Abstract
Bacterial keratitis is the most common type among all types of infectious keratitis. Currently, antibiotics are the main-stay of treatment. The objective of this systematic review is to review published clinical studies which discuss the adjunctive treatment of bacterial keratitis to guide clinical decision-making. We reviewed the role of a variety of medications and surgeries which can help in managing bacterial keratitis complications, which include as thinning, perforation, and impaired wound healing. We have included appropriate animal and laboratory studies, case reports and case series, and randomized clinical trials regarding each therapy.
Keywords: Adjunctive therapy, bacterial keratitis, corneal ulcer
Introduction
Bacterial keratitis is the most common type of infectious keratitis and accounts for approximately 65%–90% of all microbial keratitis.[1,2] Bacterial keratitis is one of the most serious ocular infections and may lead to devastating complications.[3] Early and prompt medical treatment is needed to avoid complications. The goal of adjuvant therapies is to improve visual and anatomical outcomes. However, limited information exists on the efficacy and safety of these types of therapies, their outcomes, such as vision and epithelial healing, and complications such as corneal melting, perforation, and vascularization.
In this paper, we examine the evidence for bacterial keratitis adjunct therapies, with specific regards to outcomes and complications. Corneal stromal collagen cross-linking and corticosteroid are not included here because they are covered by other articles in this issue.
Methods
PubMed was searched for “adjunct therapy” and “bacterial keratitis,” as well as specific types of treatment. Abstracts and original articles were reviewed.
Povidone-iodine
A 5% povidone-iodine (PVI) is a complex of polyvinylpyrrolidone and triiodide ions that is widely used as a preoperative disinfectant before ophthalmic surgery to reduce the risk of infection due to its broad-spectrum microbicidal effect against bacteria, virus, parasites, and fungi.[4,5] The free iodine part of PVI (0.1%, 1%, and 5%) is a strong oxidizing agent that inhibits cell growth and destabilize membrane integrity of host cells and microorganisms through immediate irreversible inhibition of mitochondrial dehydrogenase and intracellular esterase activity.[6]
Katz et al.[7] performed a randomized clinical trial (RCT) of povidone in 358 patients with microbial keratitis. Patients with corneal ulcers were randomized into two groups, receiving either standard antibiotic therapy alone (173 patients) or standard therapy plus PVI 2.5% every 2 h for 2 weeks (185 patients). Patients were followed for 2–4 months following treatment initiation. Almost two-thirds of patients in both groups had a final corrected visual acuity of 20/20 in the affected eye. Nearly 43.8% of patients in standard antibiotic therapy group improved by four or more lines, whereas only 31.7% of patients in PVI group improved by four or more lines. Regarding final visual acuity, 3.9% in the standard therapy and 6.9% in the PVI group had corrected visual acuity worse than 20/400, whereas 9.4% in the standard therapy and 13.1% in the PVI group had corrected visual acuity worse than 20/60. With regard to corneal scar formation, the difference was not statically significant.
Gregori et al.[8] performed a small RCT on the effect of 5% PVI (Betadine) versus placebo (preservative-free artificial tears) on effectiveness to decrease bacterial counts before starting antimicrobial agents. Eighteen patients were randomized to Betadine group and 17 to the placebo group. Bacterial culture was performed before and after application of one drop of PVI or preservative-free artificial tears. Initially, 44% were culture-positive in PVI group versus 53% of control group (P = 0.16). Seventeen percent of patients who received PVI showed fewer bacterial colonies on culture after the drop, whereas 41% of patients in the placebo group had fewer colonies. The difference was not statistically significant (P = 0.15). Their study showed that a single application of 5% PVI drop did not reduce bacterial load. This poor effect may be due to the lack of deep penetration of PVI into the deep corneal stroma.
These two RCTs suggest that PVI, in two different concentrations, has no significant beneficial effect either on established bacterial keratitis or in reducing bacterial load.
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy requires breathing of pure oxygen (~100%) at a pressure found on the surface of the earth at sea level, which is defined to be one atmosphere absolute through oxygen mask.[9] Currently, hyperbaric oxygenation has been used as an adjunctive treatment for ophthalmic diseases such as central retinal artery occlusion, nonhealing corneal edema, anterior segment ischemia, and rhino-orbital-cerebral mucormycosis; and nonophthalmic diseases related to hypoxic conditions.[10]
Price and Stevens[11] reported hyperbaric oxygen therapy in a patient with rhino-cerebral mucormycosis who had not responded to medical treatment to improve tissue oxygenation and prevent acidosis to achieve a better survival rate in a patient who had refused any surgical options.
To the best of our knowledge, no RCTs are available in the literature that discusses the effect of hyperbaric oxygen therapy in bacterial keratitis. Chong et al.[12] reported a 30-year-old female with culture-proven soft contact lens associated Pseudomonas keratitis who was getting progressively worse despite topical, oral, and intravenous antibiotics. On her 3rd day, hyperbaric oxygen therapy was started for 90 min daily in addition to her antibiotics therapy. Twenty-four hours later, her vision improved from counting fingers to 6/24. Hyperbaric oxygen therapy continued to complete a course of 3 days. Patient discharged with the vision of 6/9.
Further studies into the therapeutic effect of hyperbaric oxygen therapy as adjunctive therapy to antibiotics are needed to prove its clinical efficacy and to determine the safe dose to avoid ocular and systematic complications.
Cyanoacrylate Glue
Cyanoacrylates are esters of cyanoacrylic acid which is used as a tissue adhesive in the closure of impending or frank corneal perforations, to avoid or postpone keratoplasty. It is available in different preparation which include butyl-2-cyanoacrylate (Indermil; Sherwood, Davis and Geck, St. Louis, MO, USA); butyl-2-cyanoacrylate (Histoacryl; BBraun, Melsungen, Germany); N-butyl-2-cyanoacrylate (Histoacryl Blue; BBraun, Melsungen, Germany); N-butyl-cyanoacrylate (Nexacryl; Closure Medical, Raleigh, NC, USA); and 2-octyl-cyanoacrylate (Dermabond; Closure Medical, Raleigh, NC, USA). Biocompatibility of cyanoacrylate is directly related to the number of carbon atoms and length of the alkyl chain. However, the tensile strength of N-butyl-cyanoacrylate (e.g., histoacryl) is greater than longer chain (e.g., octyl-cyanoacrylate/dermabond).[13]
In addition to its structural support and ability to address perforations mechanically, cyanoacrylate also inhibits epithelial collagenase through inhibition of polymorphonuclear leukocytes and can have affects against bacteria as well.[14] Eiferman and Snyder found a bacteriostatic effect of butyl-2-cyanoacrylate glue against Gram-positive bacteria both in vitro and in vivo but did not find any activity against Gram-negative organisms.[15] They found that the maximum activity was before polymerization. It is believed that the lipopolysaccharide capsule, which surrounds the cell wall of Gram-negative bacteria, may act as a barrier to the glue.
De Almeida Manzano et al.[16] studied the in vitro antimicrobial properties of ethyl-cyanoacrylate against different microorganisms: Staphylococcusaureus (resistant to multiple antibiotics, including methicillin); Staphylococcus aureus; coagulase-negative Staphylococcus sp.; Streptococcus pyogenes; Streptococcus pneumoniae; Pseudomonas aeruginosa (resistant to multiple antibiotics); P. aeruginosa; Escherichia coli; and Enterococcus faecalis, by culturing on different appropriate solid culture media and placing ten drops of ethyl-cyanoacrylate on each plate. Each plate was incubated for 24 h. Bactericidal activity was determined by measuring the inhibition zone if present. Bactericidal analysis showed the most bactericidal activity against S. pneumoniae. Other species demonstrated less bactericidal activity, and ethyl-cyanoacrylate had no effect on either strain of P. aeruginosa.
Cyanoacrylate has several potential roles as an adjunct therapy for bacterial keratitis. It is certainly helpful for mechanical closure of the world and stabilization of corneal perforations from infectious and noninfectious etiologies, including corneal melting from immune mechanisms. It appears to possess antibacterial activity against a number of bacterial species, and further investigation would improve our understanding of this benefit.
Amniotic Membrane Transplantation
Amnion part of human placenta has anti-inflammatory, anti-fibrosis, anti-angiogenesis, and antibacterial properties.[17] It also promotes reepithelization by reinforcing basal epithelial cell adhesion, induction of epithelial cell migration, promotion of both differentiation and proliferation of conjunctival and limbal epithelial progenitor cells, prevention of epithelial apoptosis, and reduction of keratocyte apoptosis.[18,19] Furthermore, amniotic membrane (AM) can reduce pain associated with the epithelial defect.[18]
AM has been used in different ocular pathologies such as conjunctival surface reconstruction (e.g., pterygium removal),[20] persistent epithelial defect,[21] and chemical injury.[20] The main use of AM in bacterial keratitis has been to promote wound healing, such as for persistent epithelial defects following presumed adequate antibiotic treatment. The basement membrane of amniotic membrane resembles the basement membrane of conjunctiva and cornea, which works as a scaffold for epithelial cells migration and as a mechanical protection against friction with eyelid movement. Furthermore, AM contains growth factors such as transforming growth factor (TGF) α, TGF β1, β2, and β3, human chorionic growth factor, epidermal growth factor (TGF), and β fibroblast growth factor, which promote epithelial differentiation and prevent apoptosis. AM also contains collagen Type IV, V, and VII, in addition to laminin and fibronectin. Collagen Type V helps the epithelial cell to anchor to the corneal stroma. Laminin aids in adhesion of epithelial cells to the stroma. The role of fibronectin is to facilitate epithelial cell migration, adhesion, and cellular differentiation.[19,22,23,24]
In a rat model of microbial keratitis due to S. aureus, Barequet et al. and associates found less corneal haze and neovascularization after using AM with antibiotic in comparison to antibiotics alone.[25]
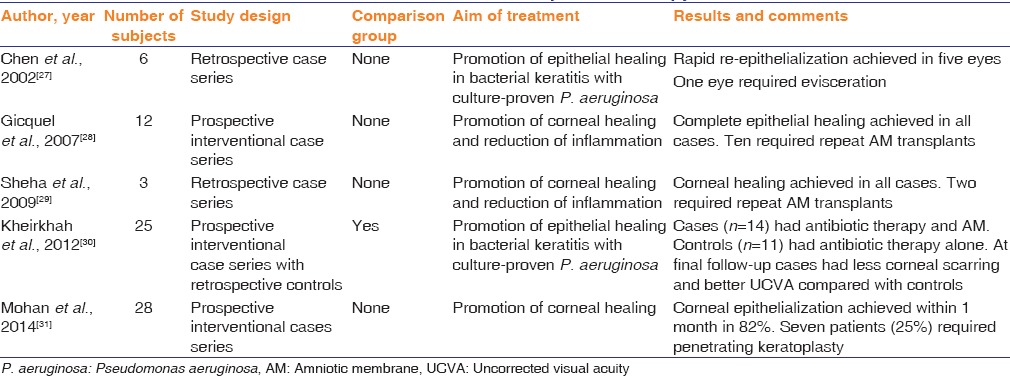
Several studies have examined the beneficial effect of AM for wound healing in bacterial keratitis. Chen et al.[26] published a retrospective case series of patients who had rapid healing of epithelial defect following application of AM, after pseudomonas-related bacterial keratitis. Five patients out of six had a rapid reepithelization; one patient required evisceration. Gicquel et al.[27] conducted a prospective interventional case series of the effect of AM on corneal healing and reduction of inflammation after microbial keratitis in twelve patients. All of them achieved complete reepithelization after one or two AM transplantations. Sheha et al.[28] reported similar results in three patients.
Kheirkhah et al.[29] conducted a prospective interventional case series (14 eyes) with retrospective controls (11 eyes), comparing the role of AM applied after 2–3 days of antibiotic therapy with patients who received antibiotic therapy alone for Pseudomonas keratitis. Cryopreserved AM was used in a combined technique, in which an inlay graft is placed on top of a damaged basement membrane, followed by an overlay patch to cover the whole cornea. They found that equal healing time and best-corrected visual acuity in cases and controls, but better uncorrected visual acuity and less corneal scar density in the AM group.
Mohan et al.[30] published a prospective interventional case series on 28 eyes. They found that complete reepithelization was achieved within 1 month in 82% of patients with 25% of eyes requiring keratoplasty. Unfortunately, the lack of a control group makes the outcomes of this series difficult to interpret.
Table 1 summarizes publications using AM as an adjunctive therapy for bacterial keratitis. Although the results in some series look promising, the lack of a comparison group in almost all the studies makes interpretation of the benefit of AM difficult to impossible. A randomized controlled study is warranted to help determine the potential benefits of this adjunct therapy.
Table 1.
Publications on the role of amniotic membrane as adjunctive therapy for bacterial keratitis
Matrix Metalloproteinases Inhibitors
Bacterial keratitis can lead to stromal thinning secondary to extracellular matrix destruction by elaboration of degradative proteases. Matrix metalloproteinases (MMPs) is one group of proteases, which include collagenases, gelatinases, and stromelysins.[32,33] Inhibition of MMPs may be helpful in the prevention of stromal destruction. Proposed MMP inhibitors which have been proposed for use in microbial keratitis include tetracyclines, acetylcysteine, ascorbate, and sodium ethylenediaminetetraacetic acid. Currently, no RCTs were performed to prove their efficacy on human corneal tissue in bacterial keratitis. In fact, despite MMP inhibition being proposed as a beneficial adjunct to corneal ulceration since the late 1980s, there is a paucity of publications involving animal models or humans reported in the literature.
The only MMP inhibitor data involving microbial keratitis in humans found on literature search is doxycycline. McElvanney[33] reported two cases of culture-proven pseudomonas bacterial keratitis treated oral doxycycline 100 mg twice daily, in addition to topical ofloxacin 0.3% and ceftazidime 5%. He found that stabilization of corneal melting occurred, but this report offers little or nothing in regard to evidence of the benefit of doxycycline.
Despite in vitro data and suppositions that MMP inhibitors may be beneficial, more data are needed overall. Well-performed RCTs are needed for promising agents.
Mitomycin C
Mitomycin C (MMC) is an antimetabolite isolated from Streptomyces caespitosus. It is converted in tissues into an alkylating agent and results in DNA alkylation in all phases of the cell cycle. Furthermore, it inhibits RNA and protein synthesis.[34] MMC has been used in refractive surgery to reduce postoperative corneal haze and scaring due to its anti-fibroblast activity.[35,36]
Kwan et al.[37] found that MMC has a broad-spectrum antimicrobial activity against a broad range of bacteria including E. coli, S. aureus, and P. aeruginosa in both rich and minimal media.
The results thus obtained from laboratory studies are limited. Clearly, further research will be required to validate any beneficial or harmful effect of MMC on human corneas in bacterial keratitis.
Autologous Serum Eye Drops
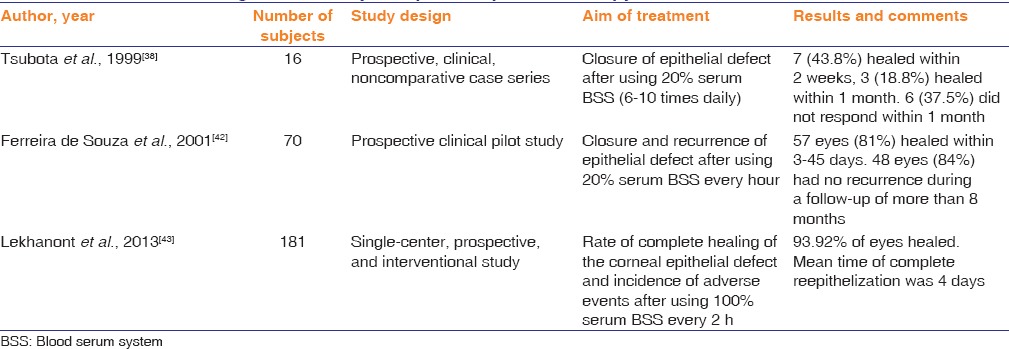
Autologous serum eye drops have beneficial effects on the corneal epithelium because it contains growth factors, vitamins, and immunoglobulins.[38] It lacks antigenicity since it is typically prepared without preservatives and is derived from the patient's own serum. Serum eye drops have been used for severe dry eyes,[39] persistent epithelial defects,[38] and superior limbal keratoconjunctivitis.[40]
Several prospective series have suggested a therapeutic effect on noninfectious-related epithelial defect with different concentrations of serum blood serum system (range between 20% and 100%). However, none of these publications have included a comparison group, and hence, it is difficult to impossible to determine if there was a beneficial effect. Table 2 lists these studies and their results.
Table 2.
The role of autologous serum eye drops as adjunctive therapy for bacterial keratitis
Cryotherapy
Currently, cryotherapy is still used in variety of ophthalmological diseases such as ocular surface squamous neoplasia, retinopathy of prematurity, retinal breaks, anterior chamber epithelial downgrowth, eyelid warts, and glaucoma surgeries. Cryotherapy produces a stimulus which causes a sudden decrease in the local tissue temperature and metabolism, which results in bacterial death and may activate an immune response.
Alpren et al.[41] found cryotherapy as an effective bactericidal treatment on experimental pseudomonas-related keratitis in 42 eyes of guinea pigs and rabbits, either alone or in combination with topical antibiotics. Eyes were divided into two groups. They used one freeze-thaw cycle for 6 s followed by topical antibiotics for one group, and antibiotic (tobramycin) alone for the second group. When the corneas were cultured, they found that all corneas treated with tobramycin alone had positive bacterial cultures, whereas 57% (24 of 42 eyes) had no growth on cultures after the cryotherapy followed by tobramycin.
Eiferman et al.[44] reported three cases of perforated Pseudomonas corneal keratitis with a scleral extension which were treated with penetrating keratoplasty and cryotherapy to the remaining cornea and sclera. All of the cases showed dramatic improvement.
Based on animal studies, cryotherapy can have a potentially beneficial effect on bacterial keratitis reaching the sclera. More research about cryotherapy on the human cornea is still necessary before obtaining a definitive answer for its efficacy and safety on human corneas, especially endothelial damage.
Conjunctival Flap
Many authors consider the conjunctival flap as a biological patch because of its protective, tropic and analgesic properties.[45,46] Using conjunctival flap to treat corneal disease was first described by Gundersen.[45]
Srivastava et al.[47] performed a conjunctival flap for 37 eyes with central and eccentric perforation <4 mm in diameter with or without iris prolapse. The conjunctival flap was successful in 30 cases in saving the eye for future penetrating keratoplasty. They found that the outcome depends on the site, site of perforation, and any cataractous lens changes. A retrospective, noncomparative case series was reported by Khodadoust and Quinter[48] involving fifty eyes that had a conjunctival flap for the treatment of chronic corneal ulcers. At the time of conjunctival flap, 19 of 50 eyes had a perforated corneal ulcer, whereas 31 eyes had not perforated. Eleven patients had bacterial keratitis. They found that 94% of the patients had a stable conjunctival flap for up to 2 years. Sixty-one percent of patients received a corneal graft 6–24 months postoperatively. Unfortunately, there was no information about the outcomes of the corneal graft after conjunctival flap.
Several reports of the conjunctival flap as treatment of open globe injury with tissue loss and for fungal keratitis. Conjunctival flap considered one of the oldest methods to treat corneal perforation when access to corneal graft is not possible. Further studies are needed to validate the role of the conjunctival flap as a treatment before keratoplasty in cases of bacterial keratitis.
Cycloplegic
Cycloplegic medications are commonly used to relieve the pain and to prevent posterior synechia formation that is often associated with iritis accompanying bacterial keratitis. Currently, there are no recorded randomized clinical studies investigating the effect of cycloplegic agents on bacterial keratitis.
Discussion
This paper presented information on adjunct treatments for bacterial keratitis. Unfortunately, despite a tradition of using these kinds of agents in microbial keratitis, there is little evidence of benefits in the literature. Studies should be carried out to enhance the study whether these kinds of treatments can improve the anatomical and visual outcomes of bacterial keratitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shah A, Sachdev A, Coggon D. Geographic variations in microbial keratitis: An analysis of the peer-reviewed literature. Br J Ophthalmol. 2011;95:762–7. doi: 10.1136/bjo.2009.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keay L, Edwards K, Naduvilath T. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–16. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Karsten E, Watson SL, Foster LJ. Diversity of microbial species implicated in keratitis: A review. Open Ophthalmol J. 2012;6:110–24. doi: 10.2174/1874364101206010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol. 1982;15:635–9. doi: 10.1128/jcm.15.4.635-639.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamora JL. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg. 1986;151:400–6. doi: 10.1016/0002-9610(86)90477-0. [DOI] [PubMed] [Google Scholar]
- 6.Chou SF, Lin CH, Chang SW. Povidone-iodine application induces corneal cell death through fixation. Br J Ophthalmol. 2011;95:277–83. doi: 10.1136/bjo.2010.189407. [DOI] [PubMed] [Google Scholar]
- 7.Katz J, Khatry SK, Thapa MD, Schein OD, Kimbrough Pradhan E, LeClerq SC, et al. A randomised trial of povidone-iodine to reduce visual impairment from corneal ulcers in rural Nepal. Br J Ophthalmol. 2004;88:1487–92. doi: 10.1136/bjo.2004.044412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregori NZ, Schiffman JC, Miller DM, Alfonso EC. Clinical trial of povidone-iodine (Betadine) versus placebo in the pretreatment of corneal ulcers. Cornea. 2006;25:558–63. doi: 10.1097/01.ico.0000214220.67872.b1. [DOI] [PubMed] [Google Scholar]
- 9.Jain KK. Physical, physiological, and biochemical aspects of hyperbaric oxygenation. In: Jain KK, editor. Textbook of Hyperbaric Medicine. Germany: Hogrefe & Huber; 2009. pp. 9–20. [Google Scholar]
- 10.Oguz H, Sobaci G. The use of hyperbaric oxygen therapy in ophthalmology. Surv Ophthalmol. 2008;53:112–20. doi: 10.1016/j.survophthal.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Price JC, Stevens DL. Hyperbaric oxygen in the treatment of rhinocerebral mucormycosis. Laryngoscope. 1980;90(5 Pt 1):737–47. [PubMed] [Google Scholar]
- 12.Chong R, Ayer CJ, Francis IC, Coroneo MT, Wolfers DL. Adjunctive hyperbaric oxygen in pseudomonas keratitis. Br J Ophthalmol. 2007;91:560–1. doi: 10.1136/bjo.2006.095448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vote BJ, Elder MJ. Cyanoacrylate glue for corneal perforations: A description of a surgical technique and a review of the literature. Clin Exp Ophthalmol. 2000;28:437–42. doi: 10.1046/j.1442-9071.2000.00351.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiyoo N. Interaction between corneal invasion of polymorphonuclear leukocytes and corneal epithelium. Nippon Ganka Gakkai Zasshi. 1990;94:445–56. [PubMed] [Google Scholar]
- 15.Eiferman RA, Snyder JW. Antibacterial effect of cyanoacrylate glue. Arch Ophthalmol (Chicago, Ill: 1960) 1983;101:958–60. doi: 10.1001/archopht.1983.01040010958022. [DOI] [PubMed] [Google Scholar]
- 16.de Almeida Manzano RP, Naufal SC, Hida RY, Guarnieri LO, Nishiwaki-Dantas MC. Antibacterial analysis in vitro of ethyl-cyanoacrylate against ocular pathogens. Cornea. 2006;25:350–1. doi: 10.1097/01.ico.0000183490.16131.e3. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J Transplant. 2014;4:111–21. doi: 10.5500/wjt.v4.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ucakhan OO, Koklu G, Firat E. Nonpreserved human amniotic membrane transplantation in acute and chronic chemical eye injuries. Cornea. 2002;21:169–72. doi: 10.1097/00003226-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Al-Swailem S. The role of amniotic membrane transplantation in the treatment of chemical injuries of the eye. Middle East J Ophthalmol. 2006;13:23–37. [Google Scholar]
- 20.Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997;104:974–85. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123:303–12. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20:325–34. [PubMed] [Google Scholar]
- 23.Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20:408–13. doi: 10.1097/00003226-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85:444–9. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barequet IS, Habot-Wilner Z, Keller N, Smollan G, Ziv H, Belkin M, et al. Effect of amniotic membrane transplantation on the healing of bacterial keratitis. Invest Ophthalmol Vis Sci. 2008;49:163–7. doi: 10.1167/iovs.07-1005. [DOI] [PubMed] [Google Scholar]
- 26.Chen JH, Ma DH, Tsai RJ. Amniotic membrane transplantation for pseudomonal keratitis with impending perforation. Chang Gung Med J. 2002;25:144–52. [PubMed] [Google Scholar]
- 27.Gicquel JJ, Bejjani RA, Ellies P, Mercie M, Dighiero P. Amniotic membrane transplantation in severe bacterial keratitis. Cornea. 2007;26:27–33. doi: 10.1097/ICO.0b013e31802b28df. [DOI] [PubMed] [Google Scholar]
- 28.Sheha H, Liang L, Li J, Tseng SC. Sutureless amniotic membrane transplantation for severe bacterial keratitis. Cornea. 2009;28:1118–23. doi: 10.1097/ICO.0b013e3181a2abad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kheirkhah A, Tabatabaei A, Zavareh MK, Khodabandeh A, Mohammadpour M, Raju VK. A controlled study of amniotic membrane transplantation for acute Pseudomonas keratitis. Can J Ophthalmol. 2012;47:305–11. doi: 10.1016/j.jcjo.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Mohan S, Budhiraja I, Saxena A, Khan P, Sachan SK. Role of multilayered amniotic membrane transplantation for the treatment of resistant corneal ulcers in North India. Int Ophthalmol. 2014;34:485–91. doi: 10.1007/s10792-013-9834-3. [DOI] [PubMed] [Google Scholar]
- 31.Hossain P. The corneal melting point. Eye (London, England) 2012;26:1029–30. doi: 10.1038/eye.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: A functional balance in tissue degradation. Oral Dis. 1996;2:70–6. doi: 10.1111/j.1601-0825.1996.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 33.McElvanney AM. Doxycycline in the management of pseudomonas corneal melting: Two case reports and a review of the literature. Eye Contact Lens. 2003;29:258–61. doi: 10.1097/01.icl.0000086490.38331.58. [DOI] [PubMed] [Google Scholar]
- 34.Wakaki S, Marumo H, Tomioka K. Isolation of new fractions of antitumor mitomycins. Antibiot Chemother. 1958;8:228–40. [PubMed] [Google Scholar]
- 35.Carones F, Vigo L, Scandola E, Vacchini L. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J Cataract Refract Surg. 2002;28:2088–95. doi: 10.1016/s0886-3350(02)01701-7. [DOI] [PubMed] [Google Scholar]
- 36.Gambato C, Ghirlando A, Moretto E, Busato F, Midena E. Mitomycin C modulation of corneal wound healing after photorefractive keratectomy in highly myopic eyes. Ophthalmology. 2005;112:208–18. doi: 10.1016/j.ophtha.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Kwan BW, Chowdhury N, Wood TK. Combatting bacterial infections by killing persister cells with mitomycin C. Environ Microbiol. 2015;17:4406–14. doi: 10.1111/1462-2920.12873. [DOI] [PubMed] [Google Scholar]
- 38.Subota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999;106:1984–9. doi: 10.1016/S0161-6420(99)90412-8. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa Y, Okamoto S, Mori T, Yamada M, Mashima Y, Watanabe R, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003;31:579–83. doi: 10.1038/sj.bmt.1703862. [DOI] [PubMed] [Google Scholar]
- 40.Goto E, Shimmura S, Shimazaki J, Tsubota K. Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea. 2001;20:807–10. doi: 10.1097/00003226-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 41.lpren TV, Hyndiuk RA, Davis SD, Sarff LD. Cryotherapy for experimental Pseudomonas keratitis. Arch Ophthalmol (Chicago, Ill: 1960) 1979;97:711–4. doi: 10.1001/archopht.1979.01020010363017. [DOI] [PubMed] [Google Scholar]
- 42.Ferreira de Souza R, Kruse FE, Seitz B. Autologous serum for otherwise therapy resistant corneal epithelial defects – Prospective report on the first 70 eyes. Klin Monbl Augenheilkd. 2001;218:720–6. doi: 10.1055/s-2001-18663. [DOI] [PubMed] [Google Scholar]
- 43.Lekhanont K, Jongkhajornpong P, Choubtum L, Chuckpaiwong V. Topical 100% serum eye drops for treating corneal epithelial defect after ocular surgery. Biomed Res Int. 2013;2013:521315. doi: 10.1155/2013/521315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eiferman RA. Cryotherapy of Pseudonomas keratitis and scleritis. Arch Ophthalmol. 1979;97:1637–9. doi: 10.1001/archopht.1979.01020020205001. [DOI] [PubMed] [Google Scholar]
- 45.Gundersen T. Conjunctival flaps in the treatment of corneal disease with reference to a new technique of application. AMA Arch Ophthalmol. 1958;60:880–8. doi: 10.1001/archopht.1958.00940080900008. [DOI] [PubMed] [Google Scholar]
- 46.Saini JS, Sharma A, Grewal SP. Chronic corneal perforations. Ophthalmic Surg. 1992;23:399–402. [PubMed] [Google Scholar]
- 47.Srivastava US, Tyagi RN, Jain AK. Conjunctival flap in perforated corneal ulcers (A review of cases) Indian J Ophthalmol. 1982;30:351–2. [PubMed] [Google Scholar]
- 48.Khodadoust A, Quinter AP. Microsurgical approach to the conjunctival flap. Arch Ophthalmol (Chicago, Ill: 1960) 2003;121:1189–93. doi: 10.1001/archopht.121.8.1189. [DOI] [PubMed] [Google Scholar]


