Abstract
BACKGROUND:
Endophthalmitis after cataract surgery is a rare but vision-threatening complication. Intracameral cefuroxime (ICC) has been reported to be effective at reducing the risk, but concerns regarding the risks associated with this intervention remain.
METHODS:
Systematic review and synthesis of the literature on ICC, with a focus on the risks of therapy.
RESULTS:
Level 2a evidence was found to support the use of cefuroxime in penicillin-allergic patients. Compounding or dilutional errors are associated with ocular toxicity, but the incidence and risk of this occurrence are unknown. Level 4 evidence supports interventions that reduce the risk of dilutional errors. The association of cefuroxime injection with toxic anterior segment syndrome (TASS) is not established; Level 5 evidence supports standard measures to reduce the incidence of TASS related to cefuroxime administration.
CONCLUSION:
Cefuroxime can be administered safely to penicillin-allergic patients, and steps should be taken to reduce the risk of compounding or dilutional errors to avoid negating the benefits of this intervention. Recommended practice patterns for endophthalmitis prophylaxis should consider the risks and benefits of ICC.
Keywords: Cataract, cefuroxime, endophthalmitis, practice pattern, risk
Introduction
Postcataract surgery endophthalmitis is a relatively uncommon but devastating complication of modern cataract surgery,[1,2] and there is not complete consensus regarding the ideal practice patterns for prophylaxis.[3] The benefits of intracameral cefuroxime (ICC) have been supported by multiple studies. The European Society of Cataract and Refractive Surgeons (ESCRS) prospective multi-center interventional trial (and many other case–control, cross-sectional, and longitudinal studies) supported the hypothesis that the use of ICC at the conclusion of cataract surgery reduces the incidence of postoperative endophthalmitis.[4,5,6,7,8,9,10,11,12,13,14,15,16] It is estimated that two to four cases of endophthalmitis per 1000 surgeries can be avoided if surgeons adopt the use of ICC,[13] and the absence of an ICC prophylactic regimen at a dose of 1 mg/0.1 mL was associated with an almost 5-fold increase in the risk of postoperative endophthalmitis.[4] The injection is expedient, painless, and achieves high antibiotic concentrations in the immediate postoperative period. In addition, intracameral injection may be particularly effective in patients who sustain posterior capsule rupture[17] and would otherwise incur an increased the risk of endophthalmitis.
When considering the implementation of any intervention, cost-effectiveness is another important variable. ICC is relatively cost-effective in preventing endophthalmitis after cataract surgery. Sharifi et al. estimated the cost-effectiveness ratio for ICC to be $1403 per case of postoperative endophthalmitis prevented. Many commonly used topical antibiotics are not cost-effective compared with ICC, even under optimistic assumptions about their efficacy.[18] Another economic analysis comparing different prophylaxis regimens concluded that ICC provided the best cost-effectiveness ratio.[19] Therefore, the decision to adopt this therapy is supported by studies demonstrating efficacy and cost-effectiveness.
The final variable that must be included when determining practice patterns is risk. One weakness inherent in any meticulously performed clinical trial is that the results may not be generalizable to a more diverse patient population and varying scenarios of care. Most clinical trials make great effort to reduce the risk of enrollment in the study, and therefore the safety profile reported may be a “best-case scenario.” The purpose of this review is to provide an evidence-based review and synthesis of the literature regarding the risks of ICC when administered for the prevention of postcataract surgery endophthalmitis, thereby informing decisions regarding best practices for cataract surgery.
The categories of risk to be reviewed are:
Risk of anaphylaxis, especially in penicillin or cephalosporin allergic patients
Risk of toxicity at routine clinical doses
Risk of toxicity at increased doses due to compounding errors
Idiosyncratic reactions, including toxic anterior segment syndrome (TASS).
Methods
We performed a systematic review of the existing scientific literature using PubMed and Google Scholar. The key words used were “Cefuroxime,” “endophthalmitis” and “cataract surgeries.” There were no date or language restrictions in the electronic searches. After reviewing the abstracts for relevance 44 articles were included for review. All articles were read in full by both authors with the exception of two that were available only as abstracts. The highest level of evidence for each aspect of the intervention was assigned utilizing the Oxford Center for Evidence-Based Medicine Guidelines.[20]
Results
Risk of anaphylaxis
Cross-reactivity between penicillins and most second- and all third- and fourth-generation cephalosporins is negligible. It is generally considered safe to administer a cephalosporin with a side chain that is structurally dissimilar to that of penicillin.[21] In patients with a documented IgE-mediated reaction to penicillin, use of cephalosporins with a similar side chain should be avoided. However, cephalosporins with different side chains (such as cefuroxime) may be given.[22]
It should be noted that the ESCRS study excluded patients with penicillin or cephalosporin allergy,[4] as did several other large studies.[7,11,23] ICC injection during cataract surgery was well tolerated in a prospective study of forty penicillin-allergic patients with a negative preoperative cefuroxime skin test.[24] The use of ICC in patients with penicillin allergy was explored between 2004 and 2012 in a case–control registry study; the control group was the cohort of patients undergoing cataract surgery under a hospital policy of excluding patients with self-reported penicillin allergy. After a critical review of the literature and pilot study in 817 patients with reported penicillin allergy, this policy was altered, and all patients without a specific history of cephalosporin anaphylactic reaction were administered ICC. Out of 13,592 subsequent cataract surgeries, there were no reported cases of anaphylaxis or allergic reaction.[25] This study is limited by the registry design, which may underreport or miscategorize adverse events. A similar longitudinal observational study by Shorstein et al. reported a decreasing endophthalmitis rate after instituting a standard ICC protocol; there were no reports of anaphylaxis or allergic reaction among 12,609 surgeries, but some patients received other intracameral antibiotics, and it is unclear if penicillin-allergic patients routinely received cefuroxime or other antibiotics such as moxifloxacin or vancomycin. Barreau et al. reported a similar case–control study comparing the endophthalmitis rates before and after instituting a standard ICC regimen; patients with cefuroxime allergy were excluded, but penicillin-allergic patients received ICC. There were no cases of anaphylaxis among 2289 patients receiving ICC, but the prevalence of penicillin allergy was not reported.[5]
There are two case reports of an anaphylactic reaction in penicillin or cephalosporin allergic patients who received ICC.[26,27] The number of patients from these centers that had received ICC were not reported, preventing an estimation of the incidence of this complication.
Risks of cefuroxime toxicity at standard doses
Adverse effects that have rarely been reported with routine clinical doses include serous macular detachment, cystoid macular edema (CME), increased central foveal thickness, decreased best-corrected distance visual acuity, anterior chamber inflammation, and vitritis.[28,29] However, a prospective study found that ICC at the standard dose of 1 mg/0.1 mL did not have a statistically significant effect on postoperative macular thickness compared with nonadministration of intracameral antibiotic;[30] although, this study was under-powered to detect rare events. The ESCRS study and other longitudinal cohort studies were not designed to assess safety or adverse events as primary endpoints, but a large number of patients enrolled without a reported increase in these adverse events suggests that they are not associated, are masked by confounding factors, or are exceedingly rare.
Risks of cefuroxime toxicity at increased doses
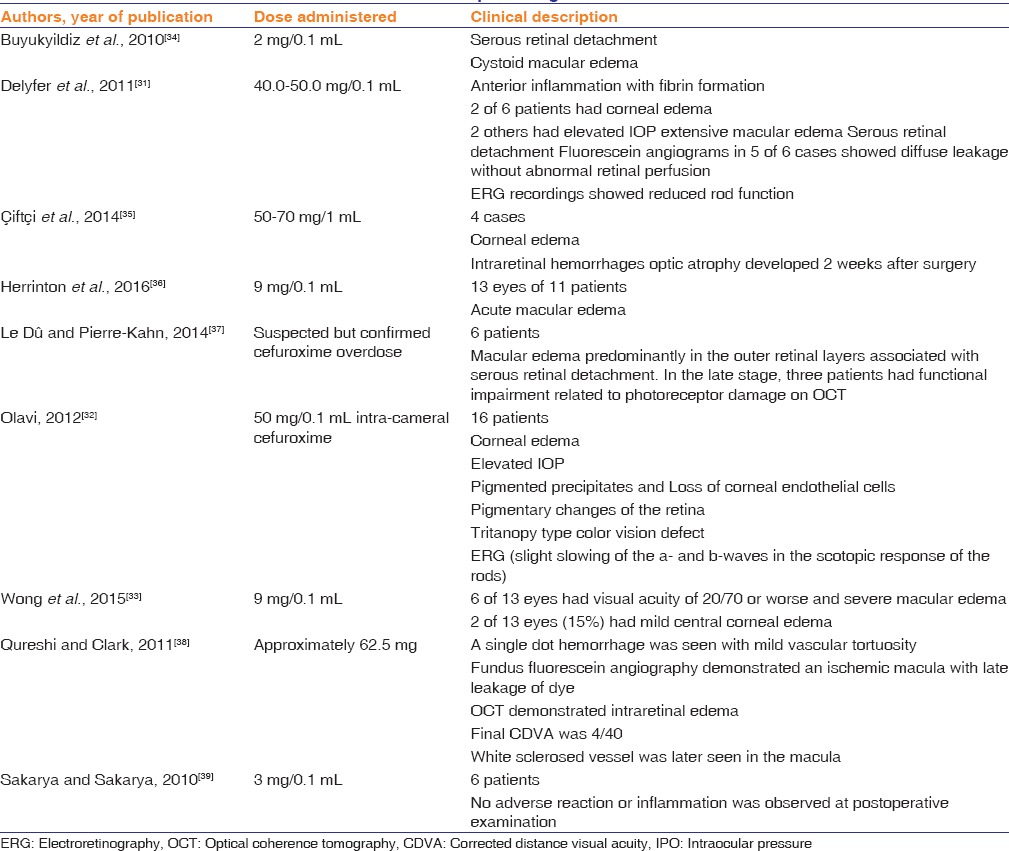
A lingering concern for some ophthalmologists is the risk of ocular toxicity attributable to inadvertent exposure to elevated concentrations (typically due to compounding errors). High doses of cefuroxime are associated with anterior and posterior segment inflammation with fibrin formation, corneal edema, elevated intraocular pressure, serous macular detachment, CME, hemorrhagic retinal infarction, and reduced rod photoreceptor cell function by electroretinography.[31,32,33,34,35] Table 1 summarizes the reported complications related to inadvertent administration of increased concentrations or volumes of cefuroxime. A limitation in assigning risk based on studies such as this is the lack of incidence data; it is unknown how many patients have received increased doses of ICC, and therefore, the rate of these adverse events is unknown. The incidence rate of compounding errors for ICC is also unknown and is presumably highly dependent on local factors such as the mechanisms of medication preparation and quality control measures.
Table 1.
Adverse events attributed to cefuroxime compounding errors
Toxic anterior segment syndrome and other idiosyncratic adverse events
TASS after cataract surgery has been reported in association with the intracameral use of cefuroxime.[18,40] Çakır et al. reported an ongoing cluster of TASS cases at a single center, with a resolution of the outbreak after discontinuing ICC in favor of intracameral moxifloxacin. As expected, there was no “rechallenge” in affected patients to determine conclusively if cefuroxime or other factor contributed to TASS. There was also no reported analysis of cefuroxime concentration to ascertain the potential role of dilution errors or other factors related to preparation. No other studies were found that studied the relationship of cefuroxime to TASS, or the effects of specific interventions to reduce the incidence of TASS related to cefuroxime.
Discussion
The efficacy of ICC as a prophylaxis for postcataract surgery endophthalmitis has been well established, but concerns regarding the risks of this intervention remain. The consensus of the literature from systemic administration of antibiotics is that the risk of cross-reactivity between second-generation cephalosporins (such as cefuroxime) and penicillin is very low. Our review of the literature supports a low rate of anaphylactic reactions, even among penicillin-allergic patients. A postal survey conducted among consultant ophthalmic surgeons working in the National Health Service Ophthalmic Departments in England revealed that of 262 consultants, 103 (37%) used cefuroxime in patients allergic to penicillin.[41] We propose that an acceptable practice pattern would be to consider ICC in all cataract surgery patients, including those with a history of penicillin allergy; administration to those patients with cephalosporin allergy may be considered, but skin testing may be indicated to identify those patients that are at increased risk of anaphylaxis and should not receive ICC. Alternative intracameral antibiotics, such as vancomycin and moxifloxacin, may reduce the risk of anaphylaxis in cefuroxime-allergic patients, but the efficacy of those interventions has been less well established.
The latest survey of members of the American Society of Cataract and Refractive Surgeons revealed that 30% of the United States ophthalmologists were utilizing intracameral antibiotics, compared to 70% of European respondents.[42] However, many US cataract surgeons believe that intra-cameral antibiotics are unnecessary, based on concerns about the methodology of the ESCRS and other studies, or that the absolute benefits do not outweigh the risks of dilution errors and toxicity.[3,43]
Not surprisingly, a clinical study demonstrated that the mathematical accuracy of a dilution protocol does not ensure dosage accuracy in a real-world clinical scenario.[44] The authors suggest that a commercial preparation would likely reduce the risk of dilution errors, but commercial preparations of intracameral antibiotic agents may not be financially viable in all health care environments. Implementation of ICC should include measures to audit and provide quality assurance of the cefuroxime dilution protocol.
Conclusion
Half of the risk-benefit equation of ICC has been solved to the satisfaction of most clinicians; the efficacy is clear. The remaining barriers to more widespread adoption include concerns about risks such as anaphylaxis, dilution errors, and toxicity (especially with noncommercial preparation[42]) and finally, the additional costs of this therapy. Since endophthalmitis is a rare event, even a slight increase in the risk of prophylactic therapy may negate the potential benefits. Our review and synthesis of the literature regarding the risks of cefuroxime therapy support the following recommendations:
Cefuroxime may be used safely in patients with penicillin allergy (Level 2a evidence)
Efforts to reduce the risk of dilution errors may include the use of a commercially prepared product, or strict quality assurance measures (Level 4 evidence)
Routine measures to reduce TASS should also apply to the use of cefuroxime (Level 5 evidence); even though, there is no proven association.
Future studies may further define the cost-effectiveness of ICC, and continued efforts to reduce the risk attributable to dilution errors are indicated. Finally, comparative studies of the efficacy and safety of cefuroxime compared to other intracameral antibiotics are needed to help define the optimal endophthalmitis prophylaxis regimen for our patients undergoing cataract surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Miller JJ, Scott IU, Flynn HW, Jr, Smiddy WE, Newton J, Miller D. Acute-onset endophthalmitis after cataract surgery (2000-2004): Incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139:983–7. doi: 10.1016/j.ajo.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, et al. Acute endophthalmitis following cataract surgery: A systematic review of the literature. Arch Ophthalmol. 2005;123:613–20. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz SG, Grzybowski A, Flynn HW., Jr Antibiotic prophylaxis: Different practice patterns within and outside the United States. Clin Ophthalmol. 2016;10:251–6. doi: 10.2147/OPTH.S100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–88. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Barreau G, Mounier M, Marin B, Adenis JP, Robert PY. Intracameral cefuroxime injection at the end of cataract surgery to reduce the incidence of endophthalmitis: French study. J Cataract Refract Surg. 2012;38:1370–5. doi: 10.1016/j.jcrs.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Daien V, Papinaud L, Gillies MC, Domerg C, Nagot N, Lacombe S, et al. Effectiveness and safety of an intracameral injection of cefuroxime for the prevention of endophthalmitis after cataract surgery with or without perioperative capsular rupture. JAMA Ophthalmol. 2016;134:810–6. doi: 10.1001/jamaophthalmol.2016.1351. [DOI] [PubMed] [Google Scholar]
- 7.García-Sáenz MC, Arias-Puente A, Rodríguez-Caravaca G, Bañuelos JB. Effectiveness of intracameral cefuroxime in preventing endophthalmitis after cataract surgery Ten-year comparative study. J Cataract Refract Surg. 2010;36:203–7. doi: 10.1016/j.jcrs.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: Outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123:295–301. doi: 10.1016/j.ophtha.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Katz G, Blum S, Leeva O, Axer-Siegel R, Moisseiev J, Tesler G, et al. Intracameral cefuroxime and the incidence of post-cataract endophthalmitis: An Israeli experience. Graefes Arch Clin Exp Ophthalmol. 2015;253:1729–33. doi: 10.1007/s00417-015-3009-z. [DOI] [PubMed] [Google Scholar]
- 10.Montan P, Lundström M, Stenevi U, Thorburn W. Endophthalmitis following cataract surgery in Sweden. The 1998 national prospective survey. Acta Ophthalmol Scand. 2002;80:258–61. doi: 10.1034/j.1600-0420.2002.800305.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Caravaca G, García-Sáenz MC, Villar-Del-Campo MC, Andrés-Alba Y, Arias-Puente A. Incidence of endophthalmitis and impact of prophylaxis with cefuroxime on cataract surgery. J Cataract Refract Surg. 2013;39:1399–403. doi: 10.1016/j.jcrs.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Behndig A, Cochener B, Güell JL, Kodjikian L, Mencucci R, Nuijts RM, et al. Endophthalmitis prophylaxis in cataract surgery: Overview of current practice patterns in 9 European countries. J Cataract Refract Surg. 2013;39:1421–31. doi: 10.1016/j.jcrs.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Kessel L, Flesner P, Andresen J, Erngaard D, Tendal B, Hjortdal J. Antibiotic prevention of postcataract endophthalmitis: A systematic review and meta-analysis. Acta Ophthalmol. 2015;93:303–17. doi: 10.1111/aos.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosa GD, Díez M. Prophylaxis of postoperative endophthalmitis with intracameral cefuroxime: A five years' experience profilaxis de la endoftalmitis postquirúrgica con cefuroxima intracamerular: Experiencia de cinco años. Arch Soc Esp Oftalmol. 2009;84:85–90. doi: 10.4321/s0365-66912009000200006. [DOI] [PubMed] [Google Scholar]
- 15.Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28:977–81. doi: 10.1016/s0886-3350(01)01269-x. [DOI] [PubMed] [Google Scholar]
- 16.Gower EW, Lindsley K, Nanji AA, Leyngold I, McDonnell PJ. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst Rev. 2013;15:CD006364. doi: 10.1002/14651858.CD006364.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39:8–14. doi: 10.1016/j.jcrs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Sharifi E, Porco TC, Naseri A. Cost-effectiveness analysis of intracameral cefuroxime use for prophylaxis of endophthalmitis after cataract surgery. Ophthalmology. 2009;116:1887–96.e1. doi: 10.1016/j.ophtha.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Linertová R, Abreu-González R, García-Pérez L, Alonso-Plasencia M, Cordovés-Dorta LM, Abreu-Reyes JA, et al. Intracameral cefuroxime and moxifloxacin used as endophthalmitis prophylaxis after cataract surgery: Systematic review of effectiveness and cost-effectiveness. Clin Ophthalmol. 2014;8:1515–22. doi: 10.2147/OPTH.S59776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. The Oxford 2011 Levels of Evidence. Vol. 1. Oxford: Centre Evidence-Based Medicine; 2011. [Last accessed on 2016 Sep 03]. Available from: http://www.cebm.net/index.aspx?o=1025 . [Google Scholar]
- 21.Campagna JD, Bond MC, Schabelman E, Hayes BD. The use of cephalosporins in penicillin-allergic patients: A literature review. J Emerg Med. 2012;42:612–20. doi: 10.1016/j.jemermed.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: A meta-analysis. Otolaryngol Head Neck Surg. 2007;136:340–7. doi: 10.1016/j.otohns.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Ng AL, Tang WW, Li PS, Li KK. Intracameral cefuroxime in the prevention of postoperative endophthalmitis: An experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol. 2016;254:1987–92. doi: 10.1007/s00417-016-3473-0. [DOI] [PubMed] [Google Scholar]
- 24.Promelle V, Jany B, Drimbea A, Jezraoui P, Milazzo S. Tolerability of intracameral cefuroxime during cataract surgery in case of penicillin allergy. J Fr Ophtalmol. 2015;38:283–7. doi: 10.1016/j.jfo.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Myneni J, Desai SP, Jayamanne DG. Reduction in postoperative endophthalmitis with intracameral cefuroxime. J Hosp Infect. 2013;84:326–8. doi: 10.1016/j.jhin.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Moisseiev E, Levinger E. Anaphylactic reaction following intracameral cefuroxime injection during cataract surgery. J Cataract Refract Surg. 2013;39:1432–4. doi: 10.1016/j.jcrs.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Villada JR, Vicente U, Javaloy J, Alió JL. Severe anaphylactic reaction after intracameral antibiotic administration during cataract surgery. J Cataract Refract Surg. 2005;31:620–1. doi: 10.1016/j.jcrs.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 28.Kontos A, Mitry D, Althauser S, Jain S. Acute serous macular detachment and cystoid macular edema after uncomplicated phacoemulsification using standard dose subconjunctival cefuroxime. Cutan Ocul Toxicol. 2014;33:233–4. doi: 10.3109/15569527.2013.835817. [DOI] [PubMed] [Google Scholar]
- 29.Xiao H, Liu X, Guo X. Macular edema with serous retinal detachment post-phacoemulsification followed by spectral domain optical coherence tomography: A report of two cases. BMC Res Notes. 2015;8:647. doi: 10.1186/s13104-015-1639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta MS, McKee HD, Saldaña M, Stewart OG. Macular thickness after cataract surgery with intracameral cefuroxime. J Cataract Refract Surg. 2005;31:1163–6. doi: 10.1016/j.jcrs.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 31.Delyfer MN, Rougier MB, Leoni S, Zhang Q, Dalbon F, Colin J, et al. Ocular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgery. J Cataract Refract Surg. 2011;37:271–8. doi: 10.1016/j.jcrs.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Olavi P. Ocular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50mg/ml intracameral cefuroxime. Acta Ophthalmol. 2012;90:e153–4. doi: 10.1111/j.1755-3768.2010.02103.x. [DOI] [PubMed] [Google Scholar]
- 33.Wong DC, Waxman MD, Herrinton LJ, Shorstein NH. Transient macular edema after intracameral injection of a moderately elevated dose of cefuroxime during phacoemulsification surgery. JAMA Ophthalmol. 2015;133:1194–7. doi: 10.1001/jamaophthalmol.2015.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buyukyildiz HZ, Gulkilik G, Kumcuoglu YZ. Early serous macular detachment after phacoemulsification surgery. J Cataract Refract Surg. 2010;36:1999–2002. doi: 10.1016/j.jcrs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Çiftçi S, Çiftçi L, Dag U. Hemorrhagic retinal infarction due to inadvertent overdose of cefuroxime in cases of complicated cataract surgery: Retrospective case series. Am J Ophthalmol. 2014;157:421–5.e2. doi: 10.1016/j.ajo.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Herrinton LJ, Shorstein NH, Paschal JF, Liu L, Contreras R, Winthrop KL, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123:287–94. doi: 10.1016/j.ophtha.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Dû B, Pierre-Kahn V. Early macular edema after phacoemulsification and suspected overdose of cefuroxime: Report of six cases. J Fr Ophtalmol. 2014;37:202–10. doi: 10.1016/j.jfo.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi F, Clark D. Macular infarction after inadvertent intracameral cefuroxime. J Cataract Refract Surg. 2011;37:1168–9. doi: 10.1016/j.jcrs.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 39.Sakarya Y, Sakarya R. Cefuroxime dilution error. Eur J Ophthalmol. 2010;20:460–1. doi: 10.1177/112067211002000232. [DOI] [PubMed] [Google Scholar]
- 40.Çakir B, Celik E, Aksoy NÖ, Bursali Ö, Uçak T, Bozkurt E, et al. Toxic anterior segment syndrome after uncomplicated cataract surgery possibly associated with intracamaral use of cefuroxime. Clin Ophthalmol. 2015;9:493–7. doi: 10.2147/OPTH.S74249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nanavaty MA, Wearne MJ. Perioperative antibiotic prophylaxis during phaco-emulsification and intraocular lens implantation: National survey of smaller eye units in England. Clin Exp Ophthalmol. 2010;38:462–6. doi: 10.1111/j.1442-9071.2010.02279.x. [DOI] [PubMed] [Google Scholar]
- 42.Chang DF, Braga-Mele R, Henderson BA, Mamalis N, Vasavada A. ASCRS Cataract Clinical Committee. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: Results of the 2014 ASCRS member survey. J Cataract Refract Surg. 2015;41:1300–5. doi: 10.1016/j.jcrs.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Liesegang TJ. Intracameral antibiotics: Questions for the United States based on prospective studies. J Cataract Refract Surg. 2008;34:505–9. doi: 10.1016/j.jcrs.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 44.Lockington D, Flowers H, Young D, Yorston D. Assessing the accuracy of intracameral antibiotic preparation for use in cataract surgery. J Cataract Refract Surg. 2010;36:286–9. doi: 10.1016/j.jcrs.2009.08.034. [DOI] [PubMed] [Google Scholar]


