Abstract
Even though reactive oxygen species (ROS) have been implicated in SLE pathogenesis, the contributory role of ROS, especially the consequences of oxidative modification of proteins by lipid peroxidation-derived aldehydes (LPDAs) such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE) in eliciting an autoimmune response and disease pathogenesis remains largely unexplored. MRL/lpr mice, a widely used model for SLE, spontaneously develop a condition similar to human SLE, whereas MRL+/+mice with the same MRL background, show much slower onset of SLE. To assess if the differences in the onset of SLE in the two substrains could partly be due to differential expression of LPDAs and to provide evidence for the role of LPDA-modified proteins in SLE pathogenesis, we determined the serum levels of MDA-/HNE-protein adducts, anti-MDA-/HNE-protein adduct antibodies, MDA-/HNE-protein adduct specific immune complexes, and various autoantibodies in 6-, 12- and 18-week old mice of both substrains. The results show age-related increases in the formation of MDA-/HNE-protein adducts, their corresponding antibodies and MDA-/HNE-specific immune complexes, but MRL/lpr mice showed greater and more accelerated response. Interestingly, a highly positive correlation between increased anti-MDA-/HNE-protein adduct antibodies and autoantibodies was observed. More importantly, we further observed that HNE-MSA caused significant inhibition in antinuclear antibodies (ANA) binding to nuclear antigens. These findings suggest that LPDA-modified proteins could be important sources of autoantibodies and CICs in these mice, and thus contribute to autoimmune disease pathogenesis. The observed differential responses to LPDAs in MRL/lpr and MRL+/+mice may, in part, be responsible for accelerated and delayed onset of the disease, respectively.
Keywords: lipid peroxidation, malondialdehyde, 4-hydroxynonenal, protein adducts, autoantibodies
Introduction
Systemic lupus erythematosus (SLE) is a chronic complex systemic autoimmune disease (AD), characterized by high levels of non-organ-specific autoantibodies directed against a multitude of self-antigens leading to deposition of immune complexes (ICs) and then destruction of end-organs, such as kidneys [1–6]. This chronic life-threatening disorder which affects a large population, is much more common in females and is one of leading causes of death among young and middle-aged women [1,2,6–8]. It is widely accepted that the etiology of SLE is multifactorial, including genetic, hormonal and environmental triggers. However, the molecular mechanisms underlying this systemic disorder are not fully understood.
Many mechanistic aspects of SLE could be addressed by using experimental animals which represent as model of certain states of immune system. A convenient and widely accepted model for SLE is MRL/MpJ-Faslpr/J (MRL/lpr) mouse, which spontaneously develops a condition that has many similarities to human SLE, such as developing marked hypergamma-globulinemia, production of numerous autoantibodies [anti-nuclear antibodies (ANA), anti-double-stranded DNA antibodies (anti-dsDNA) and anti-Smith antibodies (anti-Sm)], circulating immune complexes (CICs), lymphadenopathy and glomerulonephritis [9–13]. The female MRL/lpr mice develop a more severe form of disease which is associated with accelerated mortality compared to their male counterparts [10]. On the other hand, MRL/MpJ (MRL+/+) mouse, which shares the same MRL background with MRL/lpr mouse, also serves as model for delayed development of SLE. MRL+/+mice spontaneously develop various autoantibodies after several months, and SLE late in the second year of life [1,14–16], whereas MRL/lpr mice have accelerated appearance of various autoantibodies (only a few weeks) and develop glomerulonephritis at an early age of 4–5 months [9–11,16]. Although the lpr gene causes lymphadenopathy and autoantibody formation in a variety of mouse strains, full development of SLE is seen only in mice with the MRL background [9].
In recent years, increasing evidence has surfaced in support of a potential role of reactive oxygen species (ROS) in the pathogenesis of SLE and other ADs [4,6,16–23]. Increased lipid peroxidation, an oxidative degeneration of polyunsaturated fatty acids by ROS, is reported in SLE and other ADs [4,6,19,21,22,24–26]. Lipid peroxidation-derived aldehydes (LPDAs) such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE), bind covalently with proteins to form MDA- and HNE-modified protein adducts [25,27–30]. Interestingly, higher levels of MDA-/HNE- protein adducts have been observed in SLE patients [6,18,19,24,25,31], suggesting a potential role of these adducts in SLE. Previous studies in our laboratory using autoimmune-prone MRL+/+mice treated with trichloroethene (TCE) also suggested an association between oxidative stress and autoimmune responses [16,32,33].
Even though increased ROS formation has drawn considerable attention as the potential mechanism in the pathogenesis of SLE, the contributory role of ROS, especially the consequences of oxidative modification of proteins by MDA and/or HNE in eliciting an autoimmune response and contribution to SLE pathogenesis and progression remains largely unexplored. In the current study, using two congenic MRL mouse substrains (MRL+/+and MRL/lpr) which differ in the onset of autoimmune lesions, we have focused on evaluating the contribution of LPDAs-modified proteins in the disease pathogenesis. By evaluating the age-dependent responses in the two sub-strains, we not only provide evidence for differential formation of MDA-/HNE-protein adducts and their corresponding antibodies with increasing age in the two MRL substrains, but also much accelerated and greater response in MRL/lpr mice. For the first time, we also provide data showing a remarkable differential formation of immune complexes specific to MDA-/HNE-protein adducts and significant inhibition in the binding of ANA to nuclear antigens by LPDA-protein adducts.
Methods
Animals
Five-week old female MRL+/+and MRL/lpr mice, purchased from The Jackson Laboratories (Bar Harber, ME), were housed in plastic cages on a bedding of wood chips at the University of Texas Medical Branch (UTMB) animal house facility maintained at ~ 22 °C, 50–60% relative humidity, and a 12 hours light/dark cycle. The animals were provided standard lab chow and drinking water ad libitum and acclimated for one week. A time-response study of both MRL+/+and MRL/lpr mice was conducted by euthanizing the animals under nembutal (sodium pentobarbital) anesthesia at the ages of 6, 12 and 18 weeks. At euthanasia, blood was withdrawn from inferior vena cava. Individual sera, obtained following blood clotting and centrifugation, were stored in small aliquots at −80 °C till further analysis.
Quantitation of MDA- and HNE-protein adducts in the serum
For the quantitation of MDA- and HNE-protein adducts in the sera from both MRL+/+and MRL/lpr mice, competitive ELISAs were performed as described earlier [6,27,33]. Briefly, flat bottomed 96-well microtiter plates were coated with MDA-/HNE-ovalbumin adducts or ovalbumin (0.5 μg/well) overnight at 4 °C. For the competitive ELISA, rabbit antisera [anti-MDA (1:2000 diluted) or anti-HNE (1:3000 diluted); Alpha Diagnostics Int’l, San Antonio, TX] were incubated with test samples (standards or unknown) at 4 °C overnight. The coated plates were blocked with a blocking buffer (Sigma, St. Louis, MO) for 2 hours at room temperature (RT), then a 50 μl aliquot of each of the above-mentioned incubation mixtures was added to duplicate wells and incubated for 2 hours at RT. After washing, 50 μl of goat anti-rabbit IgG-horseradish peroxidase (HRP; 1:2000 diluted, Millipore, Billerica, MA) was added and incubated for 1 hour at RT. After washing, 100 μl of TMB peroxidase substrate (KPL, Gaithersburg, MD) was added to each well. The reaction was stopped after 10 minutes by adding 100 μl 2 M H2SO4 and the absorbance was read at 450 nm on a Bio-Rad Benchmark plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA).
ELISAs for anti-MDA- and anti-HNE-protein adduct specific antibodies in the serum
ELISAs to analyze anti-MDA- and anti-HNE-protein adduct-specific antibodies in the mouse serum were performed as described earlier [16,20,32,33]. Briefly, flat-bottomed 96-well microtiter plates were coated with MDA-/HNE-ovalbumin adducts or ovalbumin (0.5 μg/well) overnight at 4 °C. The plates were washed with Tris-buffered saline-Tween 20 (TBST) and the nonspecific binding sites were blocked by incubating with Tris-buffered saline (TBS) containing 1% bovine serum albumin (BSA; Sigma) at RT for 1 hour. After washing extensively with TBST, 50 μl of 1:100 diluted mouse serum samples were added to duplicate wells of the coated plates and incubated at RT for 2 hours. The plates were washed 5 times with TBST and then 50 μl of rabbit anti-mouse IgG-HRP (1:2000 in TBS; Chemicon, Temecula, CA) was added and incubated at RT for 1 hour. After washing, 100 μl of TMB peroxidase substrate (KPL) was added to each well. The reaction was stopped after 10 minutes by adding 100 μl 2 M H2SO4 and the OD was read at 450 nm on a Bio-Rad Benchmark plus microplate spectrophotometer (Bio-Rad Laboratories). The samples with net OD values (the difference between OD for MDA-/HNE-ovalbumin and ovalbumin) greater than 0.2, 0.4, or 0.6 were graded as moderately positive (+), highly positive (++), or strongly positive (+++), respectively [16,20,32].
Anti-nuclear, anti-ssDNA, anti-dsDNA and anti-SM antibodies in the serum
Serum levels of ANA, anti-single stranded DNA (anti-ssDNA), anti-dsDNA and anti-Sm antibodies were determined by using mouse-specific ELISA kits (Alpha Diagnostic Int’l) as described earlier [15,20,32,33].
Quantification of CICs in serum
Serum levels of CICs were determined by using mouse-specific ELISA kits (Alpha Diagnostic Int’l) following manufacturer’s manual.
Determination of MDA-/HNE-protein adduct specific CICs in the sera
The immune complexes of MDA-/HNE-protein adducts with their respective specific antibodies in the sera of two MRL substrain mice were analyzed using specific ELISAs. Briefly, flat-bottomed 96-well plates were coated with rabbit anti-MDA- or anti-HNE-protein adduct antibodies (1:1000 diluted; Alpha Diagnostics Int’l) overnight at 4 °C. The plates were washed with TBST and the non-specific binding sites were blocked with TBS containing 1% BSA (Sigma) at RT for 1 hour. After washing extensively with TBST, 100 μl of diluted serum samples were added to duplicate wells of the coated plates and incubated at RT for 2 hours. The plate was washed five times with TBST and then rabbit anti-mouse IgG-HRP was added and incubated at RT for 1 hour. After washing, 100 μl of TMB substrate was added to each well. The reaction was stopped after 10 minutes by adding 100 μl 2M H2SO4 and the OD was read at 450 nm on a Bio Rad Benchmark plus microplate spectrophotometer (Bio-Rad Laboratories).
Determination of inhibitory effects of LPDA-protein adducts on ANA binding with nuclear antigens
To determine the effect of LPDA-protein adducts on ANA binding with nuclear antigens, sera from 18-week MRL/lpr mice were used as the source of ANA for determining the inhibition in ANA binding and using mouse-specific ANA ELISA kits (Alpha Diagnostic Int’l) [15,20,32,33]. To achieve this, serum samples were incubated with LPDA-protein adducts overnight before performing assay for ANA. Briefly, 100 μl of 1:50 diluted sera [in phosphate buffered saline (PBS)] were incubated with 100 μl of PBS only, 100 μl of mouse serum albumin (MSA; 10 μg/ml in PBS), 100 μl of MDA-MSA (10 μg/ml in PBS) or 100 μl of HNE-MSA (10 μg/ml in PBS) at 4 °C overnight. The rest of the steps were similar to those described earlier [15,20,32,33].
Statistical analysis
The values presented are means ± SD. One-way ANOVA followed by Tukey-Kramer multiple comparisons test (GraphPad Instat 3 software, La Jolla, CA) was performed for the statistical analysis. Spearman’s rank correlation was used to calculate correlation coefficients between serum MDA-/HNE-protein adducts and serum CICs as well as serum anti-MDA-/HNE-protein adduct antibodies and serum ANA. A p value < 0.05 was considered to be statistically significant.
Results
Formation of MDA- and HNE-protein adducts in the sera of MRL+/+and MRL/lpr mice
To investigate the potential contribution of lipid peroxidation/oxidative stress in the induction/exacerbation of an autoimmune response, we first determined the formation of MDA-/HNE-protein adducts in the sera of 6-, 12-, and 18-week old MRL+/+ and MRL/lpr mice (Figure 1). As evident from Figure 1A, the formation of MDA-protein adducts increased with increasing age, and their levels were 50% and 67% higher, respectively, in the 12-week and 18-week old MRL+/+mice compared to the 6-week old MRL+/+mice. The formation of these adducts in MRL/lpr mice was greater compared to MRL+/+mice, and the increases in these adducts in the sera of 12- and 18-week old MRL/lpr mice were 86% and 118%, respectively, compared to the 6-week old MRL/lpr mice (p < 0.05). Remarkably, the serum levels of MDA-protein adducts in MRL/lpr mice at 6-, 12- and 18-weeks were 4.2-, 5.2- and 5.3-fold greater, respectively, than those in MRL+/+mice of the corresponding age groups (p < 0.05). Similarly, age-related increases in the levels of HNE-protein adducts in the sera of both MRL+/+and MRL/lpr mice were also observed (Figure 1B). Interestingly, levels of HNE-protein adducts in MRL/lpr mice at 6-, 12- and 18-weeks were 2.4-, 5.5- and 4.8-fold greater than those observed in MRL+/+mice (p < 0.05). Our results thus show differential age-related formation of MDA-/HNE-protein adducts in the two MRL substrains.
Figure 1.
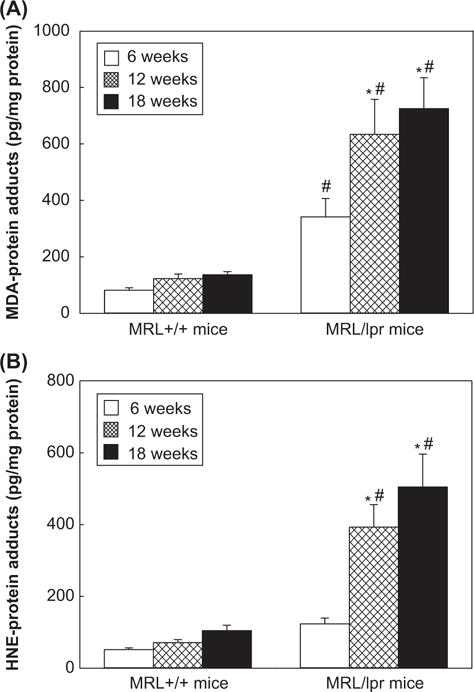
(A) MDA-protein adducts and (B) HNE-protein adducts in the sera of 6-, 12- and 18-week old MRL+/+and MRL/lpr mice. The values are means ± D. * p < 0.05 vs. 6-week old mice; # p < 0.05 vs. MRL+/+mice of respective age.
Differential induction of anti-MDA- and anti-HNE-protein adduct antibodies in the sera of MRL+/+and MRL/lpr mice
To assess if LPDA-modified proteins could contribute to an autoimmune response, anti-MDA- and anti-HNE-protein adduct antibodies were analyzed in the sera (Figure 2 and Tables I and II). There was a weak response to MDA-protein adducts in 6-week old MRL+/+mice (the levels of anti-MDA-protein adduct antibodies were close to negative controls; data not shown), but moderate increases in serum anti-MDA-protein adduct antibodies in 12- and 18-week old MRL+/+mice, which were 49% and 75% greater, respectively, compared to 6-week old MRL+/+mice (Figure 2A). The levels of these antibodies in 12- and 18-week old MRL/lpr mice increased significantly (118% and 141% increases, respectively; p < 0.05) in comparison to 6-week old MRL/lpr mice. More importantly, the levels of anti-MDA-protein adduct antibodies in all three age groups of MRL/lpr mice showed remarkable increases (4.1-, 6.1- and 5.7-fold at the ages of 6-, 12- and 18-week old, respectively, p < 0.05) in comparison to MRL+/+mice of corresponding ages. Furthermore, the number and percentage of samples positive (+), highly positive (++) and strongly positive (+++) for these antibodies showed age- and substrain-related increases in the two substrains of MRL mice (Table I).
Figure 2.
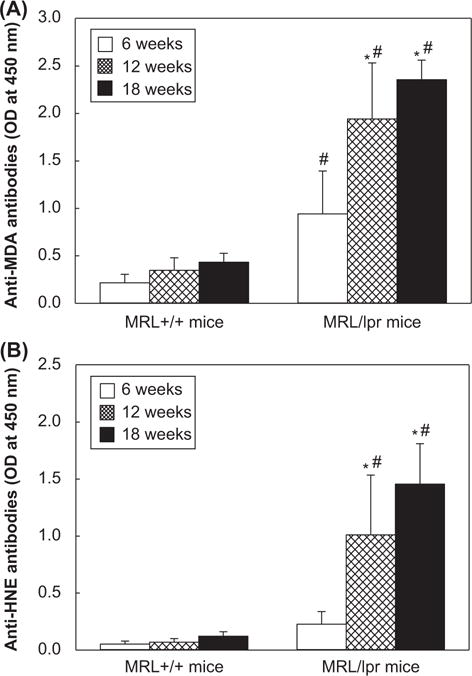
(A) Anti-MDA-protein adduct antibodies and (B) anti-HNE-protein adduct antibodies in the sera of 6-, 12- and 18-week old MRL+/+and MRL/lpr mice. The values are means ± SD. *p < 0.05 vs. 6-week old mice; # p < 0.05 vs. MRL+/+mice of respective age.
Table I.
Number and percentage of anti-MDA-protein antibody positive sera in MRL+/+and MRL/lpr mice.
| Total No. | Anti-MDA-protein antibody response*
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Response
|
+ | ++ | +++ | ||||||
|
| |||||||||
| No. | % | No. | % | No. | % | No. | % | ||
| MRL+/+mice | |||||||||
| 6-week-old | 5 | 3 | 60.0 | 3 | 60.0 | 0 | 0.0 | 0 | 0.0 |
| 12-week-old | 5 | 80.0 | 2 | 40.0 | 2 | 40.0 | 0 | 0.0 | |
| 18-week-old | 5 | 5 | 100.0 | 2 | 40.0 | 3 | 60.0 | 0 | 0.0 |
| MRL/lpr mice | |||||||||
| 6-week-old | 5 | 5 | 100.0 | 1 | 20.0 | 0 | 0.0 | 4 | 80.0 |
| 12-week-old | 5 | 5 | 100.0 | 0 | 0.0 | 0 | 0.0 | 5 | 100.0 |
| 18-week-old | 5 | 5 | 100.0 | 0 | 0.0 | 0 | 0.0 | 5 | 100.0 |
Table II.
Number and percentage of anti-HNE-protein antibody positive sera in MRL+/+and MRL/lpr mice.
| Total No. | Anti-HNE-protein antibody response*
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Response
|
+ | ++ | +++ | ||||||
|
| |||||||||
| No. | % | No. | % | No. | % | No. | % | ||
| MRL+/+mice | |||||||||
| 6-week-old | 5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 12-week-old | 5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 18-week-old | 5 | 1 | 20.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 |
| MRL/lpr mice | |||||||||
| 6-week-old | 5 | 1 | 20.0 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 |
| 12-week-old | 5 | 5 | 100.0 | 0 | 0.0 | 1 | 20.0 | 4 | 80.0 |
| 18-week-old | 5 | 5 | 100.0 | 0 | 0.0 | 0 | 0.0 | 5 | 100.0 |
Serum levels of anti-HNE-protein adduct antibodies, and the number and percentage of serum samples positive for anti-HNE-protein adduct antibodies also increased with increasing age in the two substrains but showed greater response in MRL/lpr mice, with a pattern similar to that of anti-MDA-protein adduct antibodies (Figure 2B and Table II). The age- and substrain-related increases in the levels of serum anti-MDA-/anti-HNE-protein adduct antibodies in these mice not only provide support to the increased formation of LPDA-modified protein adducts, but more importantly, suggest that the LPDA-modification of endogenous proteins elicits an immunogenic response which could potentially contribute to autoimmunity.
Production of autoantibodies in the sera of MRL+/+and MRL/lpr mice
SLE is characterized by high levels of autoantibodies, and such autoantibodies as ANA, anti-dsDNA, anti-ssDNA and anti-Sm are considered important laboratory evaluation indices of the disease [1,4,5,34–36]. Here, the autoantibodies were analyzed in the sera of 6-, 12- and 18-week old MRL+/+and MRL/lpr mice (Figure 3). There were increases in the serum levels of ANA in 12- and 18-week old MRL+/+mice (71% and 115% increases, respectively) compared to 6-week old MRL+/+mice. However, ANA levels were much greater in the 12- and 18-week old MRL/lpr mice (197% and 508% increases, respectively) in comparison to 6-week old MRL/lpr mice (p < 0.05, Figure 3A). More importantly, the ANA levels in 12- and 18-week old MRL/lpr mice were significantly higher (4.1- and 6.6-fold, respectively) than in the MRL+/+mice of the corresponding ages (Figure 3A). Furthermore, formation of anti-dsDNA, anti-ssDNA and anti-Sm antibodies in the sera of MRL mice showed a pattern similar to ANA (Figure 3B, 3C, 3D). Interestingly, the serum levels of anti-dsDNA in 6-, 12-, 18-week old MRL/lpr mice were significantly higher (4.1-, 4.5- and 3.8-fold, respectively) than those observed in MRL+/+mice of the corresponding age, suggesting an age- and substrain-related autoimmune responses in MRL+/+and MRL/lpr mice.
Figure 3.
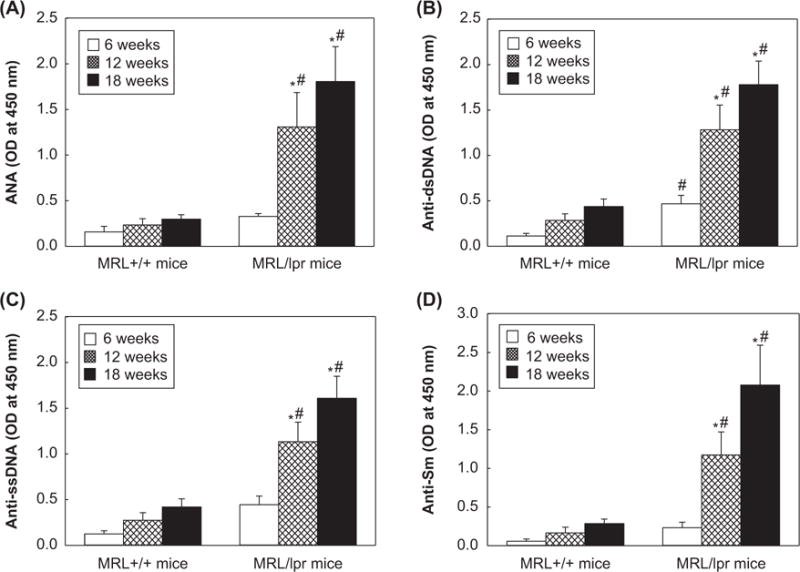
Serum levels of (A) anti-nuclear antibodies, (B) anti-dsDNA antibodies, (C) anti-ssDNA antibodies and (D) anti-SM antibodies in the 6-, 12- and 18-week old MRL+/+and MRL/lpr mice. The values are means ± SD. *p < 0.05 vs. 6-week old mice; # p < 0.05 vs. MRL+/+mice of respective age.
Correlation of anti-MDA-/HNE-protein adduct antibodies and serum autoantibodies
We evaluated relationship of the individual serum anti-MDA-/HNE-protein adduct antibodies with serum autoantibodies. As evident from Figure 6, a significant correlation was observed between serum anti-MDA-protein adduct antibodies and ANA (r = 0.81, p < 0.01; Figure 4A). Similarly, serum anti-HNE-protein adduct antibodies and ANA were also highly correlated (r = 0.82, p < 0.01; Figure 4B). Also, a remarkable direct correlation between serum anti-MDA- and anti-HNE-protein adduct antibodies and anti-dsDNA antibodies (r = 0.82 and 0.78, respectively, p < 0.01; data no shown) was observed. These findings not only reveal a direct association between anti-LPDA-modified protein antibodies and autoantibodies, but also suggest that the serum anti-MDA- and HNE-protein adduct antibodies along with autoantibodies (i.e., ANA, anti-dsDNA antibodies) may also serve as a predictive biomarker of progression of autoimmunity.
Figure 6.
Serum levels of CICs in the 6-, 12- and 18-week old MRL+/+and MRL/lpr mice. The values are means ± SD. * p < 0.05 vs. 6-week old mice; # p < 0.05 vs. MRL+/+mice of respective age.
Figure 4.
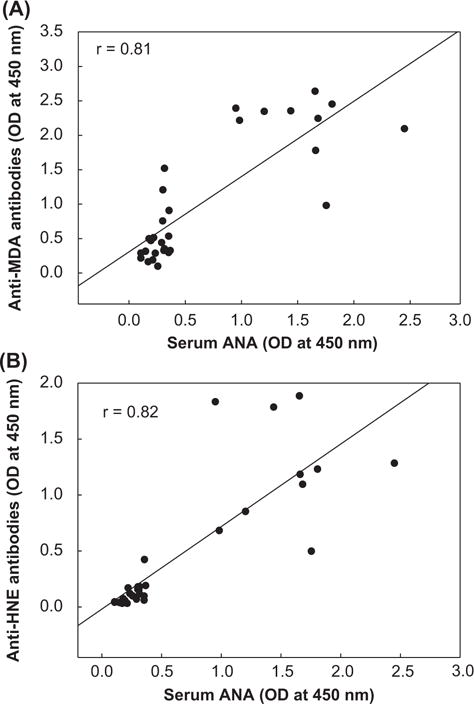
Correlation of (A) serum anti-MDA-protein adduct antibodies and (B) serum anti-HNE-protein adduct antibodies with serum ANA. Spearman’s rank correlation was used to calculate correlation coefficient.
MDA-/HNE-protein adducts have inhibitory effect on ANA binding
Since our studies showed a strong association of anti-MDA-/HNE-protein adduct antibodies and serum autoantibodies, it was of further interest to analyze if these LPDA-protein adducts affect the binding of ANA with nuclear antigens and thus, contribute to the formation of ANA. As evidenced in Figure 5, the incubation of sera from 18-week old MRL/lpr mice with MDA-MSA reduced the ANA binding with nuclear antigens by 29%, while HNE-MSA significantly decreased the ANA bindings with nuclear antigens by 35% (p < 0.05), suggesting that these adducts compete with nuclear antigens for ANA-binding activity.
Figure 5.
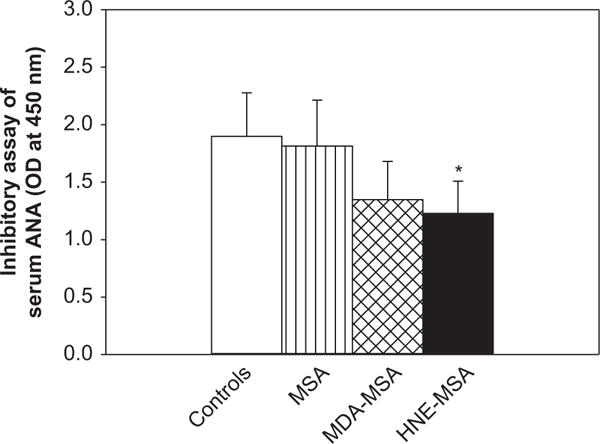
Inhibitory effect of MDA- and HNE-MSA on ANA binding to nuclear antigens. Serum from 18-week MRL/lpr mice was pre-incubated with MDA- or HNE-MSA following by ANA determination using ELISA kit (see methods). The values are means ± SD. *p < 0.05 vs. controls.
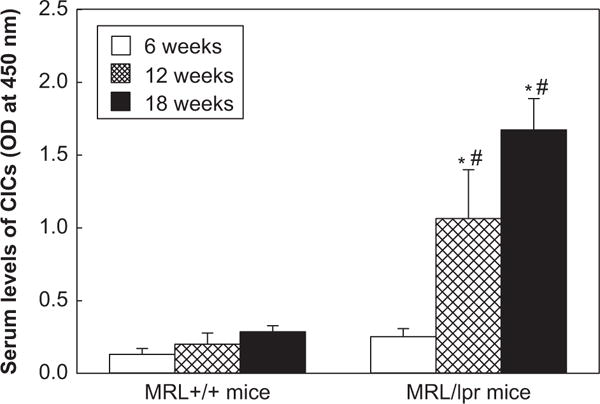
Formation of CICs in the sera of MRL+/+and MRL/lpr mice
Although the molecular mechanisms of SLE remain largely unknown, it is generally accepted that SLE is characterized by high levels of autoantibodies leading to increased formation of CICs, deposition of immune complexes and destruction of end-organs [1,4,5,10,13]. Therefore, CICs in the sera of MRL+/+and MRL/lpr were also analyzed. As shown in Figure 6, the serum levels of CICs in the MRL+/+mice at the age of 12- and 18-weeks were moderately increased (54% and 119% increases, respectively) compared to the 6-week old MRL+/+mice. However, CICs levels in the MRL/lpr mice of 12- and 18-weeks increased remarkably (322% and 564% increases, respectively; p < 0.05) compared to the 6-week old MRL/lpr mice. Moreover, the serum levels of CICs in the MRL/lpr mice of 12- and 18-weeks were 5.3- and 5.9-fold higher, respectively (p < 0.05) than the MRL+/+mice of corresponding ages. Furthermore, significant correlations were also observed between serum MDA-/HNE-protein adducts and CICs (r = 0.82, r = 0.79, p < 0.01, respectively; data not shown).
Formation of immune complexes of MDA-/HNE-protein adducts with their corresponding antibodies in the sera of MRL+/+and MRL/lpr mice
To further support the contribution of MDA-/HNE-protein adducts in the formation of LPDA-specific CICs and their role in the pathogenesis of SLE, we measured the formation of immune complexes specific for MDA-/HNE-protein adducts with their respective antibodies in the sera of MRL+/+and MRL/lpr mice. As shown in Figure 7A, there was very low formation of MDA-specific immune complexes in the sera of 6-week old MRL+/+mice (the same level of formation as negative controls, data not shown), but moderately increased formation in 12, and 18-week old MRL+/+mice (38% and 129% increases, respectively, compared to the 6-week old MRL+/+mice). Interestingly, there was greater formation of these immune complexes in the sera of 12- and 18-week old MRL/lpr mice (204% and 348% increases, respectively, p < 0.05) compared to the 6-week MRL/lpr mice. Furthermore, the serum levels of these immune complexes in the MRL/lpr mice at 6, 12- and 18-weeks were 2.9-, 6.5- and 5.7-fold higher, respectively (p < 0.05) than the MRL+/+mice of the corresponding ages. As evidenced in Figure 7B, a similar pattern in the formation of HNE-specific immune complexes was also observed.
Figure 7.
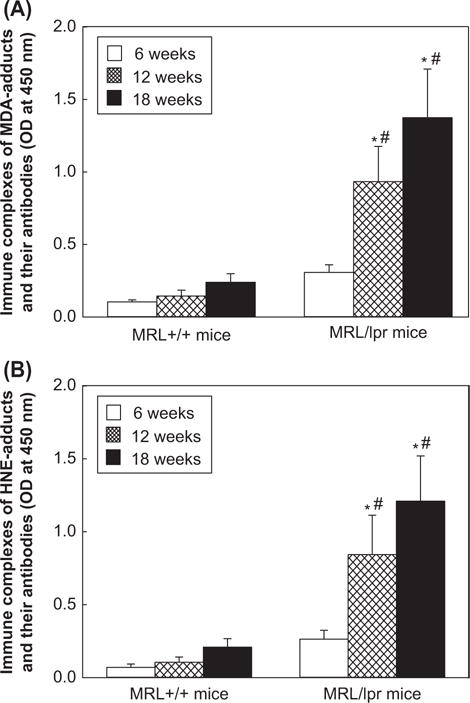
Serum levels of MDA-/HNE-protein adduct-specific immune complexes (A. MDA; B. HNE) in the 6-, 12- and 18-week old MRL+/+and MRL/lpr mice. The values are means ± SD. * p < 0.05 vs. 6-week old mice; # p < 0.05 vs. MRL+/+of respective age.
Discussion
Despite an association of high morbidity and mortality rate with SLE [1,2,6–8], the molecular mechanisms of disease pathogenesis are not fully understood. MRL/lpr mice, an extensively used model for SLE, develop various SLE features rather aggressively that are similar to those in human SLE [9,11–13,15,16], whereas MRL+/+mice, with the same MRL background as MRL/lpr mice, show much slower onset of SLE (spontaneous SLE late in the second year of life) [1,14]. We hypothesized that differences in the onset of disease features in the two substrains could partly be due to differential expression of LPDAs and consequent immunomodulatory response. To assess our hypothesis and provide evidence for the potential role of LPDA-modified proteins in the pathogenesis of ADs, we evaluated the serum levels of MDA-/HNE-protein adducts, anti-MDA-/HNE-protein adduct antibodies, CICs and MDA-/HNE-protein adduct specific immune complexes, as well as various autoantibodies in two congenic mice which differ in the onset of disease. Observed differential oxidative stress-related responses in two sub-strains with increasing age, along with the potential of LPDA-protein adducts to inhibit ANA binding to nuclear antigens suggest that LPDA could play a role in the pathogenesis of SLE.
MDA and HNE, the two major LPDAs, are highly reactive and can form MDA-/HNE-modified protein adducts via binding covalently with free amino groups of proteins and making them highly immunogenic [4,16,18,30,37–41]. Increased lipid peroxidation [4,18,19,22,24–26] and higher levels of MDA- and HNE-modified proteins have been reported in SLE patients [4,6,19,25,26,31,40]. However, significance of increased LPDA-modified proteins and their contributions to initiation and pathogenesis of SLE remain unclear. In this study, the formation of serum MDA-protein adducts and HNE-protein adducts increased with increasing age in both MRL+/+and MRL/lpr mice. However, these adducts appeared much sooner in MRL/lpr mice and also showed a robust response. The early and greater responses observed in MRL/lpr mice compared to MRL+/+suggest a potential role of these adducts not only in the development of the autoimmune response but also in early initiation of the disease.
Increasing evidence suggest that LPDA-modified proteins may trigger an immunogenic response to the self antigens, leading to a break in immune tolerance [30,40–44]. Moreover, LPDAs modification of cellular components, generating a variety of antigenic structures, also give rise to production of multi-specific antibodies that simultaneously recognize different epitopes [40,44]. In fact, antibodies generated against HNE-modified spitopes have been shown to have specificity towards native DNA [40,44], and modification of lupus-associated protein (i.e., 60-kDa Ro protein) with HNE has been shown to increase the antigenicity and to facilitate epitope spreading [43]. In the current study, we found age-dependent increases in the antibodies to MDA-/HNE-protein adducts in both MRL substrains, but remarkably accelerated and greater levels in MRL/lpr mice. Interestingly, the pattern of increased anti-MDA-/HNE-protein adduct antibodies in this study was strongly associated with the serum autoantibodies such as ANA and anti-dsDNA, suggesting that these LPDA-modified proteins are not only immunogenic and trigger the generation of antibodies, but also suggest that MDA-/HNE-protein adducts could be a source of autoantibodies contributing to the pathogenesis and progression of SLE.
SLE is characterized by high levels of non-organ-specific autoantibodies leading to formation and deposition of immune complexes and then destruction of end-organs such as kidneys and joints [1,4–6,13]. ANA includes autoantibodies directed against the extractable nuclear antigens, and the most prominent ANAs are autoantibodies which bind to dsDNA, ssDNA, histones, ribonucleoproteins (RNP) and Sm-antigens [1–4,13]. It remains to be known whether MDA-/HNE-protein adducts could also lead to production of autoantibodies and contribute to the pathogenesis of SLE. Previous studies have identified several different antigenic cross-reactivities for anti-DNA antibodies, and the sequence of an anti-HNE monoclonal antibody was highly homologous to anti-DNA autoantibodies [40,45]. Our findings in this study also show that increased anti-MDA-/HNE-protein adduct antibodies is strongly associated with the autoantibodies such as ANA and anti-dsDNA. This led us to determine if LPDA-protein adducts (neoantigens) are also recognized by anti-nuclear antibodies. Our data clearly show that pre-incubation of serum with HNE-MSA significantly decreased the ANA binding with nuclear antigens. This suggests that LPDA-modified epitopes may lead to production of multispecific antibodies which simultaneously recognize different epitopes including nuclear antigens or some of serum ANAs also have specificity towards MDA-/HNE-MSA.
Immune complexes, a hallmark and key mediators of SLE, play a central pathogenic role in causing tissue damage in the disease [1,4,5,13,46]. Previously, we hypothesized that LPDA-protein adducts might act as neoantigens eliciting autoimmune responses by stimulating T and/or B lymphocytes, then persistent increases in antibodies causing formation of immune complexes leading to ADs [6,16,20,32,33,47]. Our previous studies also have demonstrated that LPDA-protein adducts contributed to autoimmunity by activating Th1 and/or Th17 cells [20,47]. T helper (Th) cells (Th1, Th17) play central role in the pathogenesis of SLE by providing the help required for B cells to differentiate and produce a variety of pathogenic autoantibodies [47–49]. Our data show a highly positive correlation between the serum MDA-/HNE-protein adducts and serum CICs which suggests a strong association between the serum levels of these adducts and the formation of serum CICs. The results further suggest that formation of MDA-/HNE-protein adducts may serve as one of the important sources of CICs and contribute to the progression of the disease. To support our conclusion, we determined immune complexes specific for MDA-/HNE-protein adducts and found significantly increased but differential formation of these specific immune complexes in the two substrains of MRL mice, with the MRL/lpr mice showing early and greater response.
Taken together, our results clearly show age-related increases in the formation of MAD-/HNE-protein adducts, their corresponding antibodies and MDA-/HNE-specific immune complexes. Clearly, these responses were greater and more accelerated in MRL/lpr mice which also have an accelerated development of SLE in comparison to MRL+/+mice. Interestingly, the results of this study, for the first time, provide an evidence for a strong association between increased MDA-/HNE-protein adducts and CICs, as well as the highly positive correlation between increased anti-MDA-/HNE-protein adduct antibodies and autoantibodies. More importantly, our results also show that HNE-MSA caused significant inhibition in ANA binding to nuclear antigens. These findings apart from supporting immunogenic potential of MDA-/HNE-protein adducts, also suggest that these LPDA-modified proteins may be important sources of autoantibodies and CICs in these mice contributing to pathogenesis and progression of autoimmunity. The observed differential response to LPDAs in the two substrain may, in part, provide explanation for accelerated disease development. Further studies are, however, necessary to establish the novel pathways by which LPDAs break immune tolerance and contribute to an autoimmune response. It would be important to identify the LPDA-modifying proteins for establishing their role in disease pathogenesis. It would also be important to assess the usefulness of anti-MDA-/anti-HNE-protein adduct antibodies in evaluating progression and severity of ADs.
Acknowledgments
This work was supported by Grant ES016302 from the National Institute of Environmental Health Sciences (NIEHS), National Institute of Health (NIH). The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest.
References
- 1.Anam K, Amare M, Naik S, Szabo KA, Davis TA. Severe tissue trauma triggers the autoimmune state systemic lupus erythematosus in the MRL/++lupus-prone mouse. Lupus. 2009;18:318–331. doi: 10.1177/0961203308097479. [DOI] [PubMed] [Google Scholar]
- 2.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–318. doi: 10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 3.Kurien BT, Hensley K, Bachmann M, Scofield RH. Oxidatively modified autoantigens in autoimmune diseases. Free Radic Biol Med. 2006;41:549–556. doi: 10.1016/j.freeradbiomed.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Kurien BT, Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. 2008;7:567–573. doi: 10.1016/j.autrev.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol. 2009;21:489–494. doi: 10.1097/BOR.0b013e32832efff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Pierangeli SS, Papalardo E, Ansari GAS, Khan MF. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 2010;62:2064–2072. doi: 10.1002/art.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SJ, Rau LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health. 2000;90:1463–1466. doi: 10.2105/ajph.90.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 10.Field M, Brennan FM, McCarthy D, Mumford P, Maini RN. MRL-lpr/lpr mice show an impairment of IgG aggregate removal which relates to parameters of disease activity. Immunology. 1987;61:463–467. [PMC free article] [PubMed] [Google Scholar]
- 11.Reilly CM, Gilkeson GS. Use of genetic knockouts to modulate disease expression in a murine model of lupus, MRL/lpr mice. Immunol Res. 2002;25:143–153. doi: 10.1385/ir:25:2:143. [DOI] [PubMed] [Google Scholar]
- 12.Bobé P, Bonardelle D, Benihoud K, Opolon P, Chelbi-Alix MK. Arsenic trioxide: A promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice. Blood. 2006;108:3967–3975. doi: 10.1182/blood-2006-04-020610. [DOI] [PubMed] [Google Scholar]
- 13.Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, et al. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 14.Izui S, Kelley VE, Masuda K, Yoshida H, Roths JB, Murphy ED. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1985;133:227–233. [PubMed] [Google Scholar]
- 15.Khan MF, Kaphalia BS, Prabhakar BS, Kanz M, Ansari GAS. Trichloroethene-induced autoimmune response in female MRL+/+mice. Toxicol Appl Pharmacol. 1995;134:155–160. doi: 10.1006/taap.1995.1179. [DOI] [PubMed] [Google Scholar]
- 16.Khan MF, Wu X, Ansari GAS. Anti-malondialdehyde antibodies in MRL+/+mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity. Toxicol Appl Pharmacol. 2001;170:88–92. doi: 10.1006/taap.2000.9086. [DOI] [PubMed] [Google Scholar]
- 17.Hadjigogos K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003;45:7–13. [PubMed] [Google Scholar]
- 18.Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci. 2003;73:1655–1666. doi: 10.1016/s0024-3205(03)00475-2. [DOI] [PubMed] [Google Scholar]
- 19.Frostegard J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, et al. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005;52:192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, König R, Ansari GAS, Khan MF. Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4 T cells. Free Radic Biol Med. 2008;44:1475–1482. doi: 10.1016/j.freeradbiomed.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan PE, Sturgess AD, Davies MJ. Evidence for chronically elevated serum protein oxidation in systemic lupus erythematosus patients. Free Radic Res. 2009;43:117–127. doi: 10.1080/10715760802623896. [DOI] [PubMed] [Google Scholar]
- 22.Shah D, Kiran R, Wanchu A, Bhatnagar A. Oxidative stress in systemic lupus erythematosus: relationship to Th1 cytokine and disease activity. Immunol Lett. 2010;129:7–12. doi: 10.1016/j.imlet.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Shah D, Wanchu A, Bhatnagar A. Interaction between oxidative stress and chemokines: possible pathogenic role in systemic lupus erythematosus and rheumatoid arthritis. Immunobiology. 2011;216:1010–1017. doi: 10.1016/j.imbio.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, et al. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic Biol Med. 1997;23:357–360. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 25.Ben Mansour R, Lassoued S, Elgaied A, Haddouk S, Marzouk S, Bahloul Z, et al. Enhanced reactivity to malondialdehyde-modified proteins by systemic lupus erythematosus autoantibodies. Scand J Rheumatol. 2010;39:247–253. doi: 10.3109/03009740903362511. [DOI] [PubMed] [Google Scholar]
- 26.Kurien BT, Porter A, Dorri Y, Iqbal S, D’Souza A, Singh A, et al. Degree of modification of Ro60 by the lipid peroxidation byproduct 4-hydroxy-2-nonenal may differentially induce Sjögren syndrome or systemic lupus erythematosus in BALB/c mice. Free Radic Biol Med. 2011;50:1222–1233. doi: 10.1016/j.freeradbiomed.2010.10.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan MF, Wu X, Kaphalia BS, Boor PJ, Ansari GAS. Acute hematopoietic toxicity of aniline in rats. Toxicol Lett. 1997;92:31–37. doi: 10.1016/s0378-4274(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 28.Khan MF, Wu X, Boor PJ, Ansari GAS. Oxidative modification of lipids and proteins in aniline-induced splenic toxicity. Toxicol Sci. 1999;48:134–140. doi: 10.1093/toxsci/48.1.134. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Hu B, Meng Y, Zhang C, Li K, Hui C. The level of malondialdehyde-modified LDL and LDL immune complexes in patients with rheumatoid arthritis. Clin Biochem. 2009;42:1352–1357. doi: 10.1016/j.clinbiochem.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Reed TT, Pierce WM, Markesbery WR, Butterfield DA. Proteomic identification of HNE-bound proteins in early Alzheimer disease: Insights into the role of lipid peroxidation in the progression of AD. Brain Res. 2009;1274:66–76. doi: 10.1016/j.brainres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 31.D‘souza A, Kurien BT, Rodgers R, Shenoi J, Kurono S, Matsumoto H, et al. Detection of catalase as a major protein target of the lipid peroxidation product 4-HNE and the lack of its genetic association as a risk factor in SLE. BMC Med Genet. 2008;9:62–69. doi: 10.1186/1471-2350-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, Cai P, Ansari GA, Khan MF. Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response. Toxicology. 2007;229:186–193. doi: 10.1016/j.tox.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G, Ansari GA, Khan MF. Involvement of lipid peroxidation-derived aldehyde-protein adducts in autoimmunity mediated by trichloroethene. J Toxicol Environ Health, Part A. 2007;70:1977–1985. doi: 10.1080/15287390701550888. [DOI] [PubMed] [Google Scholar]
- 34.Egner W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol. 2000;53:424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 36.Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus. 2004;13:290–297. doi: 10.1191/0961203303lu1015oa. [DOI] [PubMed] [Google Scholar]
- 37.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 38.Uchida K, Szweda LI, Chae HZ, Stadtman ER. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc Natl Acad Sci USA. 1993;90:8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuma DJ. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic Biol Med. 2002;32:303–308. doi: 10.1016/s0891-5849(01)00742-0. [DOI] [PubMed] [Google Scholar]
- 40.Toyoda K, Nagae R, Akagawa M, Ishino K, Shibata T, Ito S, et al. Protein-bound 4-hydroxy-2-nonenal: an endogenous triggering antigen of anti-DNA response. J Biol Chem. 2007;282:25769–25778. doi: 10.1074/jbc.M703039200. [DOI] [PubMed] [Google Scholar]
- 41.Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuttge DM, Bruzelius M, Stemme S. T-cell recognition of lipid peroxidation products breaks tolerance to self-proteins. Immunology. 1999;98:273–279. doi: 10.1046/j.1365-2567.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scofield RH, Kurien BT, Ganick S, McClain MT, Pye Q, James JA, et al. Modification of lupus-associated 60-kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitope spreading. Free Radic Biol Med. 2005;38:719–728. doi: 10.1016/j.freeradbiomed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Uchida K. Future of toxicology-lipid peroxidation in the future: from biomarker to etiology. Chem Res Toxicol. 2007;20:3–5. doi: 10.1021/tx600304n. [DOI] [PubMed] [Google Scholar]
- 45.Spatz L, Iliev A, Saenko V, Jones L, Irigoyen M, Manheimer-Lory A, et al. Studies on the structure, regulation, and pathogenic potential of anti-dsDNA antibodies. Methods. 1997;11:70–78. doi: 10.1006/meth.1996.0389. [DOI] [PubMed] [Google Scholar]
- 46.Tung KS, DeHoratius RJ, Williams RC. Study of circulating immune complex size in systemic lupus erythematosus. Clin Exp Immunol. 1981;43:615–625. [PMC free article] [PubMed] [Google Scholar]
- 47.Wang G, Wang J, Fan X, Ansari GA, Khan MF. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose-and time-response studies in female MRL+/+mice. Toxicology. 2012;292:113–122. doi: 10.1016/j.tox.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangini AJ, Lafyatis R, Van Seventer JM. Type I interferons inhibition of inflammatory T helper cell responses in systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1108:11–23. doi: 10.1196/annals.1422.002. [DOI] [PubMed] [Google Scholar]
- 49.Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. Th17 cells can provide B cell help in autoantibody induced arthritis. J Autoimmun. 2011;36:65–75. doi: 10.1016/j.jaut.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


