Abstract
Infectious uveitis accounts for majority of the cases of uveitis in developing countries. It also encompasses an array of various microorganisms and their clinical presentations. Some of these infectious uveitic entities are familiar, while others are newly emerging in the global ophthalmic world. Many of these entities are also a major cause of morbidity and mortality, and appropriate, timely management is required to save not the eye, but life of the patient. This review highlights the ocular manifestations of various infectious uveitic entities, relevant to the ophthalmologist.
Keywords: Acute retinal necrosis, dengue, infectious uveitis, syphilis, toxoplasmosis, tuberculosis
Introduction
Infectious uveitis remains an enigma for the treating ophthalmologists. Risk of vision-robbing complications, higher rate of recurrences, absence of effective local therapies, and difficulty in diagnosis are the major challenges in the management of infectious uveitis. This article reviews salient features of some common infectious uveitic entities and their management. Furthermore, description of infectious entity such as human immunodeficiency virus (HIV)-related eye disease is beyond the scope of this article and was not included in this write-up.
Epidemiology of Infectious Uveitis in Developing Countries
Infectious uveitis accounts for majority of the cases of uveitis in developing countries, often up to 50% of the cases.[1] Epidemiology of infectious uveitis varies considerably by geographic location around the world and can be attributed to the differences in regional distribution of various microorganisms. Other important factors are differences in climatic conditions, host factor, and regional customs. Table 1 enlists the pattern of distribution of infectious uveitis in developing countries.
Table 1.
Common causes of infectious uveitis in developing countries
Ocular Toxoplasmosis
Ocular toxoplasmosis is caused by the protozoan parasite Toxoplasma gondii. The parasite has been reported to infect approximately 13%–50% of the world's population.[10] Transmission of the disease can occur to human being through following routes: by ingestion of undercooked meat of an intermediate host (lamb, pork, and beef), by inadvertent contamination of hands when disposing of cat litter trays and then subsequent transfer onto food, or by transplacental or vertical transmission of the parasite to fetus.[11] Children are usually infected by eating dirt (pica) containing spore of the parasite. Recent report showed that water contamination plays an important role in the transmission of the disease in endemic areas.
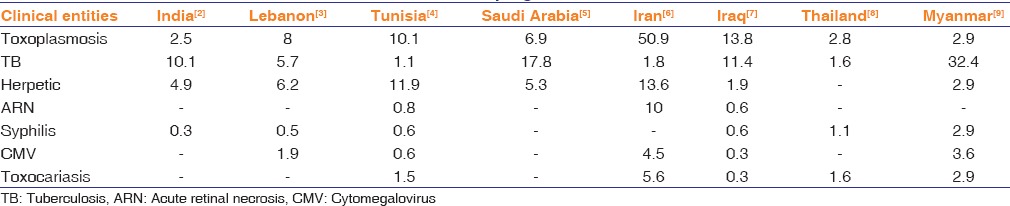
Ocular toxoplasmosis typically manifests as a localized necrotizing retinitis.[12] Characteristic lesion can be seen as a gray-white focus of retinal necrosis at the edge of a preexisting pigmented chorioretinal scar associated with severe vitritis [Figure 1]. Visualization of white retinal lesion hazily through a dense vitritis has given the dictum “headlight in the fog” for ocular toxoplasmosis. The associated iritis can be quite severe with granulomatous features and may be associated with increased intraocular pressure (IOP).[12] Atypical presentations such as retinal vasculitis, neuroretinitis, vitritis, iritis, and papillitis may also be seen. Ocular toxoplasmosis in immunocompromised individuals causes a severe, fulminant disease often with central nervous system involvement, which carries a poor prognosis and may be rapidly fatal if untreated.
Figure 1.
Fundus photograph of left eye showing active chorioretinitis of ocular toxoplasmosis at edge of a healed scar
Diagnosis of ocular toxoplasmosis is almost always clinical.[13] An acute T. gondii infection can be demonstrated by detection of specific immunoglobulin M (IgM) or IgA antibodies, or both, in the blood.[14] IgM usually appears in the 1st week after infection, peaks at 1 month, and disappears after 9 months. The demonstration of local synthesis of specific antibodies is a valuable diagnostic tool in ocular toxoplasmosis. Intraocular production of antibody can be calculated by the Goldmann–Witmer coefficient.[15] Polymerase chain reaction (PCR) and recently, real-time PCR have been utilized as a rapid and sensitive technique for the diagnosis of ocular toxoplasmosis. The use of intraocular antibody titer along with PCR yields higher sensitivity.[16]
The most common treatment for ocular toxoplasmosis consists of pyrimethamine-sulfadiazine combination and corticosteroids. This therapy consists of an initial dose of 75–100 mg of pyrimethamine daily for 2 days, followed by a 25–50 mg dose daily and a 2–4 g of sulfadiazine daily for 2 days, followed by a 500 mg to 1 g dose every 6 h as well as 5 mg of folinic acid daily for 4–6 weeks.[17] Oral prednisolone (1 mg/kg daily) is given from the 3rd day of therapy and tapered over 2–6 weeks.[18] Other treatment regimens include quadruple drug therapy (classic regimen plus clindamycin) as well as single or combined use of clindamycin, trimethoprim-sulfamethoxazole, spiramycin, minocycline, azithromycin, atovaquone, and clarithromycin. Intravitreal clindamycin with intravitreal corticosteroid is also used to treat ocular toxoplasmosis. However, repeated injections are required due to shorter half-life of intravitreal clindamycin.[19]
Ocular Toxocariasis
Ocular toxocariasis is caused by Toxocara canis or less frequently by Toxocara cati. Transmission to humans occurs by ingestion of embryonated eggs or larvae. After ingestion, the larvae migrate through the intestinal wall, reach the bloodstream, and thus reach to various organs (i.e., eye) where they induce an inflammatory reaction.[20]
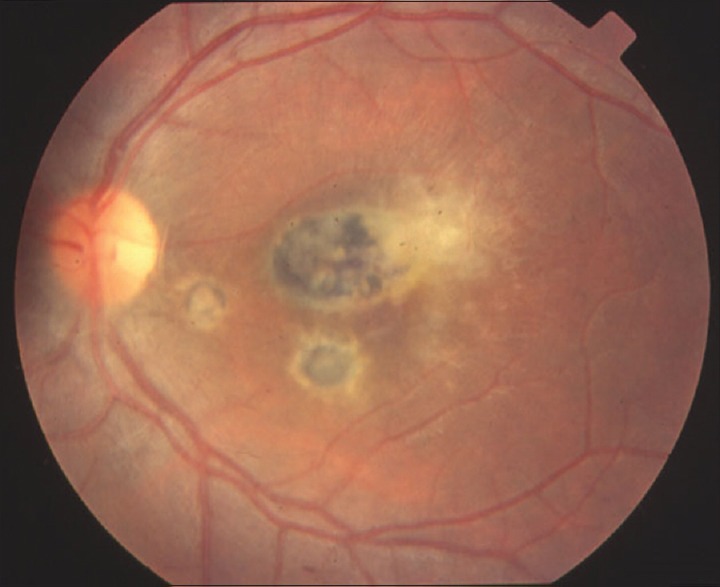
Ocular toxocariasis is an important cause of childhood visual morbidity. Typically, it affects one eye only, bilateral cases being <40%.[21] Anterior segment clinical findings may vary. The most common presentation is a posterior pole granuloma, a focal, whitish subretinal or intraretinal inflammatory mass usually <1-disc diameter with or without pigmentation, with varying degree of overlying vitreous haze. Peripheral granulomas present with varying degree of pigmentary changes and surrounding membranes [Figure 2]. Sometimes, the inflammation can be diffuse leading to snow banking. These granulomas can be connected with the optic nerve head or posterior pole by a fibrocellular band which can lead to tractional or combined retinal detachment. Nematode endophthalmitis is a severe manifestation with diffuse inflammation which may even lead to phthisis or panophthalmitis. Other rare manifestations include optic neuritis, diffuse chorioretinitis or diffuse unilateral subacute neuroretinitis, and motile subretinal larvae.[22] The characteristic feature of ocular toxocariasis is intraocular migration of the granuloma, continuous or discontinuous. Ocular complications include tractional retinal detachment, epiretinal membrane, cystoids macular edema, and macular hole formation.
Figure 2.
Montage photograph of an eye with peripheral granuloma in ocular toxocariasistoxocariasis
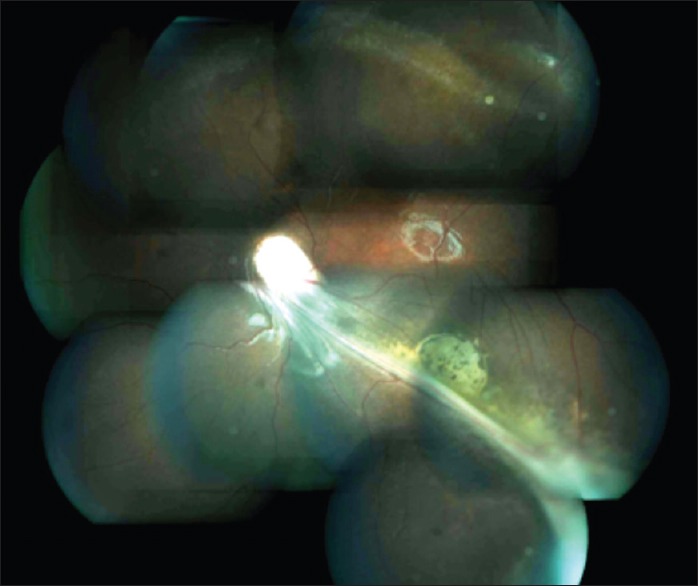
Figure 3.
Fundus photograph of patient of military tuberculosis showing multiple choroidal tubercles
Diagnosis is basically clinical. Ancillary test includes ultrasound showing the hyperechoic granuloma with tractional band. Serological diagnosis is performed by enzyme-linked immunosorbent assay (ELISA) based on the excretory-secretory antigens of T. canis.[23] The ELISA at a serum titer >1: 32 has 78% sensitivity for the detection of toxocariasis. Elevated serum IgE also provides a clue to diagnosis.
The first line of treatment is controlling the inflammation with systemic and topical steroids. Antihelminthic therapy with albendazole, though recommended for systemic toxocariasis, the role in ocular toxocariasis has not established. Report of destroying the larva by retinal laser photocoagulation has been reported.[24]
Syphilis
Syphilis is a systemic infection caused by the spirochete bacterium Treponema pallidum. There has been a recent reemergence of syphilis as a coinfection with HIV. Syphilis has been found to increase the risk of HIV transmission. HIV infection, in turn, can alter the natural history of syphilis, making the diagnosis of syphilis more difficult. Syphilis is considered as “the great imitator” as it has plethora of clinical manifestations and thus mimics many diseases.
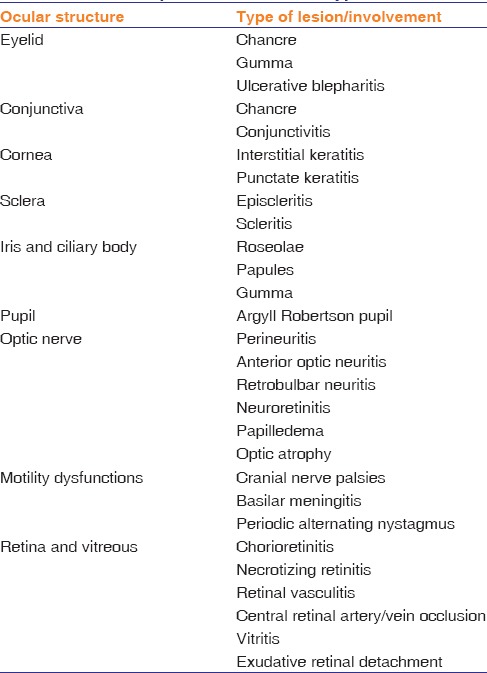
Syphilis has a complex and variable course, three progressive clinical stages with chronological overlap – primary, secondary, and tertiary syphilis. Latent syphilis is characterized by positive serological tests with no clinical evidence of infection. Ocular syphilis rarely manifests in primary stage, except as chancres of the eyelid and conjunctiva. The secondary stage may have ocular involvement in the form of keratitis, iris nodules, iridocyclitis, episcleritis, scleritis, chorioretinitis, and vitritis.[25] Ocular syphilis is more frequent in the late, latent, and tertiary stages. Syphilis should be suspected in any case of unexplained ocular inflammation, and many a times, ocular involvement can be the only presenting feature of systemic syphilis. Table 2 summarizes the spectrum of ocular involvement in syphilis.
Table 2.
Clinical spectrum of ocular syphilis
The diagnosis of syphilitic posterior uveitis requires high index of suspicion. Dark-field microscopy involves direct visualization of T. pallidum by examining exudates from tissue specimens with compound microscope with a dark field condenser. Using specific gene target primers and probes, PCR has been reported to detect T. pallidum genomes from ulcerative lesion swabs, lymph node aspirates, cerebrospinal fluid (CSF), blood, vitreous aspirate, etc.[26] Serological testing can be divided into two groups: nontreponemal tests, which detect nonspecific treponemal antibody (e.g., venereal diseases research laboratory test, rapid plasma reagin test), and treponemal tests, which detect specific treponemal antibody (e.g., T. pallidum hemagglutination assay and fluorescent treponemal antibody-absorbed).[14] Nontreponemal tests are nonspecific and may yield false positive results due to cross-reactivity. They are usually used as screening tests for syphilis and can be used to monitor disease activity and efficacy of treatment. Treponemal tests use recombinant treponemal antigens and unlike nontreponemal tests cannot be used to assess disease activity and treatment outcome. These tests are primarily used to confirm the diagnosis of syphilis in a patient with reactive nontreponemal tests. They are more specific and have lower incidence of false positivity.
The United States Center for Disease Control and Prevention (CDC) recommends performing a lumbar puncture to evaluate for neurosyphilis in all individuals with ocular syphilis.[27] Furthermore, all patients with primary and secondary syphilis should be tested for HIV infections, and in areas with high prevalence of HIV, such patients should be retested for HIV infection in 3 months if first HIV test was negative.
Parenteral penicillin G is the drug of choice for treating all stages of syphilis. Syphilis is treated according to the CDC guidelines. According to the CDC recommendations, ocular syphilis warrants treatment like neurosyphilis even if the CSF study is normal. The standard regime of treatment is intravenous (IV) aqueous crystalline penicillin G 18–24 million units/day for 10–14 days. Another recommended regimen is procaine penicillin G 2.4 million units intramuscular (IM) once daily plus probenecid 500 mg orally four times a day, both for 10–14 days.[27] In patients with penicillin allergy ceftriaxone 2 g daily either IM or IV for 10–14 days is a good alternative.
Ocular Tuberculosis
Ocular tuberculosis (TB) is thought to be caused by either direct invasion of the eye by tubercle bacilli or a hypersensitivity response to the tubercular antigen. As with other forms of extrapulmonary TB, a primary pulmonary focus is usually not found in ocular TB.[28]
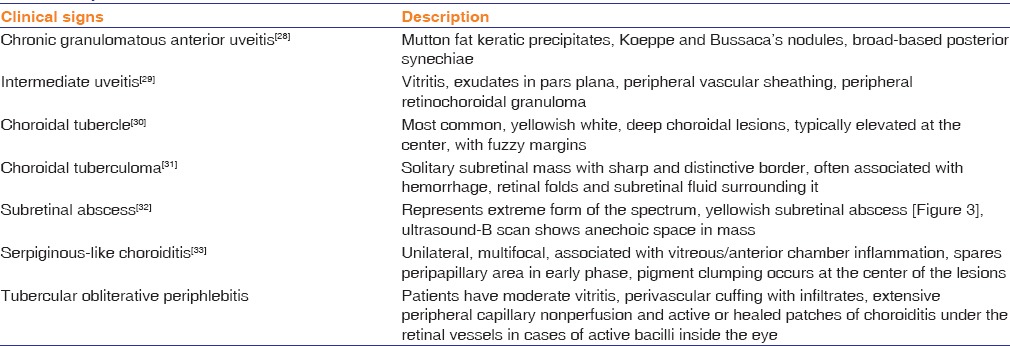
Ocular TB has a plethora of clinical presentations.[28] A brief summary of common ocular involvement in TB is highlighted in Table 3.
Table 3.
Spectrum of ocular tuberculosis
The definitive diagnosis includes isolation of Mycobacterium tuberculosis from ocular sample, which is not feasible in most of the time. Diagnosis of ocular TB in common clinical practice includes tuberculin skin sensitivity test (TST), interferon-gamma release assay, and radiological evidence of pulmonary involvement with the help of X-ray chest or high-resolution computerized tomography (chest).
For definitive diagnosis, PCR for M. tuberculosis from aqueous or vitreous tap can be done. Now, the sensitivity and specificity have been improved using real-time PCR and nested PCR. Histopathological evaluation for caseating granuloma can be advised in case of enucleated specimen or from retinal biopsy specimen if obtained in cases of unclear diagnosis with above tests.
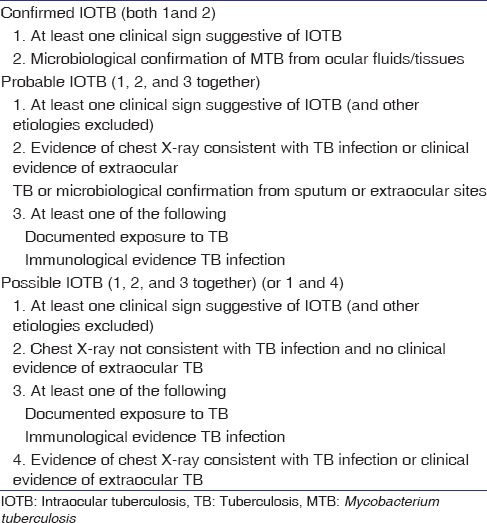
Gupta et al. have proposed a classification of intraocular TB[34] [Table 4]. The classification scheme is based on clinical signs, ocular and systemic investigations, and response to antitubercular therapy (ATT) over a period of 4–6 weeks.
Table 4.
Classification of ocular tuberculosis
ATT should be used in the presence of strong clinical suspicion and strongly positive TST (>15 mm). The schedule is same as for other extrapulmonary TB therapy with four drug regimens of isoniazid, ethambutol, rifampicin, and pyrazinamide for even 12–18 months. ATT decreases the antigen load; thus, the severity of hypersensitivity reaction. ATT is combined with oral steroids or immunosuppressive therapy in a tapering dose over 6–12 weeks. ATT has been shown to reduce the recurrence rate of uveitis.
Herpetic Uveitis
In humans, herpetic uveitis is primarily caused by three herpes viruses, herpes simplex virus (HSV), varicella zoster virus (VZV), and cytomegalovirus (CMV). The spectrum of herpetic uveitis includes anterior uveitis, scleral inflammation, necrotizing retinopathy, etc.
HSV anterior uveitis usually present with corneal involvement. Both granulomatous and nongranulomatous anterior uveitis can occur. Both sectoral and diffuse iris involvement can occur because of viral-induced endarteritis.[35] IOP rise can occur due to trabeculitis. VZV uveitis can occur without corneal involvement. The iris atrophy is usually sectoral and occurs due to an occlusive vasculitis. Usually, VZV uveitis occurs within the first 10 days of zoster, but chronic uveitis can also occur. The characteristic skin lesions help in diagnosis, especially involvement of the tip of nose (Hutchinson's sign).
CMV anterior uveitis should be considered in cases suggestive of herpetic anterior uveitis, but not responding to corticosteroids and high dose of acyclovir. The characteristic finding is a nummular keratic precipitate. CMV-associated anterior uveitis has no corneal scars, no posterior synechiae, no flare or fibrin, and no posterior segment involvement.[36]
Diagnosis is basically clinical. Aqueous humor aspirates may be analyzed for the local production of antibodies directed against HSV or VZV by the ELISA method. Detecting viral DNA in aqueous by PCR is confirmatory, but it yields positive result in only 30% of suspected herpetic anterior uveitis cases.[37]
Treatment is given with topical corticosteroids and cycloplegics. Oral antiviral is thought to be beneficial as it achieves therapeutic concentration in aqueous humor.[38] The initial dose is 800 mg five times/day for 7–10 days for VZV-associated uveitis and 400 mg five times/day for 7 days for HSV-associated uveitis.
Acute Retinal Necrosis
Acute retinal necrosis (ARN) generally affects healthy, immunocompetent young adults regardless of sex or race. It usually begins with unilateral disease. Second eye becomes involved in one-third cases usually within 1–6 weeks. ARN is caused by multiple members of the herpes virus family, mainly VZV, HSV Type 1, and Type 2, and rarely CMV and Epstein–Barr virus.
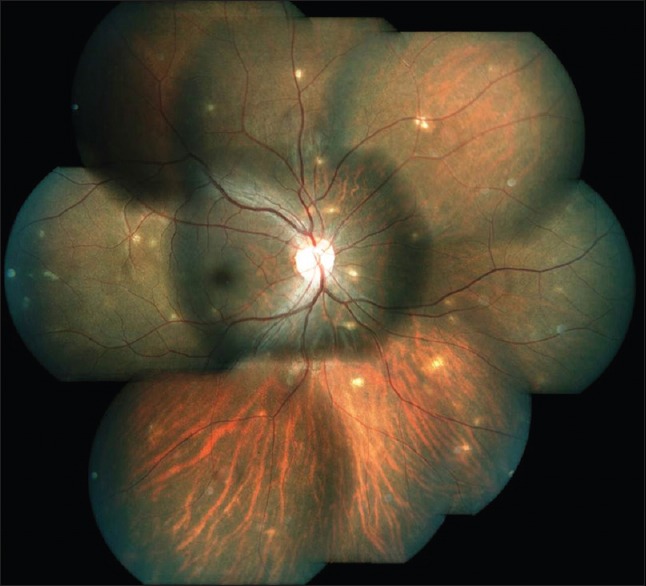
Early symptoms are usually very minimal and patients may complain irritation, redness, photophobia, and floaters. Anterior segment examination reveals mild to moderate anterior uveitis. The characteristic triad of lesions consists of moderate to severe vitritis, confluent necrotizing retinitis, and vasculitis. Ophthalmoscopically, the retinal necrosis appears as well-demarcated, multifocal patches of yellowish-white infiltrates in the periphery at the level of deep retina and retinal pigment epithelium [Figure 4]. The border between the necrosis and normal retina appears well-defined, smooth, and geographic. Over a few weeks, retinal necrosis becomes confluent and progresses rapidly and circumferentially toward the posterior pole. As the retinal necrosis and sloughing progress, resultant debris accumulation and inflammation of vitreous ensues often giving rise to a dense vitreous haze.
Figure 4.
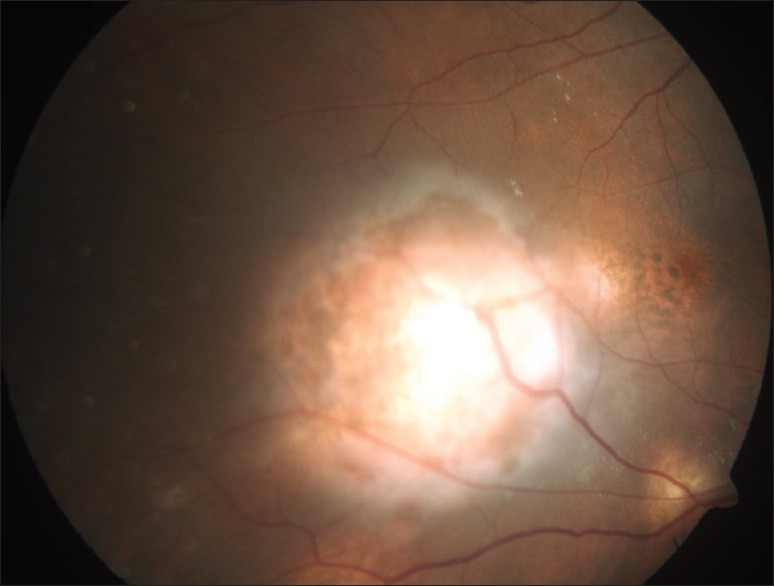
A case of tuberculous subretinal abscess
Retinal thinning and atrophy starts at the peripheral margins and gradually moves centrally. Meanwhile, retinal pigment epithelium perturbation develops with areas of clearing, forming a characteristic “Swiss-cheese appearance.” The thin atrophic retina often contributes to the development of retinal holes or tears. Retinal holes, combined with vitreous traction, lead to retinal detachment, and thus, retinal detachments in ARN patients have both a rhegmatogenous and tractional component.
Diagnosis of ARN is usually based on the clinical appearance and course and PCR. With the advent of newer technique such as real-time PCR, which helps quantitative estimation of the pathogen, aqueous or vitreous specimen can be analyzed in conditions where the cause of a severe uveitis may not be obvious.
IV acyclovir is the current treatment of choice for ARN. Recommended therapeutic regimen consists of 15 mg/kg/d IV acyclovir in 3 divided doses for 7–10 days, followed by oral antiviral medication, either acyclovir (800 mg five times a day), valacyclovir (1 g three times a day), or famciclovir (500 mg three times a day) up to 6 weeks or more. In cases with acyclovir-resistant strains of HSV and VZV, ganciclovir can be used. Corticosteroid has been used to reduce the inflammatory component Topical corticosteroid can be used to treat anterior segment inflammation. Culbertson et al. recommended applying prophylactic confluent laser photocoagulation posterior to the areas of active retinitis to create a “new artificial ora serrata.” A reduction in occurrence of retinal detachment to 17% in patients with laser photocoagulation compared with 67% in nonlaser-treated patients has been reported by Sternberg and its associates. Surgical management of retinal detachment in ARN patients is often frustrating. However, with the help of techniques such as pars plana vitrectomy, air-fluid exchange, endolaser, and gas or silicone oil tamponade, varying reports of success rate has been published.[39]
Cytomegalovirus Retinitis
CMV is a ubiquitous double-stranded DNA virus and is transmitted through placental transfer, breastfeeding, saliva, sexual contact, blood transfusions, and organ or bone marrow transplants.
CMV retinitis is the most common opportunistic ocular infection in immunosuppressed individuals. CMV infection progresses from the retinal vasculature horizontally through glial cells and vertically through Mueller cells, to result in full-thickness necrosis. There is distinction between the affected area and normal retina, which allows us to measure the progression rate of CMV retinitis. The lesions in CMV retinitis spread much more slowly than HSV or varicella-zoster retinitis. Classically, three patterns of active lesions have been described: hemorrhagic (with a predominance of retinal hemorrhage), brush fire (with a progressively expanding yellow-white margin surrounding necrotic retina), and granular (with a central atrophic area surrounded by punctate white granular satellite lesions).
Three drugs are approved by the Food and Drug Administration for the treatment of CMV retinitis: ganciclovir, foscarnet, and cidofovir.[40] All three drugs act by inhibition of CMV DNA polymerase. Because the drugs are virostatic, not virucidal, they must be continued indefinitely in patients with persistent immunosuppression. Systemic therapy is initiated with 2 weeks of twice daily IV ganciclovir 5 mg/kg or foscarnet 90 mg/kg, or once weekly IV cidofovir 5 mg/kg, followed by maintenance therapy consisting of once daily ganciclovir 5 mg/kg or foscarnet 90–120 mg/kg, or cidofovir 5 mg/kg every 2 weeks. Oral ganciclovir 1–2 g three times daily may be used instead of maintenance IV ganciclovir.
Ocular Leprosy
Leprosy is caused by Mycobacterium leprae and is more common in tropical countries. The longitudinal study on ocular leprosy found potential leprosy-related blinding pathology in 11% patients. Transmission occurs through droplets from respiratory secretions as well as ulcerative skin lesions. Susceptibility depends on age (bimodal distribution, <10 years and more than 60 years), sex (male sex), and low socioeconomic status. Lepromatous leprosy tends to be associated with more severe intraocular involvement, whereas patients with tuberculoid leprosy typically present with early involvement of the motor and sensory nerves with predominantly corneal lesions.
Patient can present with nodular episcleritis, diffuse scleritis, or episcleritis which is an immune reaction. This diffuse variety usually has associated iridocyclitis and keratitis. Other findings include iris pearls, ocular hypotony, diffuse choroiditis, focal retinal pigment epithelial alterations. Diagnosis is clinical with PCR or histopathology having a supportive role. Management is basically with multidrug therapy, with corticosteroids and cycloplegics. Clofazimine 100 mg TDS is also helpful.
Emerging Infections
With tremendous advancements in the field of microbiological diagnostics, various newer infectious agents have been detected worldwide in the last few decades. Salient clinical manifestations of few common emerging infectious uveitic entities are enlisted in Table 5.
Table 5.
Ocular manifestations of few common emerging infectious entities
Dengue virus is a mosquito-borne single-positive stranded RNA virus of the family Flaviviridae. Dengue fever is an important cause of morbidity and mortality in humans. Common ocular manifestations of dengue virus include focal chorioretinitis with macular edema, foveolitis, and periphlebitis. In addition, patients can have vitritis, anterior uveitis, intermediate uveitis, and uncommonly, optic neuritis or papillitis.[41] Foveolitis has been described as discrete, well-defined, yellow-orange subretinal lesions with striae in the fovea. Diagnosis is done by history, clinical examination, and supportive evidence of dengue fever by serology. Most patients improve spontaneously though some may require corticosteroid therapy. Usually, patients retain a central visual scotoma. Chikungunya virus, an alphavirus of the family Togaviridae with a single-stranded RNA genome, can present with conjunctivitis, nongranulomatous anterior uveitis, as well as granulomatous uveitis, keratitis with dendritic lesions.[42] Posterior segment involvement can manifest as retinitis, choroiditis, neuroretinitis, and optic neuritis. Retinitis in chikungunya retinitis usually involves posterior pole and may present with minimal vitritis, retinal hemorrhages, retinal edema, and associated retinal vessel involvement with cotton wool spots [Figure 5]. West Nile virus is prevalent in Africa, Europe, Australia, and Asia. It usually presents with a bilateral or rarely unilateral multifocal chorioretinitis with vitritis. The distribution of chorioretinal lesions is usually in linear arrays or scattered. Other manifestations include occlusive retinal vasculitis and optic neuritis.[43,44] In both chikungunya and West Nile virus, diagnosis is clinical with supporting evidence by ELISA.[45] Both of them have a self-limiting course with usually good visual recovery. Supportive treatment is given with oral and topical steroids. Cat scratch disease caused by Bartonella henselae usually presents with neuroretinitis, multifocal choroiditis, or vasculitis.[46] Condition is usually bilateral and ocular symptoms start 2–3 weeks after constitutional symptoms. Diagnosis is clinical with typical inoculation papule, supported by positive ELISA or immunofluorescent assay for B. henselae. Many physicians elect not to treat mild to moderate systemic cat scratch disease in the immunocompetent host. However, in more severe disease, a 10–14-day course of doxycycline, erythromycin, trimethoprim-sulfamethoxazole, rifampin, or IM gentamicin is often administered.
Figure 5.
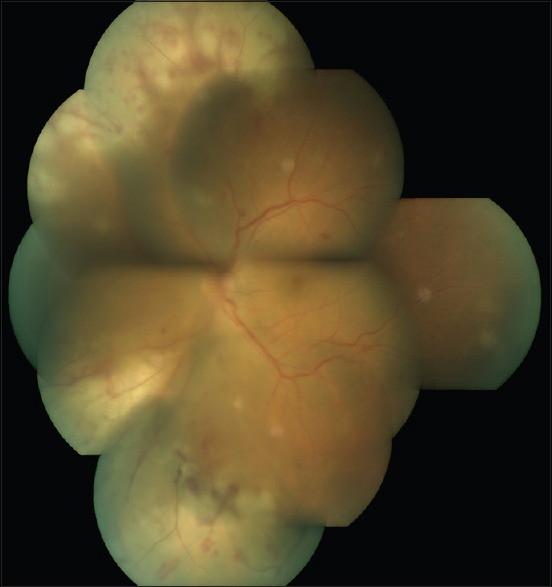
Montage photograph of a case acute retinal necrosis. Note the area of confluent necrotizing retinitis
Diagnosis and management of infectious etiology remain a real challenge to the ophthalmologists. Diagnosis of infectious uveitis requires high index of suspicion. Early identification of these conditions and initiation of appropriate antimicrobial therapy are of critical importance. On the other hand, administration of systemic or regional corticosteroid in absence of proper antimicrobial therapy can be detrimental.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rathinam SR, Namperumalsamy P. Global variation and pattern changes in epidemiology of uveitis. Indian J Ophthalmol. 2007;55:173–83. doi: 10.4103/0301-4738.31936. [DOI] [PubMed] [Google Scholar]
- 2.Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in North India. Indian J Ophthalmol. 2004;52:121–5. [PubMed] [Google Scholar]
- 3.Abdulaal M, Antonios R, Barikian A, Jaroudi M, Hamam RN. Etiology and Clinical Features of Ocular Inflammatory Diseases in a Tertiary Center in Lebanon. Ocul Immunol Inflamm. 2014:1–7. doi: 10.3109/09273948.2014.902077. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Khairallah M, Yahia SB, Ladjimi A, Messaoud R, Zaouali S, Attia S, et al. Pattern of uveitis in a referral centre in Tunisia, North Africa. Eye (Lond) 2007;21:33–9. doi: 10.1038/sj.eye.6702111. [DOI] [PubMed] [Google Scholar]
- 5.Hamade IH, Elkum N, Tabbara KF. Causes of uveitis at a referral center in Saudi Arabia. Ocul Immunol Inflamm. 2009;17:11–6. doi: 10.1080/09273940802491850. [DOI] [PubMed] [Google Scholar]
- 6.Rahimi M, Mirmansouri G. Patterns of uveitis at a tertiary referral center in Southern Iran. J Ophthalmic Vis Res. 2014;9:54–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Shakarchi FI. Pattern of uveitis at a referral center in Iraq. Middle East Afr J Ophthalmol. 2014;21:291–5. doi: 10.4103/0974-9233.142263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silpa-Archa S, Noonpradej S, Amphornphruet A. Pattern of uveitis in a referral ophthalmology center in the central district of Thailand. Ocul Immunol Inflamm. 2014:1–9. doi: 10.3109/09273948.2014.943773. [DOI] [PubMed] [Google Scholar]
- 9.Win MZ, Win T, Myint S, Shwe T, Sandar H. Epidemiology of uveitis in a tertiary eye center in Myanmar. Ocul Immunol Inflamm. 2016:1–6. doi: 10.3109/09273948.2015.1133839. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Petersen E, Kijlstra A, Stanford M. Epidemiology of ocular toxoplasmosis. Ocul Immunol Inflamm. 2012;20:68–75. doi: 10.3109/09273948.2012.661115. [DOI] [PubMed] [Google Scholar]
- 11.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: From animals to humans. Int J Parasitol. 2000;30:1217–58. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park YH, Nam HW. Clinical features and treatment of ocular toxoplasmosis. Korean J Parasitol. 2013;51:393–9. doi: 10.3347/kjp.2013.51.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodds EM. Toxoplasmosis. Curr Opin Ophthalmol. 2006;17:557–61. doi: 10.1097/ICU.0b013e32801094ca. [DOI] [PubMed] [Google Scholar]
- 14.Majumder PD, Sudharshan S, Biswas J. Laboratory support in the diagnosis of uveitis. Indian J Ophthalmol. 2013;61:269–76. doi: 10.4103/0301-4738.114095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garweg JG, Jacquier P, Boehnke M. Early aqueous humor analysis in patients with human ocular toxoplasmosis. J Clin Microbiol. 2000;38:996–1001. doi: 10.1128/jcm.38.3.996-1001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahalakshmi B, Therese KL, Madhavan HN, Biswas J. Diagnostic value of specific local antibody production and nucleic acid amplification technique-nested polymerase chain reaction (nPCR) in clinically suspected ocular toxoplasmosis. Ocul Immunol Inflamm. 2006;14:105–12. doi: 10.1080/09273940500545692. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert RE, See SE, Jones LV, Stanford MS. Antibiotics versus control for toxoplasma retinochoroiditis. Cochrane Database Syst Rev. 2002;1:CD002218. doi: 10.1002/14651858.CD002218. [DOI] [PubMed] [Google Scholar]
- 18.Jasper S, Vedula SS, John SS, Horo S, Sepah YJ, Nguyen QD. Corticosteroids as adjuvant therapy for ocular toxoplasmosis. Cochrane Database Syst Rev. 2013;4:CD007417. doi: 10.1002/14651858.CD007417.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baharivand N, Mahdavifard A, Fouladi RF. Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: A prospective randomized clinical trial. Int Ophthalmol. 2013;33:39–46. doi: 10.1007/s10792-012-9634-1. [DOI] [PubMed] [Google Scholar]
- 20.Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: Diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010;104:3–23. doi: 10.1179/136485910X12607012373957. [DOI] [PubMed] [Google Scholar]
- 21.Park SP, Park I, Park HY, Lee SU, Huh S, Magnaval JF. Five cases of ocular toxocariasis confirmed by serology. Korean J Parasitol. 2000;38:267–73. doi: 10.3347/kjp.2000.38.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arevalo JF, Espinoza JV, Arevalo FA. Ocular toxocariasis. J Pediatr Ophthalmol Strabismus. 2013;50:76–86. doi: 10.3928/01913913-20120821-01. [DOI] [PubMed] [Google Scholar]
- 23.Woodhall D, Starr MC, Montgomery SP, Jones JL, Lum F, Read RW, et al. Ocular toxocariasis: Epidemiologic, anatomic, and therapeutic variations based on a survey of ophthalmic subspecialists. Ophthalmology. 2012;119:1211–7. doi: 10.1016/j.ophtha.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Zygulska-Machowa H, Ziobrowski S. A case of ocular toxocariasis treated by xenon photocoagulation. Klin Oczna. 1987;89:213–4. [PubMed] [Google Scholar]
- 25.Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25:513–8. doi: 10.1097/ICU.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 26.Westeneng AC, Rothova A, de Boer JH, de Groot-Mijnes JD. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann-Witmer coefficient in aqueous analysis. Am J Ophthalmol. 2007;144:781–5. doi: 10.1016/j.ajo.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. MMWR Morb Mortal Wkly Rep. 2015;64:34–51. [Google Scholar]
- 28.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis – An update. Surv Ophthalmol. 2007;52:561–87. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Babu K, Bhat SS. Unilateral snow banking in tuberculosis-related intermediate uveitis. J Ophthalmic Inflamm Infect. 2014;4:4. doi: 10.1186/1869-5760-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annamalai R, Biswas J. Bilateral choroidal tuberculoma in miliary tuberculosis – Report of a case. J Ophthalmic Inflamm Infect. 2015;5:4. doi: 10.1186/s12348-014-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta V, Gupta A, Sachdeva N, Arora S, Bambery P. Successful management of tubercular subretinal granulomas. Ocul Immunol Inflamm. 2006;14:35–40. doi: 10.1080/09273940500269939. [DOI] [PubMed] [Google Scholar]
- 32.Majumder PD, Biswas J, Bansal N, Ghose A, Sharma H. Clinical profile of patients with tubercular subretinal abscess in a tertiary eye care center in Southern India. Ocul Immunol Inflamm. 2016:1–5. doi: 10.1080/09273948.2016.1199709. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Nazari Khanamiri H, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. 2013;58:203–32. doi: 10.1016/j.survophthal.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23:7–13. doi: 10.3109/09273948.2014.967358. [DOI] [PubMed] [Google Scholar]
- 35.Takase H, Kubono R, Terada Y, Imai A, Fukuda S, Tomita M, et al. Comparison of the ocular characteristics of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Jpn J Ophthalmol. 2014;58:473–82. doi: 10.1007/s10384-014-0340-6. [DOI] [PubMed] [Google Scholar]
- 36.Jap A, Chee SP. Viral anterior uveitis. Curr Opin Ophthalmol. 2011;22:483–8. doi: 10.1097/ICU.0b013e32834be021. [DOI] [PubMed] [Google Scholar]
- 37.Shoughy SS, Alkatan HM, Al-Abdullah AA, El-Khani A, de Groot-Mijnes JD, Tabbara KF. Polymerase chain reaction in unilateral cases of presumed viral anterior uveitis. Clin Ophthalmol. 2015;9:2325–8. doi: 10.2147/OPTH.S93655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung SO, Patterson A, Rees PJ. Pharmacokinetics of oral acyclovir (Zovirax) in the eye. Br J Ophthalmol. 1984;68:192–5. doi: 10.1136/bjo.68.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy R, Pal BP, Mathur G, Rao C, Das D, Biswas J. Acute retinal necrosis: Clinical features, management and outcomes – A 10 year consecutive case series. Ocul Immunol Inflamm. 2014;22:170–4. doi: 10.3109/09273948.2013.819928. [DOI] [PubMed] [Google Scholar]
- 40.Tan BH. Cytomegalovirus treatment. Curr Treat Options Infect Dis. 2014;6:256–270. doi: 10.1007/s40506-014-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim WK, Mathur R, Koh A, Yeoh R, Chee SP. Ocular manifestations of dengue fever. Ophthalmology. 2004;111:2057–64. doi: 10.1016/j.ophtha.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 42.Mahendradas P, Avadhani K, Shetty R. Chikungunya and the eye: A review. J Ophthalmic Inflamm Infect. 2013;3:35. doi: 10.1186/1869-5760-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakri SJ, Kaiser PK. Ocular manifestations of West Nile virus. Curr Opin Ophthalmol. 2004;15:537–40. doi: 10.1097/01.icu.0000143687.45232.f1. [DOI] [PubMed] [Google Scholar]
- 44.Garg S, Jampol LM. Systemic and intraocular manifestations of West Nile virus infection. Surv Ophthalmol. 2005;50:3–13. doi: 10.1016/j.survophthal.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Shukla J, Saxena D, Rathinam S, Lalitha P, Joseph CR, Sharma S, et al. Molecular detection and characterization of West Nile virus associated with multifocal retinitis in patients from Southern India. Int J Infect Dis. 2012;16:e53–9. doi: 10.1016/j.ijid.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Solley WA, Martin DF, Newman NJ, King R, Callanan DG, Zacchei T, et al. Cat scratch disease: Posterior segment manifestations. Ophthalmology. 1999;106:1546–53. doi: 10.1016/S0161-6420(99)90452-9. [DOI] [PubMed] [Google Scholar]


