Abstract
Objective: This study used the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) method to evaluate the percentage of antioxidant activity (%AA) of green tea (GT) and sodium ascorbate (SA) gel in three concentrations (10%, 20% and 30%), and the influence of these concentrations on the microshear bond strength (μ-SBT) values of bleached enamel, using 10% carbamide peroxide (CP).
Materials and methods: Eighty intact third molars were selected to perform the μ-SBT test, and were randomly divided into eight experimental groups: G1: positive control – no treatment; G2: negative control – bleached with CP; G3: PC +10% GT; G4: PC +20% GT; G5: PC +30% GT; G6: PC +10% SA; G7: PC +20% SA and G8: PC +30% SA. After applying the treatments, adhesive procedures were performed using Single Bond 2 and Filtek Z350XT. After 24 h, the samples were tested in a universal testing machine until fracture. The %AA was assessed in triplicate by DDPH method using a UV–VIS spectrophotometer.
Results: In the μ-SBT, ANOVA revealed no significant difference between the antioxidants evaluated (p = .625), but did reveal significant effects of the concentrations studied (p = .007). A negative correlation was observed between μ-SBT and solution concentrations. The values of %AA were from 90.58 to 96.75.
Conclusions: The reverse results occurred in μ-SBT values, only when the antioxidants were used in a 10% concentration and, %AA did not influence μ-SBT.
Keywords: Enamel, adhesion, dental bleaching, green tea, antioxidant activity
Introduction
Several studies have shown that carbamide and hydrogen peroxide may adversely affect the bond strength of composite to tooth structure when adhesive procedures are performed immediately after the bleaching treatment.[1–3] On the other hand, a waiting time of 24 h to two weeks after bleaching procedures restores the bond strength.[2,4,5] Nonetheless, the time requirement of today’s patients, who need ever-quicker treatments, must be taken into consideration, making the waiting time recommended by the literature impractical in some cases, and leaving the use of antioxidants as the indicated procedure. Antioxidants react with the free radicals that remain in the tooth structure after the bleaching treatment, removing them from the structure and increasing the bond strength of restorative dental material to substrate.[6]
A method used to resolve this problem involves the use of antioxidants or reducing agents. Consistent studies have been published recently that evaluate these antioxidants, including sodium ascorbate (SA),[7] grape seed extract,[8] alpha-tocopherol (vitamin E),[9] catalase, sodium bicarbonate,[10] ethanol,[11] acetone,[12] and, more recently, green tea (GT).[13,14] A study by Berger et al. [13] showed that 10% GT in gel form presents similar results to 10% SA, and concluded that this could be an alternative to reversing the bond strength values for bleached teeth; however, the antioxidant power of GT has not yet been verified.
Many studies have evaluated the effect of antioxidants on the bond strength of bleached teeth. However, evidence lacks on the antioxidant activity of GT as an option to overcome the reduced bond strength of bleached teeth, as compared with the antioxidant activity of SA. This study used the DDPH method to evaluate the percentage of antioxidant activity (%AA) of GT and SA, in different concentrations (10%, 20% and 30%), in correlation with the bond strength values of enamel bleached with 10% carbamide peroxide (CP). The hypothesis tested is that the higher the concentration of antioxidant, the greater the percentage of antioxidant activity, and, consequently, the higher the bond strength values.
Materials and methods
Sample preparation and experimental groups
The study was submitted to and approved by the Ethics Committee under protocol # 537731 (University of North Parana). Eighty extracted intact human third molars were selected for this study. The teeth were stored in 0.1% thymol solution until use. The root portion was removed and the crown was sectioned to yield two non-occlusal enamel surfaces. A total of 80 enamel specimens were obtained; only the buccal surfaces were used. The samples were embedded in an acrylic resin block, keeping only the enamel surface exposed. The labial surfaces of the samples were polished sequentially with wet 400- and 600-grit silicon carbide papers to achieve a flat, homogeneous enamel surface, without exposing the dentin in any of the samples. After polishing, the samples were washed ultrasonically in distilled and deionized water for 10 min to remove the debris left by the sandpaper on the enamel surface. Then, the samples were randomly divided into eight experimental groups (n = 10): G1: positive control – no treatment; Group 2: negative control – bleaching; Group 3 – bleaching +10% GT; Group 4 – bleaching +20% GT; Group 5 – bleaching +30% GT; Group 6 – bleaching +10% SA; Group 7 – bleaching +20% SA and Group 8 – bleaching +30% SA.
Bleaching treatment
Before beginning the bleaching treatment, individual trays were made for each sample, using 0.4-mm-thick flexible polymer in a vacuum plasticizer (P7; Bioart Equipamentos Odontológicos, São Paulo, SP, Brazil).
For the samples from Group 1 (control group), no treatment was performed on the enamel surface. For Groups G2–G8, the bleaching agent, containing 10% CP (Opalescence, Ultradent Products Inc., South Jordan, UT), was applied on the enamel surface. Approximately 0.02 mL of bleaching gel and individual trays were placed over the specimens. The bleaching gel was performed for 6 h daily for 14 days at 37 ± 1 °C, according to the manufacturer’s instructions. In the 18 remaining hours, the samples were maintained in artificial saliva at 37 ± 1 °C. Following the bleaching treatment, the bleaching gel was removed from the samples under running water.
Application of antioxidants
Green tea or SA was used as the antioxidant agent in the concentrations described previously for Groups 3–8. Approximately 0.02 mL of 10% GT (60% catechins and 5% caffeine; Fragon, São Paulo, SP, Brazil) or 10% SA (Fragon) were applied to the samples immediately after bleaching, as described above. The individual trays were placed over the samples to prevent dilution of the antioxidant gel in the artificial saliva, as reported in previous studies,[13,14] to simulate the clinical application of this technique. The antioxidant agents were used for 1 h at 37 ± 1 °C. After 1 h elapsed, the individual molds were removed and the antioxidant agents were washed from the enamel surface under running water.
Bonding procedures and microshear bond strength (μ-SBT)
The enamel surface was conditioned with 35% phosphoric acid for 30 s (Scotchbond Etchant Gel, 3M ESPE, St. Paul, MN), washed with spray air/water for 60 s, and dried with an absorbent paper filter. The adhesive system (Adper Single Bond 2; 3M ESPE) was then applied to the conditioned enamel surface, according to the manufacturer's instructions, applying two layers and a light air jet, and was photopolymerized (radii-cal, SDI, Baywater, Victoria, Australia) for 10 s.
After application of the adhesive system, a matrix (Tygon tubing – TYG-03, Saint-Gobain Performance Plastic, Maime Lakes, FL) with an outer diameter of 2 mm, 1 mm in height and 0.8 mm in internal diameter – needed to delimit the adhesion area – was placed on the adhesive enamel surface. Composite resin (Filtek Z350, 3M ESPE) was applied in a single increment into matrix, using a calcium hydroxide applicator and light-cured for 40 s (Radii-cal), to produce the resin composite cylinders. Three cylinders of each specimen were fabricated and the mean bond strength of the three cylinders was calculated per specimen. The specimens were stored at 37 °C for 24 h. Afterwards, the tubes were removed using a #15 scalpel blade, being careful not to induce any strain in the composite.
The specimens were then secured onto a microshear device adapted to a load testing machine (EMIC DL 2000, Equipamentos e Sistemas de Ensaio, São José dos Pinhais, PR, Brazil). A thin wire (0.3 mm thick) was looped around the tooth/composite resin interface and shear force was applied at a crosshead speed of 0.5 mm/min until debonding. The values were converted to MPa by dividing the force (kgF) by the adhesive interface (cm2).
The fracture surface of each specimen, after μ-SBT, was analyzed under a stereoscopic loupe (BelMicroimage Analyser; Bel Photonics, Monza, Italy) at 40× magnification, and the type of failure was determined. The types of fractures were classified as adhesive (lack of adhesion), cohesive (failure of the tooth substrate or resin composite) or mixed (adhesive and cohesive failures).
Determination of the antioxidant activity percentage (%AA)
The %AA of each substance was assessed with the DPPH free radical assay. DPPH radical scavenging activity was measured according to the previously described methodology, with some modifications.[15,16] The samples were reacted with the stable DPPH radical in an ethanol solution. The reaction mixture consisted of adding 60 μL of the sample to 2.960 mL of 0.5 mM DPPH solution dissolved in methanol. The blank consisted of 3 mL of methanol, and the control was 2.960 mL of 0.5 mM DPPH solution dissolved in methanol and added to 60 μL of water. DPPH is reduced when it reacts with an antioxidant component, which can donate hydrogen. The changes in colour (from deep violet to light yellow) were read [Absorbance (Abs)] at 517 nm after 60 min of reaction, using a UV–VIS spectrophotometer (DU 800, Beckman Coulter, Inc.). The experiment was done in triplicate for each substance. The %AA was determined according to the equation described by Mensor et al. [16]:
Statistical analysis
The μ-SBT data and %AA were analyzed by ANOVA (two-way), followed by Tukey’s multiple comparison test, considering both the antioxidants (SA and GT) and the concentrations (10%, 20% and 30%). Dunnett’s test was used to compare the experimental groups with the control groups (G1 and G2). Pearson's correlation was performed to verify the correlation between the μ-SBT and the %AA values.
Results
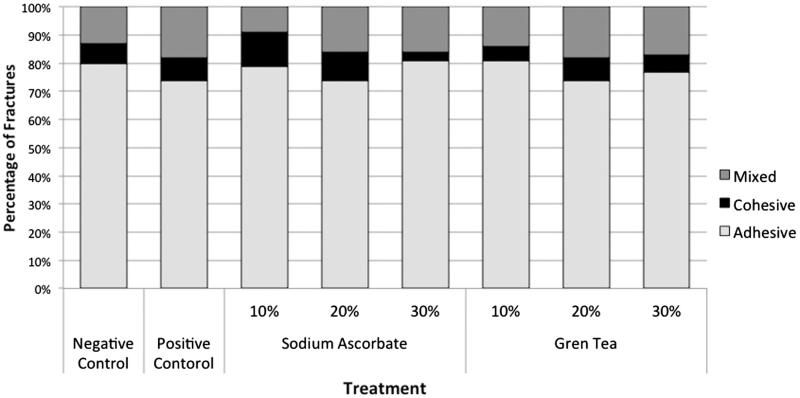
Table 1 shows the means and standard deviations of the μ-SBT measurements. ANOVA revealed no significant difference between the antioxidants (p = .625) and interaction among the factors: concentration vs. antioxidant (p = .929). However, there was a significant difference among the concentrations (p = .007). The concentration of 10% presents statistical difference to 30%, which was similarly statistical to 20% for both antioxidants. Dunnett’s test showed significant differences in μ-SBT values between the positive and negative groups and all the other groups (p = .004). In the analysis of the fracture mode, there was a predominance of adhesive failures over cohesive or mixed failures (Figure 1).
Table 1.
Means and standard deviation of bond strength (MPa).
| Antioxidants |
|||
|---|---|---|---|
| Concentrations | Sodium ascorbate | Green tea | p Values |
| 10% | 19.58 ± 4.94 A a d | 19.65 ± 4.02 A a d | .007 |
| 20% | 18.15 ± 5.40 A ab | 17.02 ± 7.8 A ab | |
| 30% | 14.39 ± 4.97 A b | 13.33 ± 5.4 A b c | |
|
p Values |
.625 |
|
|
| Positive control | 20.22 ± 3.68 | .004 | |
| Negative control | 13.15 ± 2.89 c | ||
Means followed by similar capital letters (row) are not significantly different, when comparing the antioxidant agents within each concentration using Tukey’s test (p < .05).
Means followed by similar lower-case letters (column) are not significantly different, when comparing the different concentrations of each antioxidant using Tukey’s test (p < .05).
Indicates statistically significant differences between the groups treated and the positive control, using Dunnett's test (p < .05).
Indicates statistically significant differences between the groups treated and the negative control, using Dunnett's test (p < .05).
Figure 1.
Fracture mode analysis according to experimental groups.
Table 2 shows means and standard deviation of the antioxidant activity percentage. ANOVA showed no significant difference among concentrations tested (p = .166). However, a significant difference was observed between the antioxidants (p < .001), the SA presents statistically higher values than GT in each concentration. Furthermore, an interaction was found between the concentrations and the antioxidants (p = .001).
Table 2.
Means and standard deviation of antioxidant activity percentage (%AA).
| Concentration | Antioxidants |
|
|---|---|---|
| Sodium ascorbate | Green tea | |
| 10% | 96.75 ± 1.24 Aa | 90.58 ± 1.84 Ba |
| 20% | 94.16 ± 7.51 Ab | 91.87 ± 2.22 Ba |
| 30% | 96.37 ± 0.11 Aa | 91.47 ± 0.90 Ba |
Means followed by similar capital letters (row) are not significantly different, when comparing the antioxidants agents within each concentration using Tukey’s test (p<.05).
Means followed by similar lower-case letters (column) are not significantly different, when comparing the different concentrations of each antioxidant using Tukey’s test (p<.05).
When performing the correlation between the μ-SBT and %AA values, a strong negative correlation was observed (r= −.893, p = .016) between μ-SBT and solution concentrations. It was observed that the higher the concentration, the lower the μ-SBT values.
Discussion
A decrease in bond strength immediately after tooth bleaching is a concern and of great clinical significance.[17] This decrease has been attributed to the oxygen released from bleaching agents into the tooth structure.[4] It has been reported that a delay in the bonding procedure, from 24 h to two weeks after the bleaching procedure, restores the bond strength.[2,4,5] Antioxidant agents have been used to overcome this time delay.[6,8–10,13,14] Thus, in this context, this study compared different concentrations of antioxidants and correlated with %AA. Ascorbic acid was used as the gold standard, because it has been well evaluated in the literature.[6,7,18]
The enamel shear bond strength was also evaluated after use of AS or GT in 10%, 20% or 30% concentrations. As can be seen in Table 1, there was no statistically significant difference between the antioxidants tested. It was observed that both substances in 10% or 20% concentration were more effective in overcoming the reduction in bond strength values after bleaching treatment with 10% CP. However, only when the antioxidants were used at a concentration of 10% there was a statistically significant difference in comparison with the control group.
The %AA was also evaluated using the DPPH• (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical method,[16] based on how well the antioxidants in the sample can bind with DPPH•, a stable organic radical. Technically, this is a quick method that does not detect pro-oxidizing agents, and that determines only the reducing power of the compounds analyzed.[15,16] The results of %AA, the SA and the GT showed that both antioxidants showed high %AA values, i.e. greater than 90%. However, the ascorbic acid in the three concentrations is statistically superior to GT. Moreover, no statistical difference in the results was found among the GT concentrations; however, the results for SA indicated that the 20% concentration was statistically less powerful than the 10% and 30% variations. The study by Garcia et al. [15] evaluated the %AA in various antioxidant substances using the DPPH method; the same method was used in this study. The authors also found a high %AA (76.04%) to SA gel proportion and no significant differences in effectiveness for the 10% or 20% concentrations, contrary to our findings, in which the 20% concentration was statistically lower than 10% or 30%.
The hypothesis tested in this study was rejected because a greater concentration of GT or SA did not improve the bond strength values. Moreover, there was no increase in the percentage of antioxidant activity; instead, a strong negative correlation was observed. Therefore, instead of obtaining better results using AS or GT in a 30% concentration, there was a significant reduction in the bond strength values. The antioxidants tested are presented in powder form and manufactured in gel form to make them easier to apply to the tooth. We can speculate that an increase in the concentration led to a greater amount of powder in the gel, which may have been deposited on the enamel surface, and not totally removed by etching and washing, thus yielding lower bond strength values.
The study by Kimyai et al. [19] suggests that application of antioxidants in gel form can decrease the diffusion capacity. This may have occurred when we increased the concentration of the GT and SA antioxidants. Thus, we can speculate that if the present study had tested the antioxidants in solution form, it could have obtained more satisfactory results in bond strength, because of better penetration of these test substances in solution form on the surface of the bleached enamel.
The findings of this study corroborate those by Kimyai et al., [20] in which the effect of SA in 10% and 20% concentrations, and in hydrogel form, was tested on teeth bleached with 10% CP. The authors observed that both concentrations were able to reverse the bonding strength values of the bleached enamel and that a high concentration did not lead to better results. According to Table 1, the bond strength values for 10% and 20% SA were statistically similar.
The results on bond strength, using 10% GT, corroborate previous studies,[13,14] which found that 10% GT applied for 1 h to enamel bleached with 10% CP was able to reverse the bond strength values. The same was observed in several studies using 10% SA,[6,14,21,22] which reported a reversal in enamel resistance, reverting to values similar to those obtained in enamel with no bleaching treatment.
However, the study by Sasaki et al. [9] diverges from the results obtained, insofar as the authors report that SA in either gel or solution form is inefficacious for use as an antioxidant after the bleaching treatment. This divergence in results may be attributed to the variables in the bond strength test; the load applied may influence the evaluation of the resistance.[23] Furthermore, different results may be attributed to different methods of antioxidant application. In previous studies,[23,24] the antioxidant was applied for 10 min under continuous agitation; this may increase the diffusibility of the antioxidant agent on the bleached enamel surface, whereas this study used the antioxidant in gel form for 1 h, and individual trays were placed on the enamel surface, simulating a possible clinical application, following previous studies.[13,14]
Conclusion
In conclusion, based on the results found in this study, SA and GT are more effective in a concentration of 10%, and both presented high values of antioxidant activity, which can be used to overcome the values of reduced bond strength in bleached enamel.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1. Cavalli V, de Carvalho RM, Giannini M.. Influence of carbamide peroxide-based bleaching agents on the bond strength of resin-enamel/dentin interfaces. Braz Oral Res. 2005;19:23–29. [DOI] [PubMed] [Google Scholar]
- 2. Cavalli V, Reis AF, Giannini M, et al. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent. 2001;26:597–602. [PubMed] [Google Scholar]
- 3. Dishman MV, Covey DA, Baughan LW.. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994;10:33–36. [DOI] [PubMed] [Google Scholar]
- 4. Attin T, Hannig C, Wiegand A, et al. Effect of bleaching on restorative materials and restorations – a systematic review. Dent Mater. 2004;20:852–861. [DOI] [PubMed] [Google Scholar]
- 5. Unlu N, Cobankara FK, Ozer F.. Effect of elapsed time following bleaching on the shear bond strength of composite resin to enamel . J Biomed Mater Res B Appl Biomater. 2008;84:363–368. [DOI] [PubMed] [Google Scholar]
- 6. Lai SC, Tay FR, Cheung GS, et al. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002;81:477–481. [DOI] [PubMed] [Google Scholar]
- 7. Turkun M, Kaya AD.. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J Oral Rehabil. 2004;31:1184–1191. [DOI] [PubMed] [Google Scholar]
- 8. Vidhya S, Srinivasulu S, Sujatha M, et al. Effect of grape seed extract on the bond strength of bleached enamel. Oper Dent. 2011;36:433–438. [DOI] [PubMed] [Google Scholar]
- 9. Sasaki RT, Fl Rio FM, Basting RT.. Effect of 10% sodium ascorbate and 10% α-tocopherol in different formulations on the shear bond strength of enamel and dentin submitted to a home-use bleaching treatment. Oper Dent. 2009;34:746–752. [DOI] [PubMed] [Google Scholar]
- 10. Torres CRGKA, Borges BA.. The effect of antioxidant agents as neutralizers of bleaching agents on enamel bond strength. Braz J Oral Sci 2006;5:971–976. [Google Scholar]
- 11. Sung EC, Chan SM, Mito R, et al. Effect of carbamide peroxide bleaching on the shear bond strength of composite to dental bonding agent enhanced enamel. J Prosthet Dent. 1999;82:595–599. [DOI] [PubMed] [Google Scholar]
- 12. Barghi N, Godwin JM.. Reducing the adverse effect of bleaching on composite-enamel bond. J Esthet Dent. 1994;6:157–161. [DOI] [PubMed] [Google Scholar]
- 13. Berger SB, De Souza Carreira RP, Guiraldo RD, et al. Can green tea be used to reverse compromised bond strength after bleaching? Eur J Oral Sci. 2013;121:377–381. [DOI] [PubMed] [Google Scholar]
- 14. Ozelin AA, Guiraldo RD, Carvalho RV, et al. Effects of green tea application time on bond strength after enamel bleaching. Braz Dent J. 2014;25:399–403. [DOI] [PubMed] [Google Scholar]
- 15. Garcia EJ, Oldoni TL, Alencar SM, et al. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J. 2012;23:22–27. [DOI] [PubMed] [Google Scholar]
- 16. Mensor LL, Menezes FS, Leitao GG, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. [DOI] [PubMed] [Google Scholar]
- 17. Titley KC, Torneck CD, Ruse ND.. The effect of carbamide-peroxide gel on the shear bond strength of a microfil resin to bovine enamel. J Dent Res. 1992;71:20–24. [DOI] [PubMed] [Google Scholar]
- 18. Kaya AD, Turkun M, Arici M.. Reversal of compromised bonding in bleached enamel using antioxidant gel. Oper Dent. 2008;33:441–447. [DOI] [PubMed] [Google Scholar]
- 19. Kimyai S, Oskoee SS, Rafighi A, et al. Comparison of the effect of hydrogel and solution forms of sodium ascorbate on orthodontic bracket-enamel shear bond strength immediately after bleaching: an in vitro study. Indian J Dent Res. 2010;21:54–58. [DOI] [PubMed] [Google Scholar]
- 20. Kimyai S, Valizadeh H.. The effect of hydrogel and solution of sodium ascorbate on bond strength in bleached enamel. Oper Dent. 2006;31:496–499. [DOI] [PubMed] [Google Scholar]
- 21. Lai SC, Mak YF, Cheung GS, et al. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001;80:1919–1924. [DOI] [PubMed] [Google Scholar]
- 22. Muraguchi K, Shigenobu S, Suzuki S, et al. Improvement of bonding to bleached bovine tooth surfaces by ascorbic acid treatment. Dent Mater J. 2007;26:875–881. [DOI] [PubMed] [Google Scholar]
- 23. Bulut H, Kaya AD, Turkun M.. Tensile bond strength of brackets after antioxidant treatment on bleached teeth. Eur J Orthod. 2005;27:466–471. [DOI] [PubMed] [Google Scholar]
- 24. Bulut H, Turkun M, Kaya AD.. Effect of an antioxidizing agent on the shear bond strength of brackets bonded to bleached human enamel. Am J Orthod Dentofacial Orthop. 2006;129:266–272. [DOI] [PubMed] [Google Scholar]