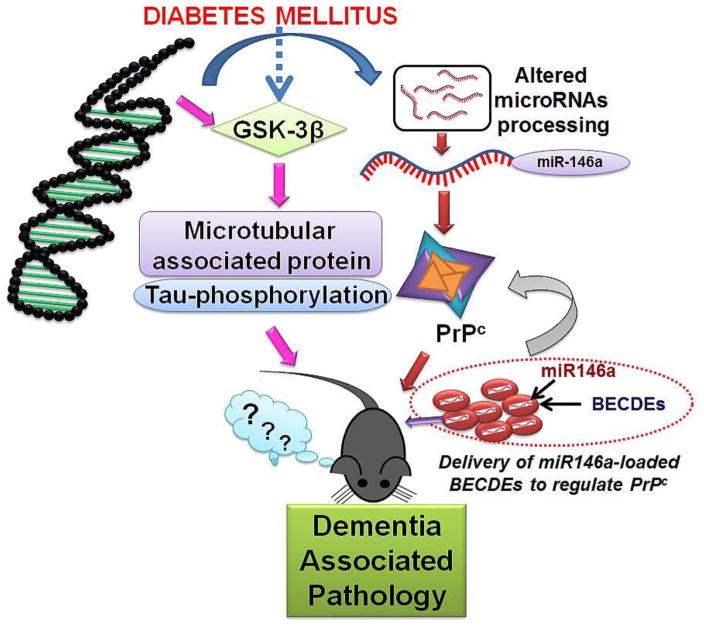
To establish whether PrPc plays a regulatory role in memory, we increased miR-146a levels in T2D db/db brains by loading a miR-146a into BECDEs and these were later administered into one of the brain’s ventricles. Exosomal delivery of therapeutic agents is rapidly gaining popularity because they are small, non-immunogenic and can cross cellular barriers while retaining therapeutic molecules (Kalani et al., 2014c) (Valadi et al., 2007). Exosomes deliver their cargo to target cells by a variety of mechanisms including: fusion, endocytosis, micropinocytosis, and phagocytosis (Marcus and Leonard, 2013). Through confocal studies, we were able to confirm that labelled-exosomal BECDEs loaded with labelled-miRNAs, reached targeted brain cells. Furthermore, in T2D db/db mice receiving miR-146a loaded BECDEs, miR-146a induced a 4 to 5 fold increase in miR-146a transcript levels and also reduced PrPc levels by 1.6 to 2 fold compared to those treated with scramble miRNA loaded BECDEs. The functional efficacy of RNA transfer between the cells via exosomes has been shown in several reports (Valadi et al., 2007). We found improvement of short-term memory function in T2D db/db mice treated with miR146a-loaded BECDEs though it was not significant and further studies are warranted with increased sample size. Although increase in PrPc leads to dementia-like pathology the reverse may not be true. Apart from PrPc that contribute to dementia like pathology, there are additional contributory pathways that involve the phosphorylation of tau and signalling molecules, which are well known to mediate pathology. Endoplasmic reticulum stress is another pathway that can contribute to development of diabetes and dementia-like pathology. Ivask et al have demonstrated that mutation in the WFS gene that encodes an endoplasmic reticulum resident transmembrane protein leads to the development of ER stress and juvenile diabetes which increases high risk of dementia like pathology (Ivask et al., 2016). The WFS gene is also important in the development of diabetes earlier in males than female (Noormets et al., 2011). Our working hypothesis is presented in figure-7.
Figure 7. Hypothesis.
Together with induced PrPc levels, which may be the resultant of decreased levels of miR-146a, microtubule associated proteins can be involved in dementia-associated pathology during type-2 diabetes. Administration of miR146a-loaded BECDEs normalized PrPc expression but could not restore memory function significantly.
To conclude, our results suggest that type-2 diabetes may promote induction of pathological events (tau-phosphorylation and PrPc upregulation) which results in behavioural alterations including: short-term memory loss and abrupt exploratory behaviour. Exosomal-based therapy with a miR-146a attenuates PrPc upregulation and improves cognitive functions which suggest that early intervention can postpone the onset of cognitive dysfunction. Therefore, additional studies are needed to further investigate age related memory loss in different age groups of mice with larger sample sizes. Also, efforts are needed to optimize the methods of miRNA loading to exosomes and delivering different concentrations of miRNAs to get optimized results.
Research Highlights.
The work describes the role of microRNA-146a in dementia-associated pathology during type-2 diabetes through the regulation of cellular prion protein
Interestingly, the result suggests that microRNA-146a loaded exosomes delivered microRNA-146a in the diabetic mice brain that regulated prion protein and memory loss.
Acknowledgments
A part of this work was published as an abstract in the meeting proceedings of Experimental Biology, 2016. The authors wish to thank research volunteer Dr. Guoxin Chu, MD (Jilin Province People’s Hospital, Changchun, China) for his help in the surgical experiments and Ms. Carissa J. Mallonee for her help in western blot experiment.
Funding: Part of this study was supported by National Institutes of Health grants HL-074185 and HL-107640 to SCT and NT. PC was supported by a post-doctoral grant from American Heart Association, 15POST23110021.
Abbreviations
- Aβ
Amyloid beta
- AchE
Acetylcholinesterase
- AD
Alzheimer’s disease
- BSA
Bovine serum albumin
- cDNA
Complimentary DNA
- DAPI
4,6-diamidino-2-phenyl-indole HCl
- DI
Discrimination Index
- FIU
Fluorescence intensity units
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GSK-3
Glycogen synthase kinase-3
- IHC
Immunohistochemistry
- LEA
Lycopersicon Esculentum agglutinin
- MAP-2
Microtubule associated protein-2
- MBEC/bEnd.3
Mouse brain endothelial cells
- miRNA or miR
MicroRNA
- NFT
Neurofibrillary tangles
- NTA
Nanoparticle tracking analysis
- PBS
Phosphate buffer saline
- P-tau
Phosphorylated tau
- PrPc/Prnp
Prion protein (cellular)
- QPCR
Quantitative PCR
- RI
Recognition Index
- RIPA
Radioimmunoprecipitation assay
- TBS
Tris-buffered saline
- TBS-T
Tris-buffered saline with Triton X-100
- T2D
Type-2 diabetes
- TSG101
Tumor susceptibility gene101
- VaD
Vascular dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012;40:10937–10949. doi: 10.1093/nar/gks832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Ji Y, Sun Y, Kascsak RJ, Kascsak RB, Mehta PD, Strittmatter SM, Wisniewski T. Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer’s disease model mouse. BMC Neurosci. 2010;11:130. doi: 10.1186/1471-2202-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WA, Zilkens RR, Starkstein SE, Davis TM, Bruce DG. Dementia onset, incidence and risk in type 2 diabetes: a matched cohort study with the Fremantle Diabetes Study Phase I. Diabetologia. 2016 doi: 10.1007/s00125-016-4127-9. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M Alzheimer’s Disease I. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends in neurosciences. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640–650. doi: 10.1111/jdi.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivask M, Hugill A, Koks S. RNA-sequencing of WFS1-deficient pancreatic islets. Physiological reports. 2016:4. doi: 10.14814/phy2.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. The international journal of biochemistry & cell biology. 2016;79:360–369. doi: 10.1016/j.biocel.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci. 2014a;107:1–7. doi: 10.1016/j.lfs.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Familtseva A, Chaturvedi P, Muradashvili N, Narayanan N, Tyagi SC, Tyagi N. Role of microRNA29b in blood-brain barrier dysfunction during hyperhomocysteinemia: an epigenetic mechanism. J Cereb Blood Flow Metab. 2014b;34:1212–1222. doi: 10.1038/jcbfm.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol. 2014c;49:590–600. doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes. 2011;60:1832–1837. doi: 10.2337/db11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders. Curr Genomics. 2009;10:154–168. doi: 10.2174/138920209788185252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus ME, Leonard JN. FedExosomes: Engineering Therapeutic Biological Nanoparticles that Truly Deliver. Pharmaceuticals. 2013;6:659–680. doi: 10.3390/ph6050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Tyagi R, Metreveli N, Tyagi SC, Lominadze D. Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:1472–1482. doi: 10.1038/jcbfm.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet RM, Polanco JC, Ittner LM, Gotz J. Tau aggregation and its interplay with amyloid-beta. Acta neuropathologica. 2015;129:207–220. doi: 10.1007/s00401-014-1371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noormets K, Koks S, Muldmaa M, Mauring L, Vasar E, Tillmann V. Sex differences in the development of diabetes in mice with deleted wolframin (Wfs1) gene. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2011;119:271–275. doi: 10.1055/s-0030-1265163. [DOI] [PubMed] [Google Scholar]
- Peila R, Rodriguez BL, Launer LJ Honolulu-Asia Aging S. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- Ramos-Rodriguez JJ, Ortiz O, Jimenez-Palomares M, Kay KR, Berrocoso E, Murillo-Carretero MI, Perdomo G, Spires-Jones T, Cozar-Castellano I, Lechuga-Sancho AM, Garcia-Alloza M. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology. 2013;38:2462–2475. doi: 10.1016/j.psyneuen.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Lovestone S. The dementias. Lancet. 2002;360:1759–1766. doi: 10.1016/S0140-6736(02)11667-9. [DOI] [PubMed] [Google Scholar]
- Saedi E, Gheini MR, Faiz F, Arami MA. Diabetes mellitus and cognitive impairments. World journal of diabetes. 2016;7:412–422. doi: 10.4239/wjd.v7.i17.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. 2011;87:146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Takashima A, Honda T, Yasutake K, Michel G, Murayama O, Murayama M, Ishiguro K, Yamaguchi H. Activation of tau protein kinase I/glycogen synthase kinase-3beta by amyloid beta peptide (25–35) enhances phosphorylation of tau in hippocampal neurons. Neuroscience research. 1998;31:317–323. doi: 10.1016/s0168-0102(98)00061-3. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Wiesmann M, Kiliaan AJ, Claassen JA. Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab. 2013;33:1696–1706. doi: 10.1038/jcbfm.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: Fit to deliver small RNA. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]