Abstract
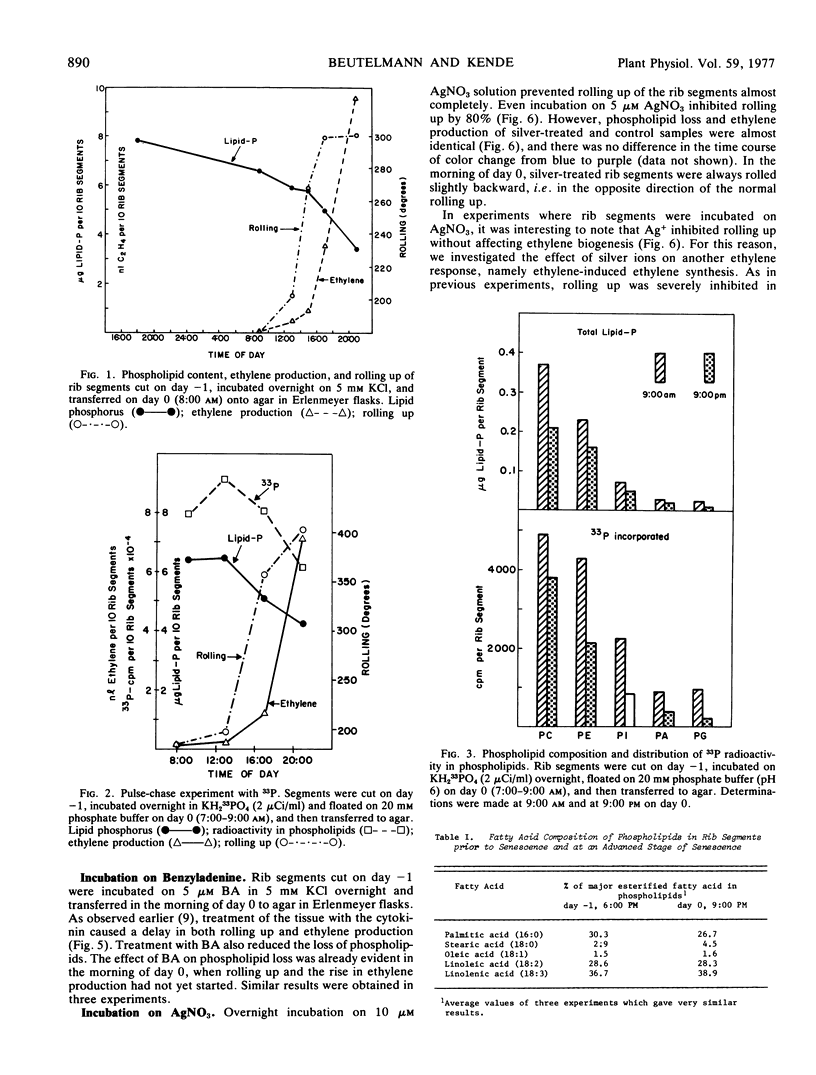
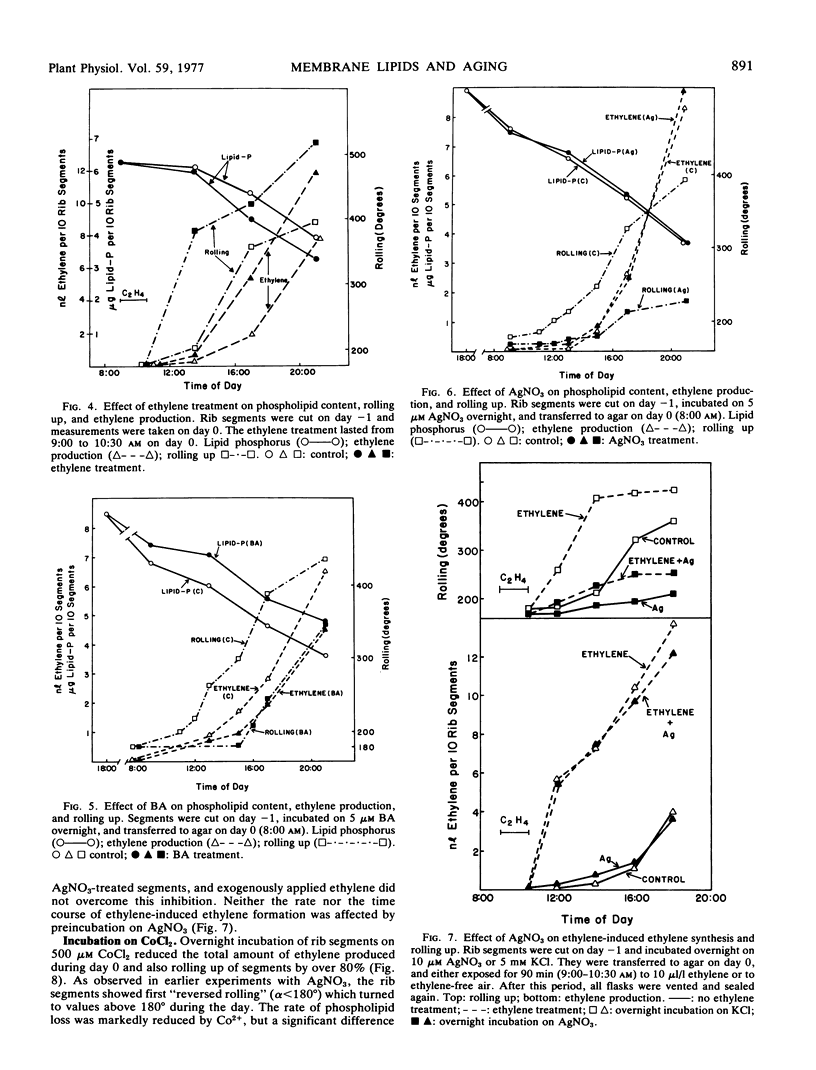
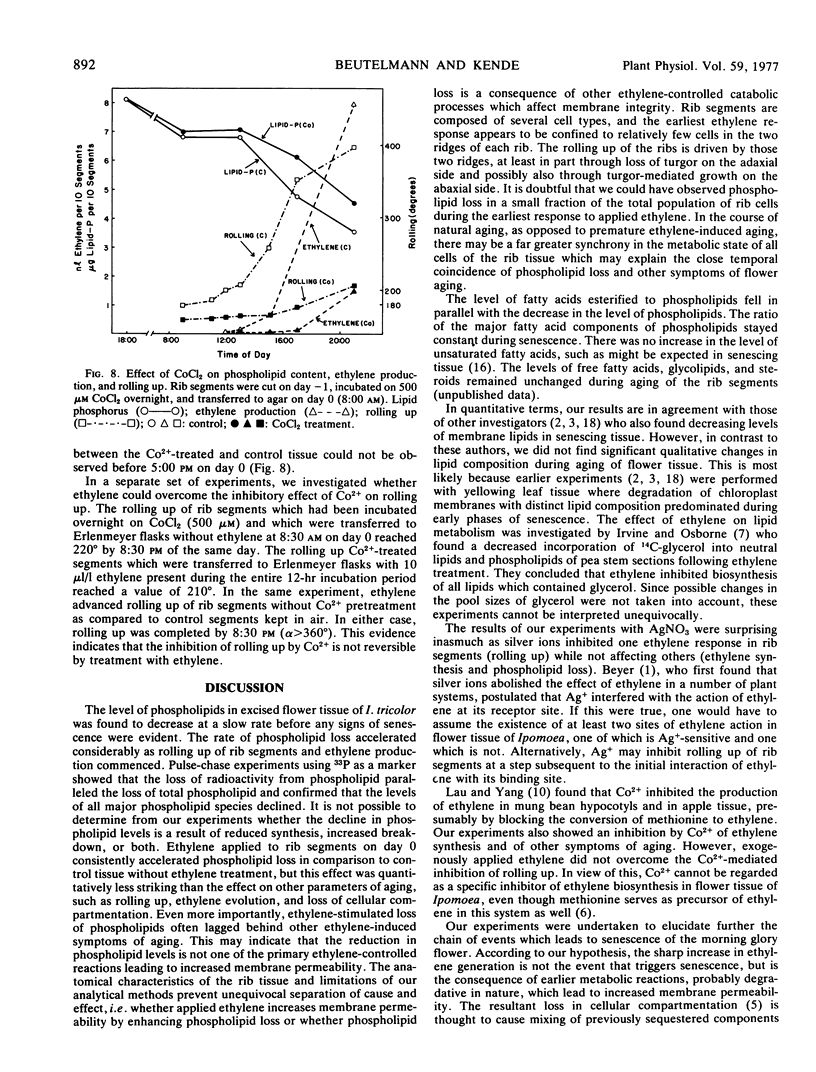
Rib segments excised from flower buds of Ipomoea tricolor Cav. pass through the same phases of senescence as the respective tissue on the intact plant. Such segments were used to correlate changes in lipid content with known symptoms of aging, such as rolling up of the ribs and ethylene formation. It was found that the level of phospholipid had already started to decline before visible signs of senescence were evident. As the segments began to roll up and to produce ethylene, the rate of phospholipid loss accelerated sharply. During the same period, the level of fatty acids esterified to phospholipids also fell by 40%. No qualitative changes in any lipid component could be detected during senescence. Labeling experiments using 33P as marker showed that the rate at which radioactivity was lost from phospholipids during aging was parallel to the rate at which the level of total phospholipids declined. Exogenously applied ethylene accelerated the loss of phospholipid and the senescence of rib segments while benzyladenine retarded both of these processes.
Ag+, which counteracts the effect of ethylene in many plants, inhibited rolling up of the rib segments but did not affect either spontaneous and ethylene-induced ethylene generation, or phospholipid loss. In contrast, Co2+, a purported inhibitor of ethylene synthesis, reduced ethylene production, rolling up, and phospholipid loss. The inhibition of ethylene-induced rolling up by Co2+ could not be overcome with exogenous ethylene, however.
Our results indicate that phospholipid loss is a marker for membrane degradation in rib segments. Changes in membrane integrity and in cellular compartmentation may be the basis for ethylene synthesis during aging of flower tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. M. A potent inhibitor of ethylene action in plants. Plant Physiol. 1976 Sep;58(3):268–271. doi: 10.1104/pp.58.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Ethylene-enhanced Ion and Sucrose Efflux in Morning Glory Flower Tissue. Plant Physiol. 1975 Apr;55(4):663–669. doi: 10.1104/pp.55.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Methionine metabolism and ethylene biosynthesis in senescent flower tissue of morning-glory. Plant Physiol. 1976 Apr;57(4):528–537. doi: 10.1104/pp.57.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Osborne D. J. The effect of ethylene on (1-14C)glycerol incorporation into phospholipids of etiolated pea stems. Biochem J. 1973 Dec;136(4):1133–1135. doi: 10.1042/bj1361133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Inhibition of ethylene production by cobaltous ion. Plant Physiol. 1976 Jul;58(1):114–117. doi: 10.1104/pp.58.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]


