Abstract
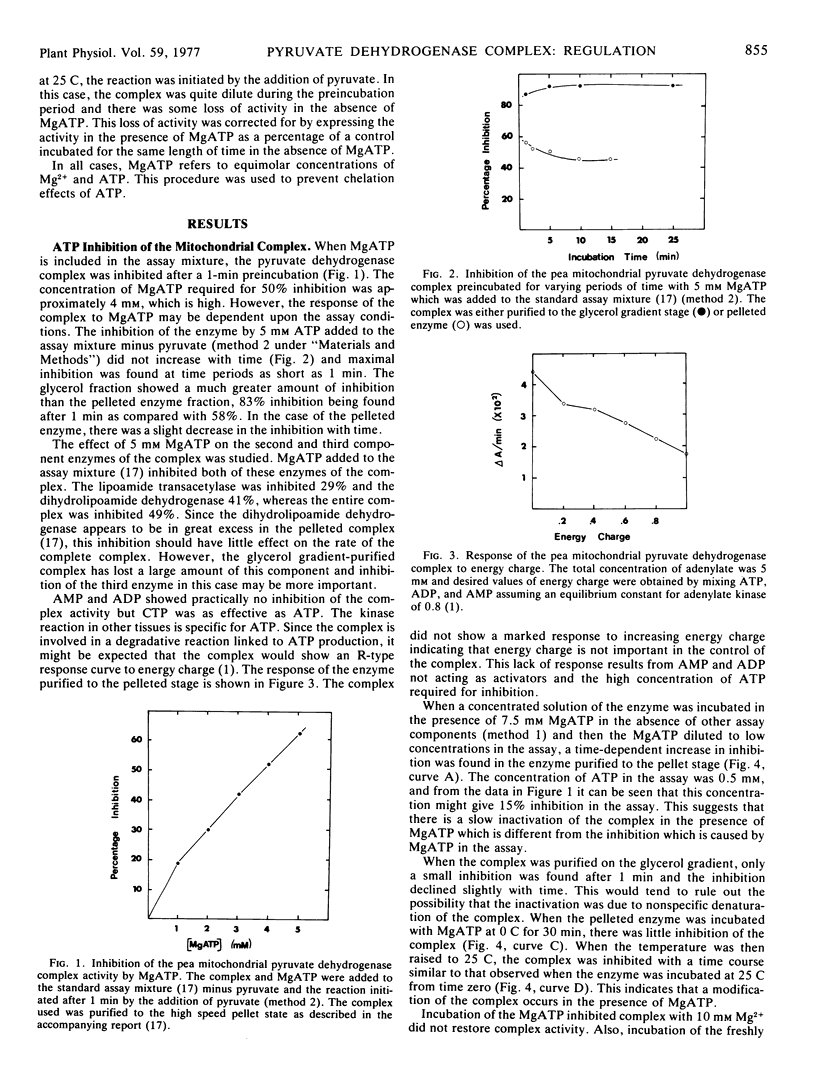
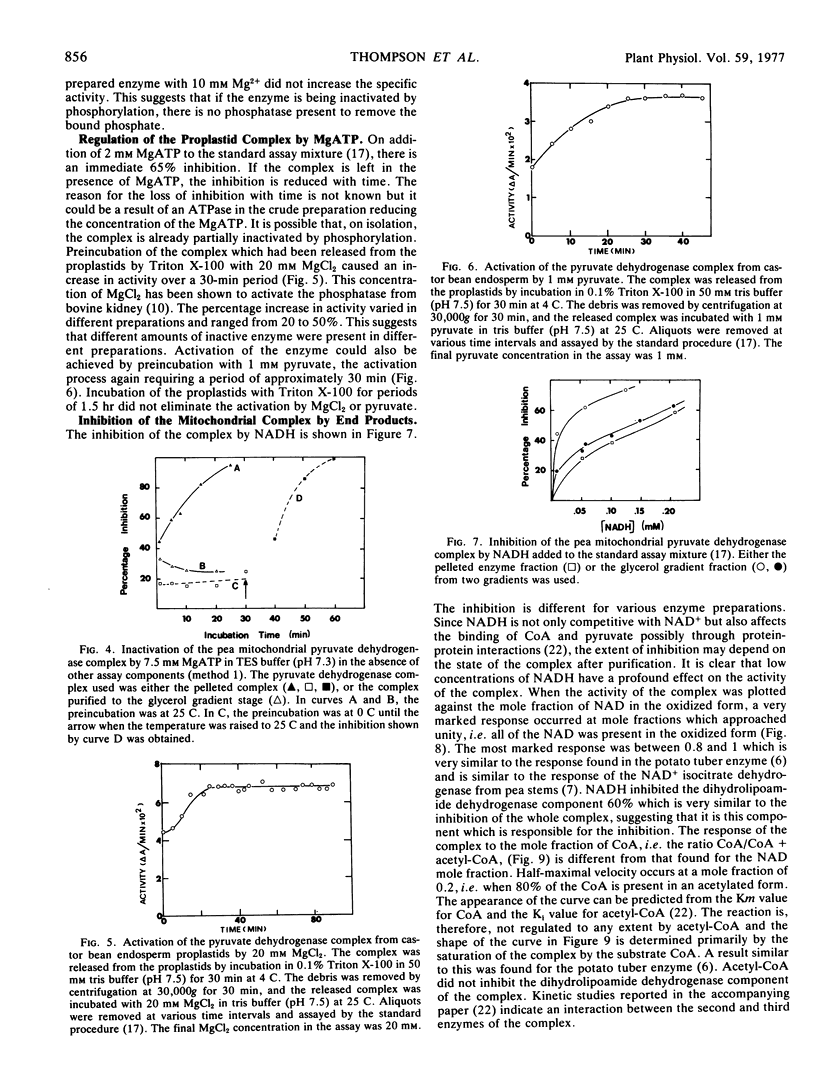
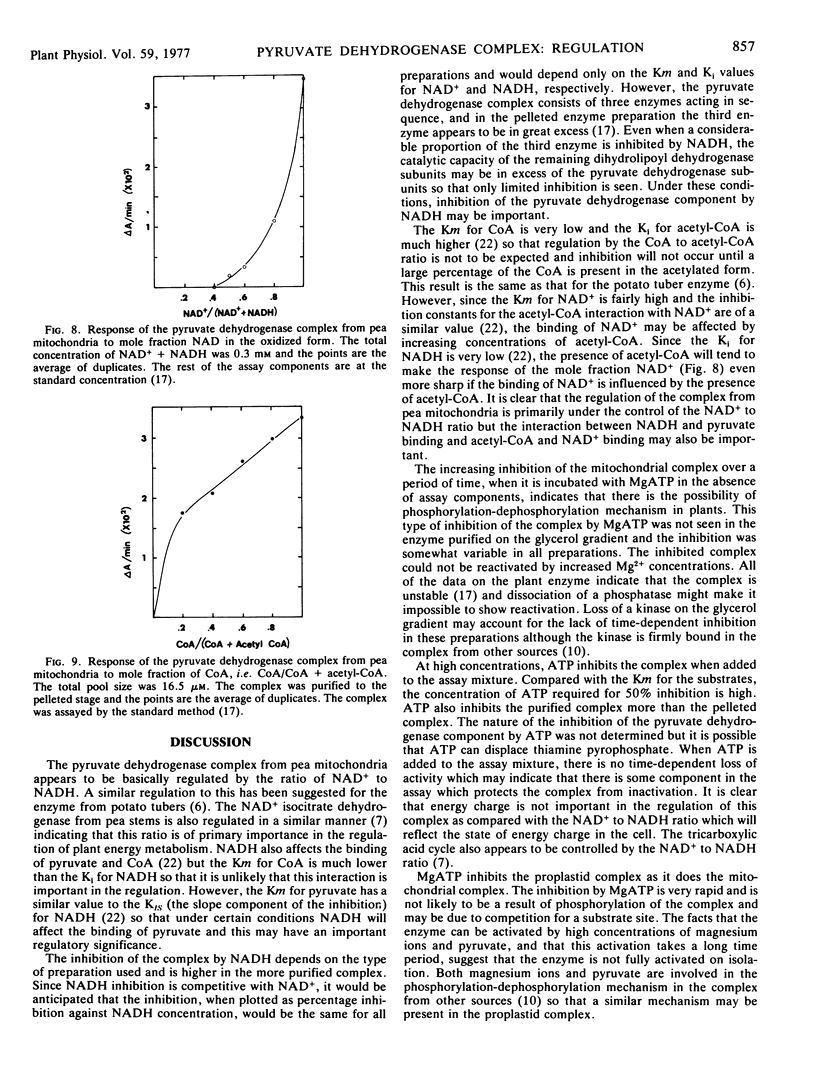
The activity of the pyruvate dehydrogenase complex from pea (Pisum sativum L.) mitochondria is inhibited when MgATP is added to the reaction mixture; 50% inhibition occurs at 4 mm ATP. The inhibition does not increase with time and is higher in the more highly purified preparations. Crude preparations of the complex show a time-dependent inactivation when incubated with 7.5 mm MgATP alone but this is not found with the more highly purified complex. This inactivation does not occur at 0 C. The complex could not be reactivated by high concentrations of Mg2+. It is suggested that a phosphorylation-dephosphorylation mechanism may occur in plants, but the phosphatase and kinase are not tightly bound to the complex and are lost on isolation. The complex does not respond in a significant manner to energy charge. The NAD+ to NADH ratio is the principal means of regulation of the complex, NADH being competitive with NAD+ for the dihydrolipoamide component. The CoA to acetyl-CoA ratio is not important in regulation.
The castor bean (Ricinus communis L.) proplastid complex is inhibited by the addition of 2 mm MgATP to the assay mixture. The inhibition is immediate, suggesting that phosphorylation of the enzyme is not involved or must be very rapid. Incubation of the complex with 20 mm MgCl2 causes an activation of the complex. Maximum activity is not expressed in this case for 30 minutes. A similar activation can be achieved by preincubating the complex with 1 mm pyruvate. These data suggest that the complex is not fully activated on isolation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E., Roach P. J., Schwedes J. S. Metabolite concentrations and concentration ratios in metabolic regulation. Adv Enzyme Regul. 1975;13:393–411. doi: 10.1016/0065-2571(75)90027-8. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Bremer J. Pyruvate dehydrogenase, substrate specificity and product inhibition. Eur J Biochem. 1969 Apr;8(4):535–540. doi: 10.1111/j.1432-1033.1969.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Bresters T. W., de Kok A., Veeger C. The pyruvate-dehydrogenase complex from Azotobacter vinelandii. 2. Regulation of the activity. Eur J Biochem. 1975 Nov 15;59(2):347–353. doi: 10.1111/j.1432-1033.1975.tb02461.x. [DOI] [PubMed] [Google Scholar]
- Crompton M., Laties G. G. The regulatory function of potato pyruvate dehydrogenase. Arch Biochem Biophys. 1971 Mar;143(1):143–150. doi: 10.1016/0003-9861(71)90194-9. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. Regulation of the nicotinamide adenine dinucleotide-specific isocitrate dehydrogenase from a higher plant. The effect of reduced nicotinamide adenine dinucleotide and mixtures of citrate and isocitrate. J Biol Chem. 1970 Aug 10;245(15):3751–3754. [PubMed] [Google Scholar]
- Guder W. G., Wieland O. H. Metabolism of isolated kidney tubules. Regulation of pyruvate dehydrogenase by metabolic substrates. Eur J Biochem. 1974 Mar 1;42(2):529–538. doi: 10.1111/j.1432-1033.1974.tb03368.x. [DOI] [PubMed] [Google Scholar]
- Hansen H. G., Henning U. Regulation of pyruvate dehydrogenase activity in Escherichia coli K12. Biochim Biophys Acta. 1966 Aug 10;122(2):355–358. doi: 10.1016/0926-6593(66)90076-2. [DOI] [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C., Löffler G., Wieland O. H. Interconversion of pyruvate dehydrogenase in the isolated perfused rat liver. Eur J Biochem. 1973 Feb 15;33(1):117–122. doi: 10.1111/j.1432-1033.1973.tb02662.x. [DOI] [PubMed] [Google Scholar]
- Portenhauser R., Wieland O. Regulation of pyruvate dehydrogenase in mitochondria of rat liver. Eur J Biochem. 1972 Dec 4;31(2):308–314. doi: 10.1111/j.1432-1033.1972.tb02534.x. [DOI] [PubMed] [Google Scholar]
- Reid E. E., Lyttle C. R., Canvin D. T., Dennis D. T. Pyruvate dehydrogenase complex activity in proplastids and mitochondria of developing castor bean endosperm. Biochem Biophys Res Commun. 1975 Jan 6;62(1):42–47. doi: 10.1016/s0006-291x(75)80402-5. [DOI] [PubMed] [Google Scholar]
- Reid E. E., Thompson P., Lyttle C. R., Dennis D. T. Pyruvate dehydrogenase complex from higher plant mitochondria and proplastids. Plant Physiol. 1977 May;59(5):842–848. doi: 10.1104/pp.59.5.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. R., Old L. O., Reed L. J. Regulatory properties of pyruvate dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1968 May 10;31(3):495–500. doi: 10.1016/0006-291x(68)90504-4. [DOI] [PubMed] [Google Scholar]
- Schwartz E. R., Reed L. J. Regulation of the activity of the pyruvate dehydrogenase complex of Escherichia coli. Biochemistry. 1970 Mar 17;9(6):1434–1439. doi: 10.1021/bi00808a019. [DOI] [PubMed] [Google Scholar]
- Shen L. C., Atkinson D. E. Regulation of pyruvate dehydrogenase from Escherichia coli. Interactions of adenylate energy charge and other regulatory parameters. J Biol Chem. 1970 Nov 25;245(22):5974–5978. [PubMed] [Google Scholar]
- Siess E., Wittmann J., Wieland O. Interconversion and kinetic properties of pyruvate dehydrogenase from brain. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):447–452. doi: 10.1515/bchm2.1971.352.1.447. [DOI] [PubMed] [Google Scholar]
- Thompson P., Reid E. E., Lyttle C. R., Dennis D. T. Pyruvate dehydrogenase complex from higher plant mitochondria and proplastids: kinetics. Plant Physiol. 1977 May;59(5):849–853. doi: 10.1104/pp.59.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais U., Gillmann U., Ullrich J. Isolation and characterisation of pyruvate dehydrogenase complex from brewer's yeast. Hoppe Seylers Z Physiol Chem. 1973 Oct-Nov;354(10-11):1378–1388. doi: 10.1515/bchm2.1973.354.2.1378. [DOI] [PubMed] [Google Scholar]
- Walajtys E. I., Gottesman D. P., Williamson J. R. Regulation of pyruvate dehydrogenase in rat liver mitochondria by phosphorylation-dephosphorylation. J Biol Chem. 1974 Mar 25;249(6):1857–1865. [PubMed] [Google Scholar]
- Wieland O. H., Hartmann U., Siess E. A. Neurospora crassa pyruvate dehydrogenase: interconversion by phosphorylation and dephosphorylation. FEBS Lett. 1972 Nov 1;27(2):240–244. doi: 10.1016/0014-5793(72)80630-6. [DOI] [PubMed] [Google Scholar]
- Wieland O., Jagow-Westermann B. v. ATP-dependent inactivation of heart muscle pyruvate dehydrogenase and reactivation by Mg(++). FEBS Lett. 1969 Jun;3(4):271–274. doi: 10.1016/0014-5793(69)80156-0. [DOI] [PubMed] [Google Scholar]


