Abstract
Background
The STTR treatment cascade provides a framework for research aimed at improving the delivery of services, care and outcomes of PLWH. The development of effective approaches to increase HIV diagnoses and engage PLWH in subsequent steps of the treatment cascade could lead to earlier and sustained ART treatment resulting in viral suppression. There is an unmet need for research applying the treatment cascade to improve outcomes for those with criminal justice involvement.
Methods
The Seek, Test, Treat, and Retain (STTR) criminal justice (CJ) cohort combines data from 11 studies across the HIV treatment cascade that focused on persons involved in the criminal justice system, often but not exclusively for reasons related to substance use. The studies were conducted in a variety of CJ settings and collected information across 11 pre-selected domains: demographic characteristics, CJ involvement, HIV risk behaviors, HIV and/or Hepatitis C infections, laboratory measures of CD4 T-cell count (CD4) and HIV RNA viral load (VL), mental illness, health related quality of life (QoL), socioeconomic status, health care access, substance use, and social support.
Results
The STTR CJ cohort includes data on 11,070 individuals with and without HIV infection who range in age from 18 to 77 years, with a median age at baseline of 37 years. The cohort reflects racial, ethnic and gender distributions in the U.S. CJ system, and 64% of participants are African-American, 12% are Hispanic and 83% are men. Cohort members reported a wide range of HIV risk behaviors including history of injection drug use and, among those who reported on pre-incarceration sexual behaviors, the prevalence of unprotected sexual intercourse ranged across studies from 4% to 79%. Across all studies, 53% percent of the STTR CJ cohort reported recent polysubstance use.
Conclusions
The STTR CJ cohort is comprised of participants from a wide range of CJ settings including jail, prison, and community supervision who report considerable diversity in their characteristics and behavioral practices. We have developed harmonized measures, where feasible, to improve the integration of these studies together to answer questions that cannot otherwise be addressed.
Keywords: Criminal justice, HIV, Data harmonization.
Background
STTR treatment cascade
The Seek, Test, Treat and Retain (STTR) treatment or HIV care cascade is a challenging yet potentially beneficial response to addressing HIV in an era of effective treatments [1–3]. This approach requires reaching out to at-risk individuals who have not been tested for HIV recently (Seek), engaging them in HIV testing (Test), initiating persons living with HIV (PLWH) on antiretroviral therapy (ART) and other treatment services (Treat), and facilitating uninterrupted HIV care (Retain) [1, 2]. The STTR treatment cascade provides a framework for research aimed at improving the delivery of services, care and outcomes of PLWH. There is substantial dropout across each cascade step, and it has been estimated that only ~19% of PLWH in the United States (US) are aware of their HIV diagnosis, engaged in care, on ART, and have an undetectable viral load (VL) [1], although more recent numbers suggest improvements [4, 5]. The development of effective approaches to increase HIV diagnoses and engage PLWH in subsequent steps of the treatment cascade could lead to earlier and sustained ART treatment resulting in viral suppression. Improvements in the STTR treatment cascade have the potential to benefit the health of PLWH and improve public health by reducing HIV transmission [2].
The relevance of understanding the STTR treatment cascade across all CJ settings
In the US, there are two main types of correctional facilities—jails, that typically are administered by city or county governments, and hold people awaiting trials or serving shorter sentences (generally under two years), and prisons that typically are administered by state and federal governments and hold people serving longer sentences or who have had their parole or probation revoked. Community supervision includes pretrial, probation and parole. Pretrial refers to people awaiting trial before receiving a criminal conviction or acquittal. Probation refers to adults who have been placed on supervision in the community, typically through local or state court systems; about half of individuals on probation also serve a short jail sentence. Parole refers to people who are released early from prison to serve the remaining part of their sentence in the community [6]. The criminal histories of people in prison typically are more serious, lengthy, and varied than individuals in jail or on community supervision [7, 8].
Currently, >95% of incarcerated individuals will be released and re-enter society with nearly 80% being released to parole supervision [9]. About 1 in 35 adults in the U.S. are under CJ supervision, and the number is expected to continue to increase [10]. This trend expands opportunities for HIV prevention and treatment, especially in the public health realm [11]. Specifically, the estimated HIV prevalence among individuals incarcerated in the US prison system is 2-3 times higher than the general population [12–16]. These statistics translate to one in seven PLWH being incarcerated each year, a figure that rises to one in five PLWH who are African-American or Hispanic [17]. Although HIV testing rates in federal and state prisons are generally high (71%), [18, 19] testing rates in jails are not (19%) [18]. Because many CJ-involved individuals pass only through jail, it is likely that many who are infected will not be offered HIV testing [18, 20]. These individuals may not know their HIV status or their potential to transmit HIV [21]. Lack of testing among this higher risk group, and early ART initiation for those who are infected, is a lost opportunity to improve individual and public health and decrease HIV transmission. Those with HIV who return to the communities where they may lack access to health care services, including screening and early diagnosis, are likely to receive HIV treatment only after the disease has progressed to advanced stages [16, 22]. As such, the Centers for Disease Control and Prevention (CDC) encourages more frequent HIV testing of persons within the CJ system, particularly those with drug-dependencies, because PLWH who learn their HIV status are less likely to spread HIV and more likely to seek medical treatment that can lower the potential for HIV transmission and reduce HIV-associated morbidity and mortality [23, 24]. Finally, while PLWH incarcerated in prisons have access to care, including ART, maintaining ART and other medical services is a significant challenge for many re-entering the community following incarceration in prison [25] and engagement in the HIV care cascade has been shown to decline substantially after release [26]. Effective interventions are essential to link individuals to appropriate post-incarceration care and enhance retention in HIV care. The CJ involvement makes this a particularly unique and important cohort for addressing key questions across the HIV treatment cascade.
Methods
Cohort development
The STTR CJ cohort is the product of the STTR Data Collection and Harmonization Initiative developed by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the Office of AIDS Research (OAR). This cohort was developed by a collaboration with researchers who harmonized and pooled data from independent prospective research studies funded under a grant mechanism focused on enhancing the STTR treatment cascade for individuals involved with the CJ system across the US [27]. This initiative produced another cohort not described here focused on vulnerable populations particularly substance users at risk for or with HIV in international and domestic settings. While the STTR initiative and its rationale have been previously described [27], the cohort itself has not yet been described.
Cohort management structure
The STTR CJ collaboration consists of a three way partnership between 1) individual researcher teams responsible for conducting the STTR studies and overseeing all enrollment and data collection; 2) a scientific officer and program officials from NIAID, NIDA, NIMH, and OAR who provide expertise and guidance regarding the data harmonization initiative; and 3) the STTR Data Coordination Center (DCC), which provides expertise in data harmonization, epidemiology, biostatistics, and HIV, harmonizes the data, supports investigations that use the harmonized data to answer novel scientific questions, and develops new methods for analysis of complex pooled data.
Cohort description
The STTR CJ cohort is unique in its focus on data from individuals involved in the CJ system and includes 11 NIAID, NIDA, NIMH, and OAR funded studies, several of which involve multiple sub-components and embedded studies. It includes single and multi-site randomized controlled and observational trials evaluating interventions to enhance care delivery along the HIV treatment cascade. It provides a large sample across CJ settings (jail, prison, community supervision) and includes both PLWH and HIV-uninfected individuals, many of whom engage in illicit drug use. Compared to single studies, the CJ cohort data provide increased statistical power to determine public health benefits of the STTR paradigm, address research questions on specific groups at risk of exiting the treatment cascade, and improve understanding of the intersection of drug use and HIV treatment. This cohort profile describes data from the resulting cohort and individual CJ studies including baseline demographic and clinical characteristics.
Data harmonization
Eleven domains were selected for data harmonization: CJ involvement; HIV risk behaviors; HIV and/or hepatitis C infection; laboratory measures of CD4+ cell count and HIV RNA viral load among those with HIV; mental health; health related quality of life; socioeconomic; health care access; substance use and alcohol use; and social support (Fig. 1). The goal is to integrate data from across studies to address research questions requiring sample sizes beyond that of a single study.
Fig. 1.
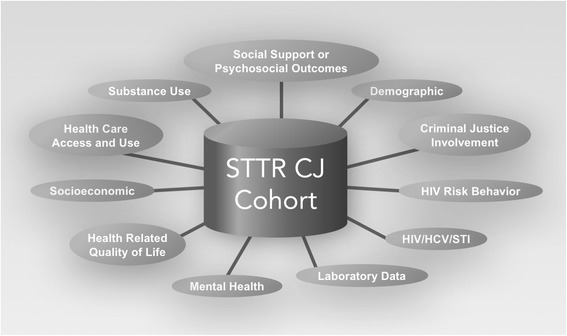
STTR CJ domains selected for data harmonization
The DCC, with investigators from individual studies, is responsible for data integration. The DCC provides technical support and oversees a web-based project collaboration and document management system; documents procedures for data collection and translation, transmission, and quality control; develops integration methods; and generates analyzable data sets. Studies typically upload data biannually. The DCC coordinates data integration from studies sometimes collected using different instruments or timeframes to ensure that the data are comparable across studies in meaning and content. To accomplish this, an examination of data types collected by each study was conducted, working closely with individual study personnel to survey each study’s protocol including how data were collected and modifications to the instruments used. Where data were comparable, codes were developed to create standardized sets of variables.
Availability of data and materials
The datasets generated during the current study are not publically available due to privacy/license concerns. However, we welcome collaboration from interested parties. We have policies to ensure multi-center analytic research proposals are developed and results analyzed collaboratively and fairly. We are guided by principles of other cohort collaborations [28–30] including that data at the DCC will be stripped of all protected health information (PHI); an individual study can choose to participate or not in any scientific aim or sub-aim; and concept proposals and completed manuscripts must be approved by the Publications and Presentations (P&P) committee. We appreciate and encourage mentorship and the use of the STTR data to enable early investigators to address meaningful questions with support to help ensure their success. Additional information can be obtained at the STTR website: https://sttr-hiv.org/cms or by contacting the STTR DCC at STTR@uw.edu.
Results
Description of the STTR CJ studies and participants
Figure 2 shows the diverse geography of the participating studies. For each of the 11 studies and their substudies, the research topic, the components of the STTR treatment cascade addressed, and the targeted participants are described in Table 1. All the steps of the treatment cascade are included in multiple studies, and a diverse array of research topics are included. The STTR CJ cohort includes studies focused on later steps in the STTR treatment cascade such as treatment and retention of PLWH and studies focused on earlier steps such as HIV testing. Therefore, the percentage of PLWH in the individual STTR CJ studies at baseline varies, ranging from 0 to 100% (Table 1), with an overall baseline total of 1888 PLWH. For each of the 11 studies and their substudies, detailed inclusion and exclusion criteria, and the study design including whether it was observational or a trial is described in Table 2. In addition to baseline information, most studies collected data at 3 and/or 6-month follow-up time points, and many include 12 month and other follow-up time points (Table 2).
Fig. 2.
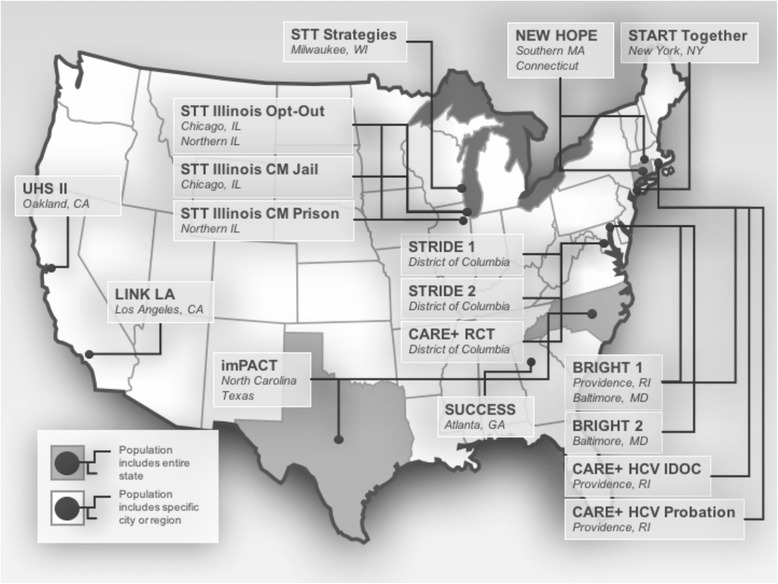
STTR CJ participating sites
Table 1.
Description of STTR CJ cohort studies including research topics, targeted study participants, and HIV status of participants
| Study | Enrollment start date | Treatment cascade | Research topic | Targeted study participants | HIV, % |
|---|---|---|---|---|---|
| BRIGHT | |||||
| BRIGHT 1 | 2011 | S, T | HIV testing | On probation or parole | 0.1 |
| BRIGHT 2 | 2011 | Tr, R | Linkage to HIV care via intensive CM | HIV-infected; on probation or parole | 100 |
| CARE+ | |||||
| RCT | 2013 | Tr, R | Linkage to HIV care via multi-component intervention | HIV-infected; in jail or recently released | 100 |
| HCV RIDOC | 2012 | S, T | Rapid HCV testing using pre-test video counseling | Short-term detainees; unknown HCV status | N/A |
| HCV PROB | 2014 | S, T | Rapid HCV testing using pre-test video counseling | On probation or parole; unknown HCV status | N/A |
| IMPACT | 2012 | Tr, R | Linkage to HIV care and engagement in clinical care via multi-component intervention | HIV-infected inmates; RNA levels <400 copies/mL, on ART; 3 months prior to prison release | 100 |
| LINK LA | 2012 | Tr, R | Linkage to HIV care via peer-based intervention | HIV-infected; in jail | 100 |
| NEW HOPE | 2011 | Tr, R | Medication assisted treatment for substance abuse | HIV-infected; opiate dependent; leaving prison & jail | 100 |
| STT | 2013 | S, T, Tr, R | HIV testing and linkage to HIV care | Jail detainees | 0.4 |
| STT ILLINOIS | |||||
| OPT OUT | 2012 | S, T | HIV testing | In jail | N/A |
| CM JAIL | 2013 | Tr, R | Linkage to, and retention in HIV care via transition CM | HIV-infected; leaving jail | 100 |
| CM PRISON | 2013 | Tr, R | Linkage to, and retention in HIV care via transition CM | HIV-infected; recently released from prison | 100 |
| START | 2011 | S, T | HIV testing and risk reduction | Substance users entering drug treatment program | N/A |
| STRIDE | |||||
| STRIDE 1 | 2011 | Tr, R | Medication assisted treatment for substance abuse | HIV-infected; opiate dependent | 100 |
| STRIDE 2 | 2014 | Tr, R | Medication assisted treatment for substance abuse | HIV-infected; opiate dependent | 100 |
| SUCCESS | 2014 | Tr, R | Linkage to, and retention in, HIV care via intensive CM | HIV-infected; jail detainees | 100 |
| UHS II | |||||
| UHS IIC | 2011 | S, T | HIV testing | People who inject drugs or smoke crack cocaine | 3.3 |
| UHS IIL | 2011 | Tr, R | Linkage to and engagement in HIV care via CM | People who inject drugs or smoke crack cocaine and have HIV | 100 |
ART antiretroviral therapy, CJ criminal justice, CM case management, N/A not applicable, HCV hepatitis C virus, HIV human immunodeficiency virus, R retain, RNA ribonucleic acid, S seek, STTR seek, test, treat and retain, T test, Tr treat, US United States
Table 2.
Study design, follow-up, and participant criteria for STTR CJ cohort studies
| Studya | Study design | Follow-up time points | Individual inclusion and exclusion criteria |
|---|---|---|---|
| BRIGHT | |||
| BRIGHT 1 | Randomized/observational study of on-site rapid HIV testing at probation/parole office vs. off-site referral at a community health center | Baseline only | Unknown HIV status; on probation or parole in Baltimore, MD or Providence/ Pawtucket, RI and residence in the Baltimore or Providence/Pawtucket area throughout the study period |
| BRIGHT 2 | RCT of case management (Project Bridge) vs. TAU | 3,6,12,15,18 months | HIV-infected; on probation or parole in Baltimore, MD and planned residence in greater Baltimore area throughout the study period |
| CARE+ | |||
| RCT | RCT of CARE+ Corrections intervention | Monthly for 24 months | Aged 18+; HIV-infected; released from the correctional facility or half-way house ≤6 months ago and living in Washington, DC metropolitan community (not a restricted setting, e.g. half-way house) or currently detained in jail with anticipated release to community (not a restricted setting); reading at 8th grade level and English-speaking |
| HCV RIDOC | Cross-sectional study of HCV testing | Baseline only | Aged 18+; self-reported as HCV negative and documented HCV infection during Department of Corrections time with anticipated release between 3 and 12 weeks from enrollment; English-speaking |
| HCV PROB | Cross-sectional study of HCV testing | Baseline only | Aged 18+; self-reported as HCV negative or unknown; on probation or parole; English speaking |
| IMPACT | RCT of IMPACT intervention vs. standard of care | 2, 6, 14, and 24 weeks | Aged 18+; HIV-infected with HIV RNA < 400 copies/mL receiving ART who were incarcerated in NC or TX and 3 months prior to release and not convicted of sexual assault, death or serious injury; English-speaking |
| LINK LA | RCT of intervention | 2, 6, 12 months | Male or transgender individuals, aged 18+; HIV infected; incarcerated in a single facility for 5+ days; residing in Los Angeles County, CA upon release; English fluency |
| NEW HOPE | Placebo-controlled RCT of. extended-release naltrexone | Monthly for 12 months | Aged 18+, HIV-infected, meeting DSM-IV criteria for opioid dependence, within CT corrections system and not pending trial for a felony, within 30 days of being released to greater New Haven, Hartford, Waterbury or Springfield areas or 30 days after release; English- or Spanish-speaking, no liver failure or grade IV hepatitis, no active opioid withdrawal, no receipt of methadone or buprenorphine/naloxone for treatment of opioid dependency, no participation in pharmacotherapy trial in the previous 30 days |
| STT | Observational study of comprehensive Seek Test Treat strategies with medical record linkage 2 years post-release | 2 years | Aged18+; admitted to a detention facility, expected to be released to Milwaukee County, WI who were willing to be tested for HIV; verbal communication in English |
| STT ILLINOIS | |||
| OPT OUT | Cross-sectional study of opt-out HIV testing | Baseline only | Aged 18+; detained in the IL corrections system |
| CM JAIL | Non-randomized study of case-management intervention vs. standard of care | 6, 12, 18 months | Aged 18+; HIV-infected; detained in IL corrections system (jail); expecting to reside in Chicago after release |
| CM PRISON | Non-randomized study of case-management intervention vs. standard of care | 6, 12, 18 months | Aged 18+; HIV-infected; recently released from IL corrections system (prison); enrollment was within 60 days of release |
| START | 1. RCT of CARE-Rapid (computer-assisted program) vs. TAU and 2. quasi-experimental study of manualized intervention vs. TAU, with follow-up at 3 months | 3 months | Men aged 18+ with either unknown or believed negative HIV status within 90 days of release from NY detention center entering a residential substance abuse treatment program |
| STRIDE | |||
| STRIDE 1 | RCT of buprenorphine vs. placebo | Monthly for 12 months | Aged 18+; HIV-infected; meeting DSM-IV criteria for opioid dependence; resident of Washington, DC with eligibility for medical entitlements; English- or Spanish-speaking; no current opiate medications for chronic pain conditions or need to be placed on such medications; no current methadone doses over 30 mg/day, no AST and ALT >5× the ULN; no pregnancy or breast-feeding; no liver dysfunction; no suicidal ideation; no participation in pharmacotherapy trial in the previous 30 days |
| STRIDE 2 | Longitudinal cohort study comparing treatment using opioid substitution therapy to no treatment | 3, 6, 9, 12 months | Aged 18+; HIV-infected; meeting DSM-IV criteria for opioid dependence; resident of Washington, DC with eligibility for medical entitlements; English-speaking |
| SUCCESS | Non-randomized pilot study of Strengths-Based case management and texting | 3, 12 months | Aged 18+; HIV-infected; detained or sentenced in jail or detention center and likely to leave within 6 weeks; no recent participation in randomized trial to improve retention in HIV care; English-speaking |
| UHS II | |||
| UHS IIC | Cross-sectional study of HIV testing | Baseline only | Aged 18+; crack cocaine or injection drug use in the past 30 days |
| UHS IIL | Longitudinal cohort study of case management (Project Bridge) vs. usual treatment | Quarterly for 24 months | Aged 18+; HIV-infected; crack cocaine or injection drug use in the past 30 days |
ALT alanine aminotransferase, ART antiretroviral therapy, AST aspartate aminotransferase, CA California, CJ criminal justice, CT Connecticut, DC District of Columbia, DSM-IV Diagnostic and Statistical Manual of Mental Disorders IV, HCV hepatitis C virus, HIV human immunodeficiency virus, IL Illinois, MD Maryland, NC North Carolina, NY New York, RCT randomized controlled trial, RI Rhode Island, RNA ribonucleic acid, TAU treatment as usual, TX Texas, ULN upper limit of normal, WI Wisconsin
aStudy acronyms as in Table 1
The STTR CJ cohort includes 11,070 individuals with and without HIV who range in age from 18 to 77 years with a median baseline age of 37 years. The STTR CJ cohort is diverse and includes substantial numbers of African-American (64%) and Hispanic (12%) individuals as well as 17% percent women (Table 3). Given differences in enrollment criteria across STTR CJ cohort studies, the percentage reporting HIV risk behaviors varies considerably (Table 4). Injection drug use (IDU) ranged from 86% of participants reporting ever use in one study, with 64% in that study reporting IDU within the 30 days prior to incarceration, to very low levels in other studies (Table 4). Reports of recent condomless anal or vaginal intercourse also varied from 4 to 79% of participants. Recent use of marijuana, cocaine, and opioids, and binge drinking were common across all studies (Table 5) with 53% reporting use of multiple substances. Information regarding nonmedical prescriptions drug use is available from 9 of the studies and substudies. The participants were recruited from a wide range of CJ settings including jail, prison, and community supervision (pretrial, parole and probation) with particularly large numbers of individuals from jail, prison, and probation (Table 6).
Table 3.
Demographic characteristics for STTR CJ cohort study participants
| Race/ethnicity, % | Self-identified gender, % | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studya | Nb | American Indian or Alaskan Native | Asian | Black or African American | Native Hawaiian or Other Pacific Islander | White | Hispanic or Latino | Some other race | Two or more races | Don’t know, refused, missing | Female | Male | Trans-gender | Don’t know, refused, missing | Age, median years (range) |
| BRIGHT | |||||||||||||||
| BRIGHT 1 | 2405 | 2.4 | 0.0 | 51.6 | 0.0 | 28.0 | 12.8 | 1.5 | 3.6 | 0.1 | 17.8 | 82.1 | 0.2 | 0.0 | 39 (18-73) |
| BRIGHT 2 | 100 | 1.0 | 0.0 | 90.0 | 0.0 | 3.0 | 3.0 | 0.0 | 3.0 | 0.0 | 22.0 | 78.0 | 0.0 | 0.0 | 45 (28-70) |
| CARE+ | |||||||||||||||
| RCT | 112 | 0.0 | 0.0 | 86.6 | 0.0 | 3.6 | 0.9 | 0.9 | 8.0 | 0.0 | 28.6 | 57.1 | 12.5 | 1.8 | 42 (19-63) |
| HCV RIDOC | 250 | 4.8 | 1.2 | 14.8 | 0.0 | 46.8 | 22.4 | 2.0 | 8.0 | 0.0 | 6.4 | 93.6 | 0.0 | 0.0 | 31 (19-58) |
| HCV PROB | 138 | 4.4 | 0.0 | 18.8 | 0.7 | 41.3 | 25.4 | 2.2 | 5.8 | 1.5 | 18.8 | 80.4 | 0.7 | 0.0 | 32 (19-68) |
| IMPACT | 381 | 2.1 | 0.0 | 66.1 | 0.0 | 19.7 | 8.1 | 0.8 | 3.2 | 0.0 | 22.0 | 75.3 | 2.4 | 0.3 | 44 (20-64) |
| LINK LA | 356 | 1.1 | 0.8 | 34.6 | 1.1 | 21.9 | 31.2 | 0.8 | 8.5 | 0.0 | 3.6 | 84.5 | 11.0 | 0.0 | 40 (21-69) |
| NEW HOPE | 123 | 0.0 | 0.0 | 22.8 | 0.0 | 12.2 | 63.4 | 0.0 | 0.8 | 0.8 | 17.9 | 81.3 | 0.8 | 0.0 | 46 (21-67) |
| STT | 3963 | 1.4 | 0.2 | 63.2 | 0.1 | 14.6 | 9.4 | 0.5 | 5.9 | 4.7 | 2.9 | 96.9 | 0.1 | 0.1 | 29 (18-73) |
| STT ILLINOIS | |||||||||||||||
| OPT OUT | 236 | 0.4 | 0.0 | 67.8 | 0.0 | 9.7 | 20.3 | 0.9 | 0.9 | 0.0 | 56.8 | 43.2 | 0.0 | 0.0 | 27 (18-60) |
| CM JAIL | 376 | 1.1 | 0.3 | 81.4 | 0.0 | 7.7 | 9.0 | 0.0 | 0.5 | 0.0 | 17.6 | 79.8 | 2.7 | 0.0 | 43 (18-74) |
| CM PRISON | 90 | 0.0 | 0.0 | 90.0 | 0.0 | 3.3 | 5.6 | 0.0 | 0.0 | 1.1 | 8.9 | 86.7 | 3.3 | 1.11 | 46 (19-64) |
| START | 195 | 1.0 | 0.5 | 46.2 | 0.0 | 21.6 | 29.2 | 0.5 | 1.0 | 0.0 | 0.0 | 100 | 0.0 | 0.0 | 38 (19-65) |
| STRIDE | |||||||||||||||
| STRIDE 1 | 50 | 0.0 | 0.0 | 90.0 | 0.0 | 0.0 | 2.0 | 0.0 | 4.0 | 4.0 | 28.0 | 68.0 | 2.0 | 2.0 | 53 (39-67) |
| STRIDE 2 | 109 | 0.0 | 0.0 | 96.3 | 0.0 | 1.8 | 0.9 | 0.0 | 0.0 | 0.9 | 46.8 | 45.9 | 7.3 | 0.0 | 51 (23-66) |
| SUCCESS | 56 | 0.0 | 0.0 | 69.7 | 0.0 | 8.9 | 3.6 | 0.0 | 7.1 | 10.7 | 7.1 | 89.3 | 3.6 | 0.0 | 38 (21-58) |
| UHS II | |||||||||||||||
| UHS IIC | 2090 | 0.6 | 0.1 | 86.0 | 0.3 | 4.7 | 6.8 | 0.2 | 1.2 | 0.2 | 41.0 | 58.8 | 0.2 | 0.0 | 49 (18-77) |
| UHS IIL | 48 | 2.1 | 0.0 | 83.3 | 0.0 | 4.2 | 6.3 | 0.0 | 4.2 | 0.0 | 29.2 | 60.4 | 10.4 | 0.0 | 47 (27-66) |
CJ, criminal justice; STTR, seek, test, treat and retain
aStudy acronyms as in Table 1
bThere are 33 participants who are included in more than one sub-study: 3 in BRIGHT 1 and BRIGHT 2, and 30 in UHSIIC and UHSIIL
Table 4.
Risk behavior characteristics for STTR CJ cohort study participants
| Injection drug use, %c | Shared injection equipment, %c | Condomless sex, %c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Studya | Nb | Ever use: yes | Ever use: don’t know, refused, missing | Recent use: yes | Recent: don’t know, refused, missing | Recent use | Recent use: don’t know, refused, missing | Recent | Recent: don’t know, refused, missing |
| BRIGHT | |||||||||
| BRIGHT 1 | 2405 | 22.4 | 0.04 | 4.3 | 12.3 | 1.1 | 12.3 | 49.9 | 12.7 |
| BRIGHT 2 | 100 | 51.0 | 10.0 | 2.0 | 16.0 | 1 | |||
| CARE+ | |||||||||
| RCT | 112 | 14.3 | 1.8 | 4.5 | 6.3 | 3.6 | 5.4 | 78.6 | 3.6 |
| HCV RIDOC | 250 | 17.6 | 10.0 | 4.4 | − | ||||
| HCV PROB | 138 | 13.0 | 4.4 | 1.5 | − | ||||
| IMPACT | 381 | − | − | − | − | ||||
| LINK LA | 356 | − | − | − | 54.8 | 3.7 | |||
| NEW HOPE | 123 | 86.2 | 3.3 | 64.2 | 3.3 | 17.9 | 4.9 | 25.2 | 3.3 |
| STT | 3963 | 10.5 | 0.8 | 6.1 | 1.2 | 3.9 | 0.9 | 49.1 | 10.8 |
| STT ILLINOIS | |||||||||
| OPT OUT | 236 | − | 7.6 | − | − | ||||
| CM JAIL | 376 | − | 8.0 | 0.3 | 2.7 | 0.3 | 28.5 | 2.7 | |
| CM PRISON | 90 | − | 8.9 | 1.1 | 3.3 | 1.1 | 38.9 | 1.1 | |
| START | 195 | − | 3.6 | − | 25.1 | 0.5 | |||
| STRIDE | |||||||||
| STRIDE 1 | 50 | 76.0 | 4.0 | 34.0 | 4.0 | 4.0 | 8.0 | 4.0 | 6.0 |
| STRIDE 2 | 109 | 49.5 | 15.6 | 3.7 | 0.9 | 17.4 | 6.4 | ||
| SUCCESS | 56 | − | 10.7 | − | 51.8 | 3.6 | |||
| UHS II | |||||||||
| UHS IIC | 2090 | 43.0 | 26.5 | − | 64.5 | 0.05 | |||
| UHS IIL | 48 | 37.5 | 22.9 | 52.1 | |||||
CJ criminal justice, STTR seek, test, treat and retain
aStudy acronyms as in Table 1
bThere are 33 participants who are included in more than one sub-study: 3 in BRIGHT 1 and BRIGHT 2, and 30 in UHSIIC and UHSIIL
cReference periods differed across studies; 30 days: STRIDE 1, NEWHOPE, START, SUCCESS, 90 days: BRIGHT 1 and 2, STRIDE 2, all STT Illinois studies, CARE + HCV RIDOC, CARE + RCT, STT, 180 days: LINK LA, UHS II. The following studies measured pre-incarceration risk behaviors: CARE+ RCT, CARE+ HCV RIDOC, LINK LA, NEW HOPE, STT, STT ILLINOIS OPT OUT, STT ILLINOIS CM JAIL, and STT ILLINOIS CM PRISON
Table 5.
Substance use distribution for STTR CJ study participants
| Alcohol use, %c,e | Substance use, %d,e | Multiple substance use, %d,e | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Studya | Nb | Any | Binge | Marijuana | Cocaine | Opioids | Stimulants | Other | ≥2 substances (including alcohol) |
| BRIGHT | |||||||||
| BRIGHT 1 | 2405 | 48.2 | 28.7 | 27.5 | 10.7 | 13.0 | 0.3 | 2.9 | 29.9 |
| BRIGHT 2 | 100 | 44.0 | 25.0 | 22.0 | 26.0 | 23.0 | 1.0 | 3.0 | 38.0 |
| CARE+ | |||||||||
| RCT | 112 | 80.4 | 60.7 | 35.7 | 37.5 | 17.0 | 8.9 | 20.5 | 54.5 |
| HCV RIDOC | 250 | 71.6 | 60.4 | 60.8 | 24.8 | 31.2 | 9.2 | 22.0 | 66.8 |
| HCV PROB | 138 | 63.8 | 37.0 | 42.0 | 15.9 | 10.9 | 2.9 | 4.4 | 39.1 |
| IMPACT | − | − | − | − | − | − | − | − | − |
| LINK LA | 356 | 53.1 | 24.7 | 54.2 | 17.1 | 10.1 | 58.1 | 9.8 | 66.3 |
| NEW HOPE | 123 | 37.4 | 22.8 | 23.6 | 77.2 | 91.9 | 0.8 | 8.9 | 81.3 |
| STT | 3963 | 46.5 | 31.4 | 37.7 | 14.8 | 13.8 | 9.8 | 14.4 | 38.9 |
| STT ILLINOIS | |||||||||
| OPT OUT | − | − | − | − | − | − | − | − | − |
| CM JAIL | 376 | 71.0 | 49.5 | 45.0 | 39.1 | 32.5 | 14.1 | 10.4 | 65.7 |
| CM PRISON | 90 | 75.6 | 53.3 | 63.3 | 44.4 | 41.1 | 13.3 | 13.3 | 77.8 |
| START | 195 | 72.8 | 53.9 | 35.9 | 21.5 | 21.0 | 1.0 | 12.3 | 47.2 |
| STRIDE | |||||||||
| STRIDE 1 | 50 | 58.0 | 22.0 | 6.0 | 24.0 | 92.0 | 0.0 | 6.0 | 58.0 |
| STRIDE 2 | 109 | 62.4 | 33.0 | 24.8 | 41.3 | 83.5 | 6.4 | 11.0 | 69.7 |
| SUCCESS | 56 | 71.4 | 48.2 | 51.8 | 48.2 | 25.0 | 21.4 | 1.8 | 57.1 |
| UHS II | |||||||||
| UHS IIC | 2090 | 79.7 | 54.6 | 61.7 | 90.8 | 47.3 | 10.6 | 12.0 | 92.3 |
| UHS IIL | 48 | 64.6 | 39.6 | 66.7 | 66.7 | 22.9 | 6.3 | 8.3 | 72.9 |
CJ criminal justice, STTR seek, test, treat and retain
aStudy acronyms as in Table 1
bThere are 33 participants who are included in more than one study: 3 in BRIGHT 1 and BRIGHT 2, and 30 in UHSIIC and UHSIIL
cAlcohol reference periods differed across studies; 30 days: LINK LA, NEW HOPE, START; UHS II; 90 days: BRIGHT 1 and 2, STT; 180 days: STT Illinois; 1 year: CARE + RCT, SUCCESS; not specified: STRIDE 1 and 2, START
dSubstance reference periods differed across studies; 30 days: LINK LA, NEW HOPE, START Together; STRIDE 1, SUCCESS, UHS II; 90 days: BRIGHT 1 and 2, CARE+ RCT and HCV RIDOC, STT Strategies, STRIDE 2; 180 days: STT Illinois
eThe following studies measured pre-incarceration alcohol and substance use: CARE+ RCT, CARE+ RIDOC, LINK LA, NEW HOPE, STT, STT JAIL, STT PRISON, and SUCCESS
Table 6.
Supervision status for STTR CJ cohort study participants
| CJ supervision status, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Studya | Nb | No current CJ supervision | Jail | Prison | Probation | Parole | Probation/parole | Other | Don’t know, refused, missing |
| BRIGHT | |||||||||
| BRIGHT 1 | 2405 | 0 | 0 | 0 | 81.3 | 15.2 | 3.5 | 0 | 0.08 |
| BRIGHT 2 | 100 | 0 | 0 | 0 | 69.0 | 25.0 | 6.0 | 0 | 0 |
| CARE+ | |||||||||
| RCT | − | − | − | − | − | − | − | − | − |
| HCV RIDOC | 250 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| HCV PROB | 138 | 0 | 0 | 0 | 97.8 | 0.7 | 0.7 | 0 | 0.7 |
| IMPACT | 381 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| LINK LA | 356 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| NEW HOPE | 123 | 0.0 | 58.5 | 27.6 | 3.3 | 0.8 | 0.0 | 5.7 | 4.1 |
| STT | 3963 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| STT ILLINOIS | |||||||||
| OPT OUT | 236 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| CM JAIL | 376 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| CM PRISON | 90 | 14.4 | 0.0 | 0.0 | 0.0 | 78.9 | 4.4 | 1.1 | 1.1 |
| START | − | − | − | − | − | − | − | − | − |
| STRIDE | |||||||||
| STRIDE 1 | 50 | 44.0 | 0.0 | 0.0 | 16.0 | 30.0 | 4.0 | 4.0 | 2.0 |
| STRIDE 2 | 109 | 78.9 | 0.0 | 0.0 | 3.7 | 10.1 | 0.0 | 6.4 | 0.9 |
| SUCCESS | 56 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| UHS II | |||||||||
| UHS IIC | 2090 | 68.0 | 0 | 0 | 23.5 | 6.1 | 2.1 | 0 | 0.2 |
| UHS IIL | 48 | 52.1 | 0 | 0 | 35.4 | 4.2 | 8.3 | 0 | 0 |
CJ criminal justice, STTR seek, test, treat and retain
aStudy acronyms as in Table 1.
bThere are 33 participants who are included in more than one study: 3 in BRIGHT 1 and BRIGHT 2, and 30 in UHSIIC and UHSIIL
Harmonization results
By combining data across multiple studies, we include individuals involved with varying aspects of the CJ system, enabling comparisons that cannot typically be done within individual studies. For example, harmonizing data enables analyses involving subgroups such as transgender individuals and other subgroups that are often too small to assess in individual studies.
A key finding from our conduct of data harmonization is the need for multiple approaches. One technique involved modern psychometric approaches such as item response theory (IRT) to co-calibrate scales. Co-calibration enables people from studies who responded to different instruments to have scores that are “calibrated together” on a single common metric. IRT provides a suite of tools to model and account for measurement precision. We use this approach to address differences in the depression and anxiety measures across studies. In contrast, we found a different approach was needed to harmonize domains like ART adherence, which are behavioral rather than latent traits. Below we describe two harmonization efforts for ART adherence and risk behaviors.
Harmonization of ART adherence measures
We used CJ studies that measured ART adherence with both the AIDS Clinical Trials Group (ACTG) questionnaire and the Visual Analogue Scale (VAS) measure to develop rules and a formula to co-calibrate both instruments. By co-calibrating adherence items from the ACTG (7 day recall) with the VAS, we were able to harmonize studies having different approaches to measuring adherence [31]. We found that 44% of PLWH in the STTR CJ cohort who were on ART reported adherence rates <95% for the prior 30 days and 38% reported having missed one or more doses in the prior 14 days. Furthermore, we found important variations not only in ART adherence in different groups, but also in how adherence measures work. The findings also highlighted problems with the VAS as a single item adherence measure and showed its validity varied across groups. These results would not have been feasible within single studies but instead were possible by harmonizing multiple studies to gain both necessary sample size and variation [32].
HIV sexual risk behavior harmonization
Eight studies administered a standardized risk behavior assessment tool to evaluate sexual risk behaviors in these reference periods: 30 or 90 days prior to incarceration for jail and prison detainees, or the previous 30 or 90 days for participants enrolled in the community. We described sexual risk behaviors of HIV-infected and uninfected participants in these studies and compared men and women [33, 34]. To test for differences in risk behaviors between men and women, we performed individual patient data meta-analysis to combine information across studies. Findings indicated a high prevalence of condomless anal or vaginal sex among PLWH and uninfected participants, particularly women with HIV. For example, the adjusted odds ratio for condomless sex was 1.84 (1.16-2.95) for women with HIV compared with men with HIV [34]. By aggregating data across studies, we were able to overcome the much lower prevalence of women in CJ populations and, in turn, in each of the individual CJ studies.
Discussion
STTR CJ is a unique cohort that combines data from 11 studies that together address steps across the HIV treatment cascade focusing on participants with CJ involvement. The STTR CJ cohort includes a large and diverse assemblage of individuals who are at risk for, or living with HIV. The pooled data cohort is a sample of men, women, and transgender individuals across different racial/ethnic groups in multiple CJ settings (prison, jail, pre-trial, community supervision) as well as participants with no CJ involvement. The intervention studies span the HIV treatment cascade and have broad geographic locations across the US with participants enrolled from 11 states and the District of Columbia. These characteristics provide the opportunity to address questions of great importance for HIV prevention and treatment particularly related to individuals involved in the CJ system, those at risk or with HIV and using substances, and the unique risks and clinical needs of specific subgroups. For example, we are particularly interested in understanding the impact of changes in the intensity of substance use on HIV care cascade outcomes such as viral suppression and adherence, the impact of incarceration and release on the HIV care continuum, and to better understand risk behaviors of individuals upon release from incarceration. Finally, the study investigators and DCC have expertise in clinical, epidemiologic, CJ, biostatistical, and data harmonization areas. This team is well-positioned to address trans-disciplinary scientific questions and identify new questions that will arise as HIV care and prevention continues to evolve, and we have developed harmonized measures to allow for combining studies to answer these questions.
There are limitations to the STTR CJ cohort data. Some of these limitations are inherent in combining data from independent studies with different enrollment criteria and different study designs. While the STTR CJ cohort includes a large number of CJ-involved PLWH or persons at risk of HIV, this combination of studies is not a representative sample of those involved in the CJ system in the US, and in particular may underrepresent rural areas. Harmonization of some variables has been difficult due to modifications made by some individual studies to standardized instruments and data collection timeframes. For example, some studies modified timeframes for behaviors that were assessed (30 days vs. 90 days) to fit their study follow-up periods. Missing data varied by participating study and some studies have limited or no data on certain domains. This means that it is not feasible to use all studies in some analyses. For example, studies varied in whether they focused on current substance use or included type of substances at the time of incarceration. HIV status also varied with studies focused on later stages in the HIV cascade more likely focusing on PLWH. Similarly, studies that focused on late stages of the cascade cannot contribute to HIV risk analyses in that all participants are PLWH. The heterogeneity of study designs in the participating studies including both observational and interventional studies makes it feasible to study all the different stages of the STTR cascade but also complicates approaches to combining studies. When merging these studies it is critical to identify the subset of the STTR CJ cohort appropriate for and able to provide data on the research questions of interest. These studies recruited from diverse source populations. For example, two studies recruited participants who were at high risk of CJ system involvement but not necessarily already involved with the CJ system at baseline. This variation prevents the naïve pooling of studies, as baseline characteristics are different between studies, and requires clustering of participants by study be accounted for using a mixed model or generalized estimating equation approach. Longitudinal follow-up duration and frequency varies with several studies having limited follow-up time although we will continue to include all additional data collected over follow-up, so long as studies remain ongoing. While this variation limits power for analyses looking at longitudinal outcomes, the STTR CJ cohort remains better powered than individual studies.
The STTR CJ cohort is designed to allow expansion for new studies and domains as needed to address important questions in the HIV research agenda particularly related to the STTR treatment cascade. New studies will be added as needed to increase demographic, geographic, or clinical diversity and broaden scientific expertise. The STTR CJ cohort welcomes collaboration from interested parties and has policies to ensure multi-center analytic research proposals are developed and results analyzed collaboratively and fairly.
Conclusions
This cohort provides a large study sample across different CJ settings (jail, prison, community supervision) and includes both persons with and without HIV, many of whom engage in illicit drug use. Compared to single studies, the combined data of the CJ cohort provides increased statistical power to determine the public health benefit of the STTR paradigm, address research questions on specific groups at risk of exiting the treatment cascade, and improve understanding of the intersection of drug use and HIV treatment.
Acknowledgements
The authors thank the other investigators, the staff, and particularly the participants in the individual STTR studies for their valuable contributions. We acknowledge the support and vision of NIDA Program Officer, Shoshana Kahana.
Funding
The opinions in this paper are those of the authors and do not reflect those of the National Institute on Drug Abuse, the National Institutes of Health, or the Department of Health and Human Services.
Research presented in this paper is the result of secondary data analysis and was supported by 5U01DA037702 from the National Institute on Drug Abuse (NIDA). Primary data collection for STTR studies was supported by grants 5R01DA030768, 5R01DA030747, 5R01DA030781, 5R01DA030771, 5R01MH094090, 5R01DA030778, 1R01DA030766, 7R01DA030770, 5R01DA030762, 5R01DA030776, 5R01DA030793, 1R01DA032059, 1R01DA032083, 1R01DA032106, 1R01DA032061, 1R01DA032110, 1R01DA032080, 1R01DA032082, 5R01DA032057, 1R01DA032098, 1R34DA035728 and 1R01DA032100. Additional support was provided by NIAID AI027757. The authors thank the other investigators, the staff, and particularly the participants of the individual STTR studies for their valuable contributions. A full list of participating STTR investigators and institutions can be found at http://www.sttr-hiv.org. All authors have reviewed this publication and consented for publication.
Availability of data and materials
Given the sensitive nature, this data is not publically available. However, as stated in the paper, those interested in collaborating or working with this project or data should contact the STTR Data Coordination Center at sttr@uw.edu.
Authors’ contributions
RC was involved in funding for the STTR cohort collaboration. RC, MG, HC all contributed to the first draft of the manuscript. MG, FA, CB, WC, PF, CG, KK, AK, IK, JL, LO, JR, SS, DS, AS, SS, FT, DW, JY all contributed to data collection. BK, LS, MLB, JACD, RMN, and RY all contributed to analyses. All authors read and approved the final manuscript.
Competing interests
The authors declared that they have no competing interests.
Consent to publish
Not applicable.
Ethics approval and consent to participate
Each study has its own local human subjects approvals and consents individuals for participation. The University of Washington Human Subjects Division (HSD) reviewed the project (EJ-46474) and declared that the deidentified data received was determined to not be human subjects and provided a waiver.
Abbreviations
- ACTG
AIDS Clinical Trials Group
- ART
Antiretroviral therapy
- CJ
Criminal Justice
- DCC
Data Coordination Center
- IDU
Injection drug use
- IRT
item response theory
- NIDA
National Institute on Drug Abuse
- NIMH
National Institute of Mental Health
- OAR
Office of AIDS Research
- STTR
Seek, Test, Treat and Retain
- US
United States
- VAS
Visual analogue scale
- VL
Viral load
Contributor Information
Redonna Chandler, Email: rchandle@mail.nih.gov.
Michael S. Gordon, Email: mgordon@friendsresearch.org
Bridget Kruszka, Email: bkruszka@uw.edu.
Lauren N. Strand, Email: lnstrand@uw.edu
Frederick L. Altice, Email: Frederick.Altice@yale.edu
Curt G. Beckwith, Email: cbeckwith@lifespan.org
Mary L. Biggs, Email: mlbiggs@uw.edu
William Cunningham, Email: wcunningham@mednet.ucla.edu.
J.A. Chris Delaney, Email: jacd@uw.edu
Patrick M. Flynn, Email: p.flynn@tcu.edu
Carol E. Golin, Email: carol_golin@med.unc.edu
Kevin Knight, Email: k.knight@tcu.edu.
Alex H. Kral, Email: akral@rti.org
Irene Kuo, Email: ikuo@gwu.edu.
Jennifer Lorvick, Email: jlorvick@rti.org.
Robin M. Nance, Email: rmnance@uw.edu
Lawrence J. Ouellet, Email: ljo@uic.edu
Josiah D. Rich, Email: jrich@lifespan.org
Stanley Sacks, Email: stansacks@mac.com.
David Seal, Email: dseal@tulane.edu.
Anne Spaulding, Email: aspauld@emory.edu.
Sandra A. Springer, Email: sandra.springer@yale.edu
Faye Taxman, Email: ftaxman@gmu.edu.
David Wohl, Email: wohl@med.unc.edu.
Jeremy D. Young, Email: youngj@uic.edu
Rebekah Young, Email: rlyoung@uw.edu.
Heidi M Crane, Email: hcrane@uw.edu.
References
- 1.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–596. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 3.Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS Res Ther. 2016;13:35. doi: 10.1186/s12981-016-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Althoff KN, Buchacz K, Hall HI, Zhang J, Hanna DB, Rebeiro P, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157(5):325–335. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horberg MA, Hurley LB, Klein DB, Towner WJ, Kadlecik P, Antoniskis D, et al. The HIV Care Cascade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDs. 2015;29(11):582–590. doi: 10.1089/apc.2015.0139. [DOI] [PubMed] [Google Scholar]
- 6.Bureau of Justice Statistics: What is the difference between probation and parole?. http://www.bjs.gov/index.cfm?ty=qa&iid=324.
- 7.Inciardi JA. The war on drugs IV: the continuing saga of the mysteries and miseries of intoxication, addiction, crime, and public policy. 4. Boston: Pearson/Allyn and Bacon; 2008. [Google Scholar]
- 8.Schmalleger F. Criminal justice today: an introductory text for the 21st century custom edn. Prentice Hall Kaplan Higher Education: Upper Saddle River; 2011. [Google Scholar]
- 9.James N. Offender reentry: correctional statistics, reintegration into the community, and recidivism. Cong Res Serv. 2015:1–16.
- 10.Glaze L, Kaeble D. Correctional populations in the United States, 2013. Bur Justice Stat Bull. 2014:1–14.
- 11.Robillard AG, Brathwaite RL, Gallito-Zaparaniuk P, Kennedy S. Challenges and strategies of frontline staff providing HIV services for inmates and releases. J Correct Health Care. 2011;17(4):344–360. doi: 10.1177/1078345811413088. [DOI] [PubMed] [Google Scholar]
- 12.Maruschak L. In: Bureau of Justice Statistics Bulletin: HIV in prisons, 2007-2008. US Department of Justice, editor. Washington, D.C: Bureau of Justice Statistics; 2009. [Google Scholar]
- 13.Maruschak L. HIV in prisons, 2000. In. Edited by US Department of Justice. Washington, D.C: Bureau of Justice Statistics Bulletin; 2002. [Google Scholar]
- 14.Rosen DL, Schoenbach VJ, Wohl DA. All-cause and cause-specific mortality among men released from state prison, 1980-2005. Am J Pub Health. 2008;98(12):2278–2284. doi: 10.2105/AJPH.2007.121855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen DL, Schoenbach VJ, Wohl DA, White BL, Stewart PW, Golin CE. Characteristics and behaviors associated with HIV infection among inmates in the North Carolina prison system. Am J Pub Health. 2009;99(6):1123–1130. doi: 10.2105/AJPH.2007.133389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westergaard RP, Spaulding AC, Flanigan TP. HIV among persons incarcerated in the USA: a review of evolving concepts in testing, treatment, and linkage to community care. Curr Opin Infect Dis. 2013;26(1):10–16. doi: 10.1097/QCO.0b013e32835c1dd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releases from US correctional facilities, 2006: declining shares of epidemic but persistent public health opportunity. PLoS One. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Center for Disease Control and Prevention (CDC). HIV testing implementation guidance for correctional settings. In.; 2009.
- 19.Maruschak LM, Berzofsky M, Unangst J. In: Medical problems of state and federal prisoners and jail inmates, 2011-12. U.S. Department of Justice Office of Justice Programs Bureau of Justice Statistics, editor. 2015. pp. 1–22. [Google Scholar]
- 20.Solomon L, Montague BT, Beckwith CG, Bailargeon J, Costa M, Dumont D, et al. Survey finds that many prisons and jails have room to improve HIV testing and coordination of postrelease treatment. Health Aff. 2014;33(3):434–442. doi: 10.1377/hlthaff.2013.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Center for Disease Control and Prevention (CDC) CDC releases revised HIV testing recommendations in healthcare settings. 2006. [Google Scholar]
- 22.Obermeyer CM, Osborn M. The utilization of testing and counseling for HIV: a review of the social and behavioral evidence. Am J Public Health. 2007;97(10):1762–1774. doi: 10.2105/AJPH.2006.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Center for Disease Control and Prevention (CDC) HIV prevention strategic plan: extended through 2010. Center for Disease Control and Prevention. 2010. [Google Scholar]
- 24.Belani H, Chorba T, Fletcher F, Hennessey K, Kroeger K, Lansky A, et al. In: Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. Center for Disease Controlet al., editors. 2012. [PubMed] [Google Scholar]
- 25.Baillargeon J, Giordano TP, Rich JD, Wu ZH, Wells K, Pollock BH, et al. Accessing antiretroviral therapy following release from prison. JAMA. 2009;301(8):848–857. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iroh PA, Mayo H, Nijhawan AE. The HIV Care Cascade before, during, and after incarceration: a systematic review and data synthesis. Am J Public Health. 2015;105(7):e5–16. doi: 10.2105/AJPH.2015.302635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandler RK, Kahana SY, Fletcher B, Jones D, Finger MS, Aklin WM, et al. Data collection and harmonization in HIV research: the seek, test, treat, and retain initiative at the National Institute on Drug Abuse. Am J Public Health. 2015;105(12):2416–2422. doi: 10.2105/AJPH.2015.302788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, et al. Cohort profile: the North American AIDS Cohort collaboration on research and design (NA-ACCORD). Int J Epidemiol. 2007; [DOI] [PMC free article] [PubMed]
- 29.May MT, Ingle SM, Costagliola D, Justice AC, de Wolf F, Cavassini M, et al. Cohort profile: antiretroviral therapy Cohort collaboration (ART-CC) Int J Epidemiol. 2014;43(3):691–702. doi: 10.1093/ije/dyt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the Centers for AIDS research network of integrated clinical systems. Int J Epidemiol. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nance R, Crane H, Golin C, Wechsberg W, Gordon M, Cunningham C, et al. Co-calibration of two validated, self-reported measures of ART adherence in the CFAR network of integrated clinical systems (CNICS) research network and STTR consortium. Miami: 10th International Conference on HIV Treatment and Prevention Adherence; 2015. [Google Scholar]
- 32.Strand L, Nance R, Chandler RK, Cunningham W, Riley ED, Mehta S et al. Initial data harmonization in the STTR HIV consortium: evaluating the relationship between antiretroviral adherence and substance use. In: Society for Epidemiologic Research (SER) Annual Meeting. Denver, CO; 2015.
- 33.Loeliger K, Biggs ML, Seal D, Gordon M, Beckwith C, Kuo I et al. Gender differences in sexual risk behaviors among HIV-infected and unifected persons involved in the U.S. criminal justice system: the STTR harmonization project. National HIV Prevention Conference. Atlanta, GA.; 2015.
- 34.Loeliger KB, Biggs ML, Young R, Seal DW, Beckwith CG, Kuo I, et al. Gender differences in HIV risk behaviors among persons involved in the U.S. criminal justice system and living with HIV or at risk for HIV: a "seek, test, treat, and retain" harmonization consortium. AIDS Behav. 2017. doi:10.1007/s10461-017-1722-9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are not publically available due to privacy/license concerns. However, we welcome collaboration from interested parties. We have policies to ensure multi-center analytic research proposals are developed and results analyzed collaboratively and fairly. We are guided by principles of other cohort collaborations [28–30] including that data at the DCC will be stripped of all protected health information (PHI); an individual study can choose to participate or not in any scientific aim or sub-aim; and concept proposals and completed manuscripts must be approved by the Publications and Presentations (P&P) committee. We appreciate and encourage mentorship and the use of the STTR data to enable early investigators to address meaningful questions with support to help ensure their success. Additional information can be obtained at the STTR website: https://sttr-hiv.org/cms or by contacting the STTR DCC at STTR@uw.edu.
Given the sensitive nature, this data is not publically available. However, as stated in the paper, those interested in collaborating or working with this project or data should contact the STTR Data Coordination Center at sttr@uw.edu.


