Abstract
We report on a simple approach for efficient NMR proton hyperpolarization of propane using parahydrogen-induced polarization (PHIP) technique, which yielded ~6.2% proton polarization using ~80% parahydrogen, a record level achieved with any hyperpolarization technique for propane. Unlike in previously developed approaches designed for continuous flow operation, where reactants (propene and parahydrogen) are simultaneously loaded for homogeneous or heterogeneous pairwise addition of parahydrogen, here a batch-mode method was applied: propene was first loaded into the catalyst-containing solution, which was followed by homogeneous hydrogenation via parahydrogen bubbling delivered at ~7.1 atm. The achieved nuclear spin polarization of this contrast agent potentially useful for pulmonary imaging was approximately 2 orders of magnitude greater than that achieved in the continuous-flow homogeneous catalytic hydrogenation, and a factor of 3–10 more efficient compared to the typical results of heterogeneous continuous-flow hydrogenations.
Keywords: hyperpolarization, parahydrogen-induced polarization, propane, hydrogenation, NMR
Graphical Abstract
Hyperpolarization of nuclear spins enables enhancement of nuclear magnetic resonance (NMR) signal by several orders of magnitude.[1] Therefore, NMR hyperpolarization is an efficient approach for overcoming the sensitivity challenges of NMR spectroscopy and magnetic resonance imaging (MRI). Production of hyperpolarized (HP) contrast agents for clinical and industrial applications is a rapidly developing field. The most widespread hyperpolarization techniques in the context of biomedical applications are dissolution dynamic nuclear polarization (d-DNP),[1b, 2] spin-exchange optical pumping (SEOP)[3] and parahydrogen-induced polarization (PHIP).[4] D-DNP is well established for production of HP solutions of metabolites (e.g. pyruvate), which can be used as contrast agents for molecular imaging of cancer[5] and other pathologies.[6] HP noble gases (e.g. 129Xe or 3He) obtained by SEOP can be employed for functional MRI of lungs[7] and other applications.[8] However, both DNP and SEOP techniques have significant drawbacks: ~ 1 h long polarization cycles, expensive and sophisticated hardware. Moreover, MRI detection of heteronuclei (i.e., other than proton) is not common for standard clinical MRI facilities. On the other hand, PHIP technique for production of proton-hyperpolarized gases obviates all of the above shortcomings, because it is very simple and instrumentationally non-demanding. In PHIP, singlet spin order of parahydrogen (p-H2) is used to create non-equilibrium spin states[4a] via pairwise addition of two atoms from the same p-H2 molecule to some asymmetric unsaturated substrate (Figure 1a). Once the symmetry of p-H2 molecule is broken as the nascent parahydrogen protons become magnetically non-equivalent in the reaction product, a non-equilibrium nuclear spin polarization is produced, which can be conveniently detected using conventional proton detection universally available on nearly all NMR spectrometers and MRI scanners.
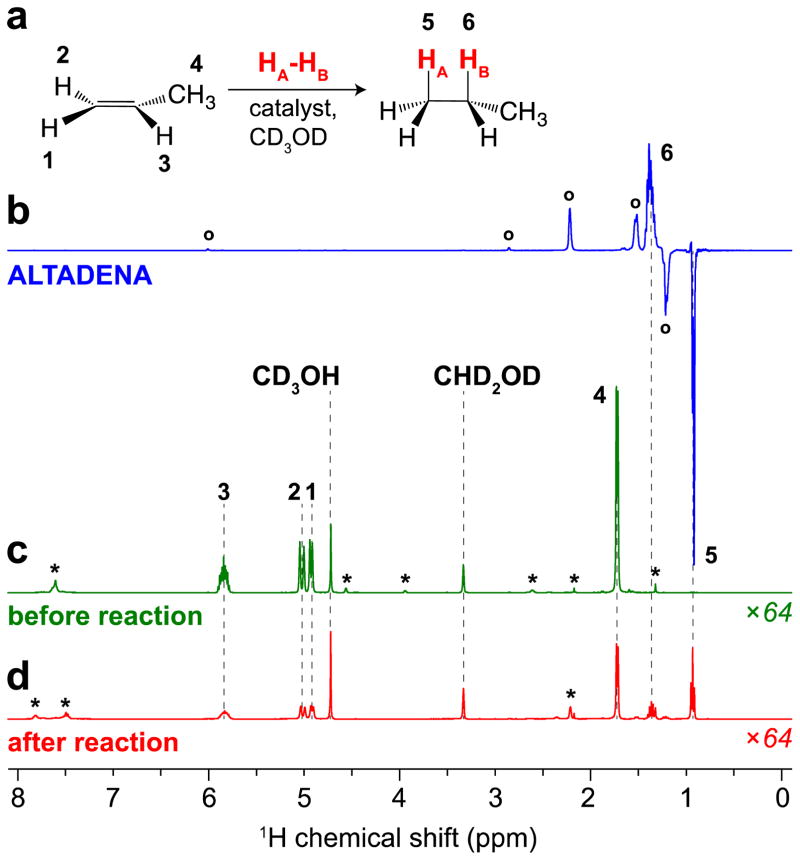
Figure 1.
(a) Scheme of p-H2 pairwise addition to propene. (b) ALTADENA[19] 1H NMR spectrum acquired after PHIP of propene with p-H2 with separate loading of reactants into the catalyst solution in CD3OD (duration of p-H2 bubbling is 8 s) corresponding to the batch-mode condition (corresponding PASADENA spectrum obtained using 50% p-H2 is provided in Figure S4). (c) 1H NMR spectrum of thermally-polarized solution with loaded propene before p-H2 bubbling. (d) 1H NMR spectrum of fully relaxed (i.e. thermally-polarized condition) reaction mixture obtained after the experiment (b). Note that spectra (c) and (d) are scaled by a factor of 64. SE = 1910 for CH2 group of propane, corresponding to %PH = 6.2 % (80% p-H2). Resonances labeled * correspond to the initial [Rh(NBD)(dppb)]BF4 complex and its reduced form. Note the additional HP resonances labeled with ° correspond to HP norbornene and norbornane due to PHIP process of these catalyst-derived compounds.[20]
Despite the initial PHIP phenomenon discovery in the 1980s,[4a] it was deemed to be relatively impractical to design molecular contrast agents with hyperpolarization pool stored on protons, because of their relatively short T1 resulting in rapid (few seconds or less) depolarization. Indeed, much of the later biomedical efforts were focused on polarization transfer from nascent parahydrogen protons to significantly slower relaxing (a minute or more) 13C sites for in vivo angiography[9] and molecular imaging[10] applications. However, the discovery of the long-lived spin states[11] (LLSS) provided a glimpse of hope that the lifetime of proton HP for contrast agents produced by PHIP could be extended. Indeed, LLSS of HP protons in gaseous propane were recently demonstrated with TLLSS reaching 5–6 s,[12] which is sufficiently long for potential biomedical use as inhalable contrast agents for functional pulmonary imaging.
HP propane is a non-toxic gas[13] and is a promising alternative to HP 129Xe, because its PHIP production is relatively inexpensive, its NMR/MRI detection does not require specialized 129Xe radiofrequency hardware and software, and it can enable 3D MRI with superb spatial (~0.5×0.5×0.5 mm3 voxel size) resolution even at nuclear spin polarization (%PH) of ~1%.[12] Increasing the %PH of propane and other HP gases is certainly required, and it is an area of extensive experimental efforts.[14]
Pairwise p-H2 addition can be achieved by either homogeneous[9] or heterogeneous (HET)[15] catalysis. The important advantage of HET-PHIP is the ability to produce pure HP gases, e.g. propane.[12a, 14a, 14c] However, the level of proton polarization of HP propane produced by HET-PHIP is relatively low at %PH~1%.[14a] An alternative approach is the biphasic gas-liquid hydrogenation of propene by bubbling its mixture with p-H2 through a catalyst solution.[16] This approach also allows producing HP propane in the gas phase in a continuous flow regime, however %PH achieved to date were relatively low, i.e. < 1%. In principle, near 100% pairwise addition of p-H2 is theoretically possible,[4a, 4b] and because of our long-term goal of using HP propane as HP inhalable contrast agent, the motivation for this work is to improve %PH of HP propane via PHIP. Herein, we explore PHIP of propane using a previously established batch-mode approach for production of injectable contrast agents,[9, 17] where catalyst and to-be-hyperpolarized substrate are loaded in the liquid phase first, which is followed by pairwise addition of p-H2 gas and produces a batch of HP contrast agent.
In this study, we utilized the [Rh(L)(dppb)]BF4 complex most widely used in PHIP experiments as a catalyst for p-H2 pairwise addition (L = NBD (norbornadiene) or = COD (cyclooctadiene), dppb = 1,4-bis(diphenylphosphino)butane). First, the simultaneous loading of propene and p-H2 into the catalyst solution in a continuous flow regime was tested, similarly to the experimental protocol used previously.[16] However, here an elevated p-H2 pressure (~7 atm vs. 1 atm) was employed (Figure 2), because it increases p-H2 concentration in the liquid phase[18] and consequently increases the rate of hydrogenation.[17b] NMR detection of HP propane was performed in the liquid phase of methanol-d4. HP NMR resonances of propane were observed under both ALTADENA[19] and PASADENA[4b] conditions with good reproducibility (> 10 experiments were repeated on the same catalyst solution portion, Figures S2 and S3). However, the NMR signal enhancements (SE) of HP propane’s methyl and methylene resonances were low (only ca. 3–10 fold, corresponding to %PH of 0.01–0.03% using ~50% p-H2).
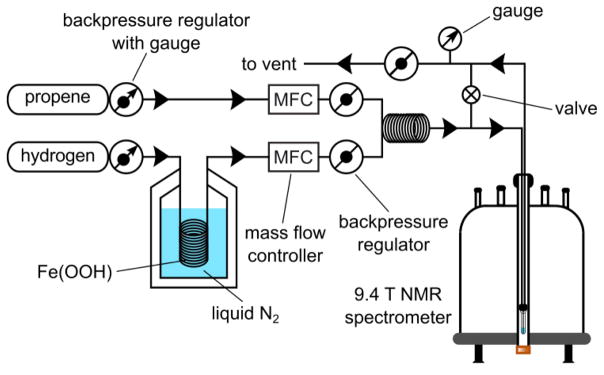
Figure 2.
The diagram of the experimental setup. In continuous-flow experiment, both gas flows are operating and enable continuous simultaneous loading of propene and p-H2 through the catalyst solution placed in the NMR tube. Normal ultra-high purity (>99.999%) hydrogen gas passes through Fe(OOH) catalyst at liquid N2 temperature (~77K) or utilizes a p-H2 generator using cryo-cooling (and producing ~80% p-H2). Both gas flows are controlled by the mass flow controllers (MFC), and the system pressure is regulated by the safety valve (labeled as ø set to ~7.1 atm) immediately before the vent. Manual valve (⊗) enables fast (in less than 1 s) cessation of gas flow through solution placed in the NMR tube. In batch-mode production method, propene gas is loaded in the solution first, and it is followed by hydrogen gas flow only. Hydrogenation is performed inside 9.4 T spectrometer under PASADENA condition[19] and in the Earth’s magnetic field under ALTADENA[4b] condition respectively.
In the batch-mode approach, propene is loaded first (by saturating the catalyst solution by propene bubbling at 1 atm pressure) followed by pressurizing the sample and bubbling with p-H2 through the solution at ~7.1 atm. This experimental protocol is ~100 times more efficient than the continuous-flow method described above, and yielded signal enhancements and polarizations as high as SE ~ 1910 and %PH ~ 6.2% under ALTADENA conditions (Figure 2) using ~80% p-H2 without taking into account possible polarization losses due to relaxation processes. Correspondingly, when ~50% p-H2 was used (Figure S8), SE and %PH were decreased to 840 and 2.7% respectively.
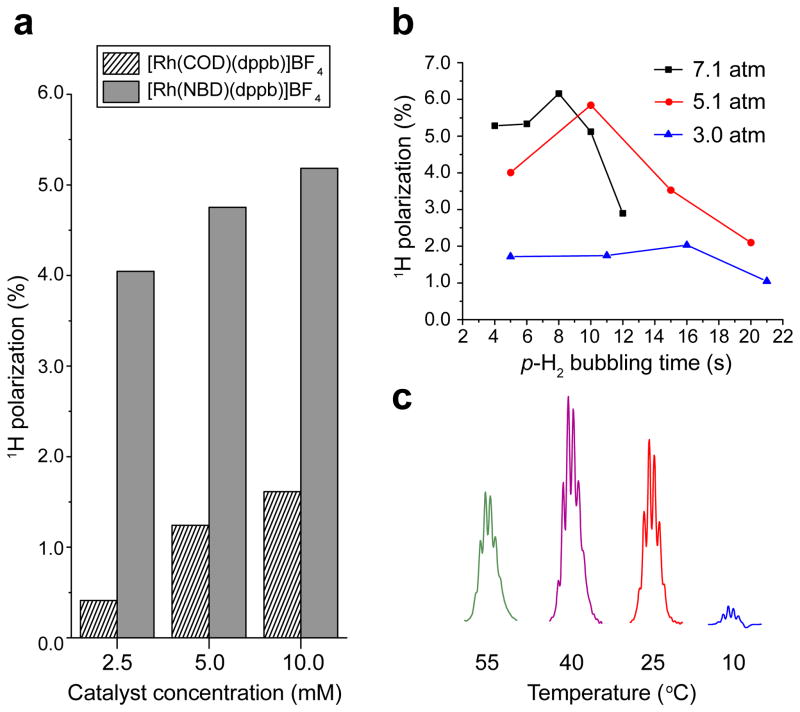
The comparison of two catalysts at several concentrations shown in Figure 3a clearly indicates that the catalyst performance with NBD ligand is significantly better than that with COD ligand. Additionally, in both cases, catalyst concentration also impacts the apparent %PH. Moreover, Figure 3b shows a definite advantage of using high p-H2 pressure for improving apparent %PH for HP propane in liquid phase hydrogenation. Furthermore, additional experiments performed at various hydrogenation temperatures exhibit a temperature dependence of %PH as well (Figure 3c).
Figure 3.
(a) Proton polarization of HP propane as a function of catalyst concentration for two representative Rh-based catalysts; duration of p-H2 bubbling is 10 s. (b) Proton polarization of HP propane as a function of p-H2 bubbling time in three different pressure regimes. (c) Optimization of propane hyperpolarization by monitoring hyperpolarized 1H signal (of -CH2- group) by varying the temperature (estimated values) of the sample (p-H2 bubbling duration is 8 s). Experimental data shown in (b) and (c) is collected for [Rh(NBD)dppb]BF4 catalyst.
The potential possible explanation of such a dramatic difference in catalyst’s performance between continuous-flow and batch mode hyperpolarization procedures is partially based on the differences in concentrations of reactants in the solution, which is in line with previously published studies. These studies reported high level of hyperpolarization achieved via batch-mode loading of substrate following by reaction with p-H2: i.e. utilizing 2-hydroxyethyl acrylate,[9] fumarate,[21] phosphoenolpyruvate,[22] and more recently vinyl acetate[17b, 23] with similar or identical catalyst, where high levels of hyperpolarization were detected for 1H[17b] or 13C[23b] nuclei (the latter is an indirect confirmation that proton polarization was high prior to polarization transfer from nascent parahydrogen protons[17b] to 13C[17b, 23b]) In case of separate loading of reactants using batch-mode production, the initial concentration of loaded propene is 330 ± 45 mM (according to reference signals of catalyst’s thermally polarized protons). On the other hand, H2 solubility in methanol is only ~28 mM at 7.1 atm.[24] Moreover, simultaneous loading of propene and excess p-H2 in the continuous flow mode also likely results in the irreversible catalyst degradation of some fraction of Rh catalyst. This is indirectly confirmed by the fact that once the propene substrate is depleted in the batch-mode procedure, and the catalyst solution is further bubbled with p-H2, the subsequent attempts to re-load the propene substrate to repeat the hyperpolarization cycle were unsuccessful (i.e. > 1 order of magnitude lower polarization signals). Furthermore, continuous-flow mode experiments utilized somewhat lower p-H2 partial pressure likely resulting in a slower production of hyperpolarized product.
The reported here %PH value of ~6.2% was obtained with ~80 % p-H2, so that utilization of 100% p-H2 would increase proton polarization to ~9% for hyperpolarized propane.[25] This value is substantially higher than typical values reported for propane or any other hydrocarbon gas hyperpolarized by PHIP so far. For PHIP, the highest reported %PH value was ~1% for HP propane.[14a, 15b] To date, no other HP techniques have reported hyperpolarization on hydrocarbon gases. We note that although the nascent proton polarization in PHIP can exceed 50% for some injectable contrast agents,[17a, 26] (i) the direct proton detection is usually not performed in situ of production inside a hyperpolarizer, and (ii) proton polarization is too short-lived to be useful for injectable contrast agents. While d-DNP can hyperpolarize proton sites in principle,[27] no reports have been shown that d-DNP can efficiently hyperpolarize any gas besides 129Xe[28] and 15N2O.[29]
The presented polarization values are likely somewhat underestimated due to residual hydrogenation of substrate during the delay between the acquisitions of the ALTADENA spectrum and the spectrum of fully relaxed solution. This delay (of >2 min) is mandatory, because HP must return back to the equilibrium state for probing propane concentration in the solution; also note that the alternative efforts of using normal H2 at room temperature yielded small (yet detectable) HP signatures of propane, and therefore are unsuitable for quantification. The T1 relaxation time constants of propane HP states induced via ALTADENA condition in solution are 22.4±0.5 s for CH2 group and 16.1±0.3 s for CH3 group respectively, which is in qualitative agreement with T1 measurements of dissolved thermally-polarized propane using inversion recovery technique (23.3±0.3 s and 19.6±0.3 s respectively). These values are significantly greater than the corresponding relaxation decay constants of HP propane in the gas phase.[12a, 30] Moreover, the decay constants could be even greater at low magnetic fields due to LLSS presence.[12a] Therefore, the production of HP propane in the liquid (vs. gas) phase using the presented batch-mode approach may be advantageous, because the decay of the HP state can potentially minimize polarization losses, and hydrogenation process can continue significantly longer without significant polarization decay losses.
Future studies are certainly warranted to optimize the HP propane production by the batch-mode approach, including catalyst improvement to yield greater degree of the pairwise addition of p-H2 and greater % conversion (up to ~100% from the 40–80% conversion levels achieved here, Figure S6), further optimization of p-H2 pressure and reaction temperature, and others. Moreover, HP propane separation from the liquid phase and filtration from residual propene, H2 and norbornene/norbornane certainly have to be addressed in the context of potential biomedical use of HP propane gas, which was not pursued in the feasibility study described here.
Experimental Section
All experimental procedures, additional NMR spectra are provided in Supporting Information (SI) for this communication.
Supplementary Material
Acknowledgments
OGS, KVK and IVK acknowledge the grant from the Russian Science Foundation (14-35-00020) for the support of homogeneous hydrogenation experiments, and FASO Russia project # 0333-2014-0001 for basic funding. DAB, AMC and EYC thank NIH 1R21EB018014, T32 EB001628, 1R21EB020323, U01 CA202229 and 1F32EB021840, NSF CHE-1416268 and CHE-1416432, DOD CDMRP W81XWH-12-1-0159/BC112431, W81XWH-15-1-0271 and W81XWH-15-1-0272, and ExxonMobil Research and Engineering Company Knowledge Build.
Footnotes
Supporting information for this article is given via a link at the end of the document.((Please delete this text if not appropriate))
References
- 1.a) Nikolaou P, Goodson BM, Chekmenev EY. Chem Eur J. 2015;21:3156–3166. doi: 10.1002/chem.201405253. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Ardenkjaer-Larsen JH. J Magn Reson. 2016;264:3–12. doi: 10.1016/j.jmr.2016.01.015. [DOI] [PubMed] [Google Scholar]; b) Comment A. J Magn Reson. 2016;264:39–48. doi: 10.1016/j.jmr.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Bouchiat MA, Carver TR, Varnum CM. Phys Rev Lett. 1960;5:373–375. [Google Scholar]
- 4.a) Bowers CR, Weitekamp DP. Phys Rev Lett. 1986;57:2645–2648. doi: 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]; b) Bowers CR, Weitekamp DP. J Am Chem Soc. 1987;109:5541–5542. [Google Scholar]; c) Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL. J Am Chem Soc. 1987;109:8089–8091. [Google Scholar]
- 5.a) Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Sci Transl Med. 2013;5:198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Brindle KM. J Am Chem Soc. 2015;137:6418–6427. doi: 10.1021/jacs.5b03300. [DOI] [PubMed] [Google Scholar]; b) Comment A, Merritt ME. Biochemistry. 2014;53:7333–7357. doi: 10.1021/bi501225t. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Koptyug IV. Mendeleev Commun. 2013;23:299–312. [Google Scholar]
- 7.Mugler JP, Altes TA. J Magn Reson Imaging. 2013;37:313–331. doi: 10.1002/jmri.23844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodson BM. J Magn Reson. 2002;155:157–216. doi: 10.1006/jmre.2001.2341. [DOI] [PubMed] [Google Scholar]
- 9.Golman K, Axelsson O, Johannesson H, Mansson S, Olofsson C, Petersson JS. Magn Reson Med. 2001;46:1–5. doi: 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharya P, Chekmenev EY, Reynolds WF, Wagner S, Zacharias N, Chan HR, Bünger R, Ross BD. NMR Biomed. 2011;24:1023–1028. doi: 10.1002/nbm.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Carravetta M, Levitt MH. J Am Chem Soc. 2004;126:6228–6229. doi: 10.1021/ja0490931. [DOI] [PubMed] [Google Scholar]; b) Warren WS, Jenista E, Branca RT, Chen X. Science. 2009;323:1711–1714. doi: 10.1126/science.1167693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Kovtunov KV, Truong ML, Barskiy DA, Koptyug IV, Waddell KW, Chekmenev EY. Chem Eur J. 2014;20:14629–14632. doi: 10.1002/chem.201405063. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kovtunov KV, Truong ML, Barskiy DA, Salnikov OG, Bukhtiyarov VI, Coffey AM, Waddell KW, Koptyug IV, Chekmenev EY. J Phys Chem C. 2014;118:28234–28243. doi: 10.1021/jp508719n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart RD, Newton PE, Baretta ED, Herrmann AA, Forster HV, Soto RJ. Environ Health Perspect. 1978;26:275–285. doi: 10.1289/ehp.7826275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Kovtunov KV, Barskiy DA, Coffey AM, Truong ML, Salnikov OG, Khudorozhkov AK, Inozemceva EA, Prosvirin IP, Bukhtiyarov VI, Waddell KW, Chekmenev EY, Koptyug IV. Chem Eur J. 2014;20:11636–11639. doi: 10.1002/chem.201403604. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sharma R, Bouchard LS. Sci Rep. 2012;2:5. doi: 10.1038/srep00277. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhou R, Zhao EW, Cheng W, Neal LM, Zheng H, Quiñones RE, Hagelin-Weaver HE, Bowers CR. J Am Chem Soc. 2015;137:1938–1946. doi: 10.1021/ja511476n. [DOI] [PubMed] [Google Scholar]; d) Zhao EW, Zheng H, Ludden K, Xin Y, Hagelin-Weaver HE, Bowers CR. ACS Catalysis. 2016;6:974–978. [Google Scholar]
- 15.a) Kovtunov KV, Zhivonitko VV, Skovpin IV, Barskiy DA, Koptyug IV. Top Curr Chem. 2013;338:123–180. doi: 10.1007/128_2012_371. [DOI] [PubMed] [Google Scholar]; b) Kovtunov KV, Beck IE, Bukhtiyarov VI, Koptyug IV. Angew Chem Int Ed. 2008;47:1492–1495. doi: 10.1002/anie.200704881. [DOI] [PubMed] [Google Scholar]; c) Koptyug IV, Kovtunov KV, Burt SR, Anwar MS, Hilty C, Han SI, Pines A, Sagdeev RZ. J Am Chem Soc. 2007;129:5580–5586. doi: 10.1021/ja068653o. [DOI] [PubMed] [Google Scholar]
- 16.Kovtunov KV, Zhivonitko VV, Skovpin IV, Barskiy DA, Salnikov OG, Koptyug IV. J Phys Chem C. 2013;117:22887–22893. [Google Scholar]
- 17.a) Goldman M, Johannesson H, Axelsson O, Karlsson M. C R Chimie. 2006;9:357–363. [Google Scholar]; b) Shchepin RV, Barskiy DA, Coffey AM, Manzanera Esteve IV, Chekmenev EY. Angew Chem Int Ed. 2016;55:6071–6074. doi: 10.1002/anie.201600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truong ML, Shi F, He P, Yuan B, Plunkett KN, Coffey AM, Shchepin RV, Barskiy DA, Kovtunov KV, Koptyug IV, Waddell KW, Goodson BM, Chekmenev EY. J Phys Chem B. 2014;18:13882–13889. doi: 10.1021/jp510825b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pravica MG, Weitekamp DP. Chem Phys Lett. 1988;145:255–258. [Google Scholar]
- 20.Kovtunov KV, Barskiy D, Shchepin RV, Coffey AM, Waddell KW, Koptyug IV, Chekmenev EY. Anal Chem. 2014;86:6192–6196. doi: 10.1021/ac5013859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya P, Chekmenev EY, Perman WH, Harris KC, Lin AP, Norton VA, Tan CT, Ross BD, Weitekamp DP. J Magn Reson. 2007;186:150–155. doi: 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY. J Am Chem Soc. 2012;134:3957–3960. doi: 10.1021/ja210639c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.a) Cavallari E, Carrera C, Boi T, Aime S, Reineri F. J Phys Chem B. 2015;119:10035–10041. doi: 10.1021/acs.jpcb.5b06222. [DOI] [PubMed] [Google Scholar]; b) Reineri F, Boi T, Aime S. Nat Commun. 2015;6:5858. doi: 10.1038/ncomms6858. [DOI] [PubMed] [Google Scholar]
- 24.Liu QS, Takemura F, Yabe A. J Chem Eng Data. 1996;41:1141–1143. [Google Scholar]
- 25.Bowers CR. In: Encycl Magn Reson. Grant DM, Harris RK, editors. John Wiley & Sons, Ltd; Chichester: 2002. pp. 750–770. [Google Scholar]
- 26.a) Goldman M, Johannesson H. C R Physique. 2005;6:575–581. [Google Scholar]; b) Chekmenev EY, Hovener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP. J Am Chem Soc. 2008;130:4212–4213. doi: 10.1021/ja7101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bornet A, Melzi R, Perez Linde AJ, Hautle P, van den Brandt B, Jannin S, Bodenhausen G. J Phys Chem Lett. 2013;4:111–114. doi: 10.1021/jz301781t. [DOI] [PubMed] [Google Scholar]
- 28.Pourfathi M, Kuzma NN, Kara H, Ghosh RK, Shaghaghi H, Kadlecek SJ, Rizi RR. J Magn Reson. 2013;235:71–76. doi: 10.1016/j.jmr.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuzma NN, Hakansson P, Pourfathi M, Ghosh RK, Kara H, Kadlecek SJ, Pileio G, Levitt MH, Rizi RR. J Magn Reson. 2013;234:90–94. doi: 10.1016/j.jmr.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barskiy DA, Salnikov OG, Kovtunov KV, Koptyug IV. J Phys Chem A. 2015;119:996–1006. doi: 10.1021/jp510572d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.