Abstract
This study determined the influence of coating with either petroleum jelly or light-cured varnish and storage medium on the flexural strength of glass-ionomer cements (GIC). The flexural strength of two glass-ionomer cements (Fuji Equia Fil and Ketac Molar Aplicap) was measured. Specimens (2 × 2 × 25 mm) were prepared in three groups: uncoated, coated with petroleum jelly, or coated with light-cured varnish (EquiaCoat) cured for 20 s using a cure lamp (Bluephase G2, Ivoclar Vivadent, Schaan, Liechtenstein). Specimens were stored for 1 week at 37 °C in water, artificial saliva or 20 mmol dm− 3 lactic acid, then flexural strength was determined in 3-pont bend. Data were analyzed by ANOVA and Tukey HSD post hoc test (p < 0.05). In addition, the mold was filled with water and the temperature change caused by the cure lamp was measured with a thermocouple. For both materials, storage in water gave the lowest flexural strength. It was slightly higher in either saliva or lactic acid, and was improved by coating in petroleum jelly. Specimens coated with light-cured varnish, that also involved heating with a cure lamp, gave the highest flexural strength. The heating effect of the lamp was demonstrated by the temperature rise in the water in the mold after light exposure from 21.9 (± 1.0) °C to 26.8 (± 1.0) °C. hence, sealing of GIC from aqueous media improves flexural strength. The cure lamp emitted heat, which may enhance the flexural strength of specimens coated with light-cured varnish.
Keywords: Coating, flexural strength, glass-ionomer
Introduction
Glass-ionomer cements (GIC) are widely used in the field of dentistry.[1] They are acid-base cements that set by reaction of a basic glass powder with an aqueous solution of polymeric acid. The polymer is typically polyacrylic acid, but acrylic-maleic acid copolymers are also used in commercial glass-ionomer cements.[2]
The setting process in glass-ionomers involves reaction of the glass powder with the acid solution. Ions (Ca2+ or Sr2+, plus Al3+) are released from the glass and react with the polymer to form metal polyacrylate units which act as cross-links between the polymer molecules and cause the cement to harden.[3] In addition, there appears to be a further reaction of the ion-depleted glass that forms an inorganic network.[4] There is growing evidence that this network is based on phosphate groups.[5]
The fact that glass-ionomers are water-based and set by reaction of water-soluble ions means that they are susceptible to attack by aqueous solutions before they have fully set. For this reason, they are generally coated immediately after placement by clinicians with varnishes [6] or petroleum jelly.[7,8] This prevents premature loss of network-forming ionic species and also loss of water. Recent studies suggest that protecting cements in this way may improve the mechanical properties of the cements.[9] In particular, they have shown that surface hardness of coated specimens is improved by coating, compared to specimens stored in water without any surface treatment.[9–11]
Coating of glass-ionomers is not beneficial for all properties. One important attribute is the release of fluoride, but studies have shown that fluoride release can be reduced by between 61 and 76% by coating with varnish.[10] Water loss is reduced, however, and this is desirable, as it prevents surface crazing and the development of an unsightly chalky appearance.
Glass-ionomers have been shown to interact with aqueous media in different ways.[12] For example, in mildly-acidic conditions they release increased amounts of ions compared with pure water, specifically calcium, aluminum, fluoride, silicate and phosphate.[13] In saliva, they have been found to show increased surface hardness,[14] due to adsorption of calcium and phosphate ions. There is also some evidence that the compressive strength of glass-ionomers varies when stored in different solutions [15] and hardness is reduced by exposure to pure water.[16]
The current paper is aimed at addressing some aspects of this question. The study reported has involved two commercial brands of glass-ionomer, stored in one of three different media, either coated or uncoated. Two options for coating have been explored, namely coating with petroleum jelly and coating with a light-cured varnish. Since the cure lamp emits heat, the extent to which it is able to heat water in the molds was determined. For the cured cements, the physical property examined has been flexural strength, since this is considered to be of particular clinical relevance.
Materials and methods
Both brands of glass-ionomer are capsulated and were mixed on a RotoMix apparatus (3M-ESPE, Seefeld, Germany). In a typical experiment, six specimens were prepared, each from an individual capsule that were mixed, then the contents extruded into a split mold of dimensions 2 × 2 × 25 mm. They were allowed to set for 10 minutes. In order to achieve a flat surface, a transparent foil was placed on the surface of the unset material, and the surface pressed with a glass microscope slide. The foils were removed about 3 minutes after mixing. Specimens receiving a coating were prepared in the same way, except for the application of the coating.
Three types of specimens were prepared; uncoated, coated with petroleum jelly, and coated with light-cured varnish. The coating (petroleum jelly, Vaseline®, Uniliver, or varnish, EquiaCoat®, GC) was applied 3 min after activation of GIC capsules to the side of the specimen exposed to the mold. For the varnish, the application was followed by curing for 20 s with a dental cure lamp (Bluephase G2, Ivoclar Vivadent, Schaan, Liechtenstein).
Specimens were removed from the molds and then stored in one of the three different media, namely pure water, artificial saliva or lactic acid. The artificial saliva consisted of sodium chloride (0.50 g dm− 3), sodium bicarbonate (4.2 g dm− 3), sodium nitrate (0.03 g dm− 3) and potassium chloride (0.20 g dm− 3). The lactic acid was at a concentration of 20 mmol dm− 3, as used in previous studies.[13,14] Storage times of 1 week were used throughout.
Following storage, flexural strength was determined in 3-point bend. Each specimen was gently ground with 1200 grit silicon carbide (SiC) paper then loaded into a Universal testing machine (Lrx Material Testing Machine, AMATEK Lloyd Instruments, UK) with the coated side facing the tensile zone where appropriate. The distance between the supporting points was 20 mm and specimen was fractured as a crosshead speed of 1 mm/min (Figure 1).
Figure 1.
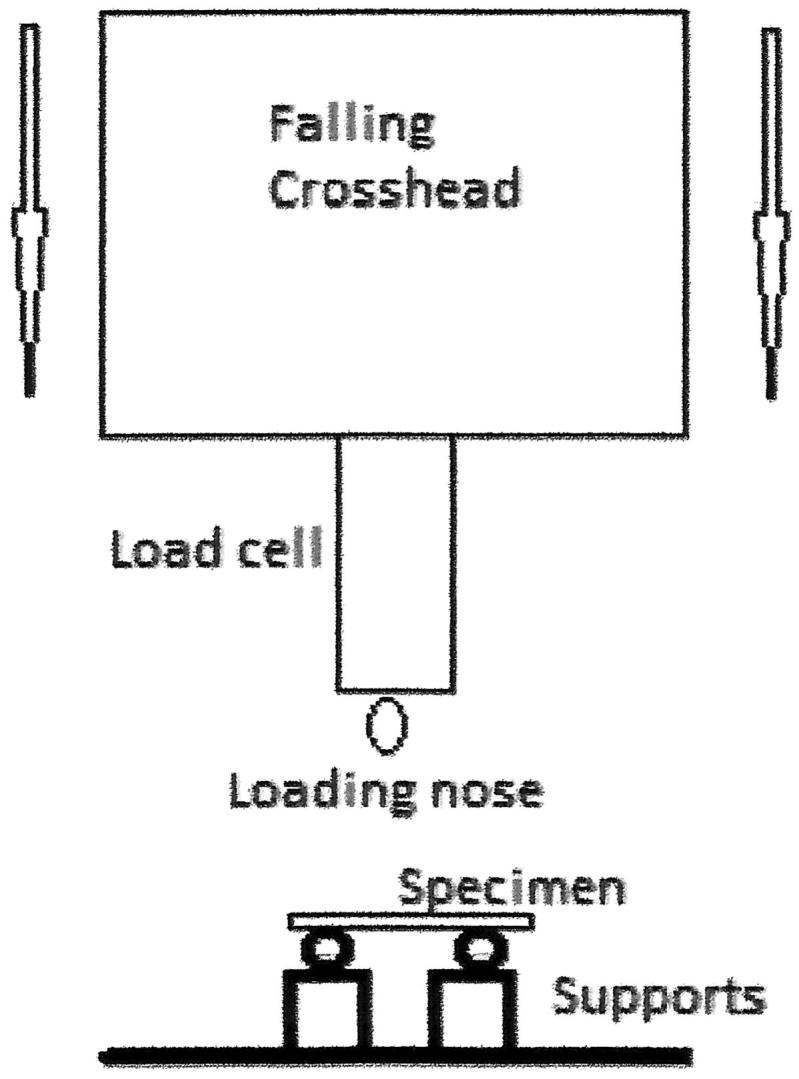
Test assembly for three-point bending test.
Data were analyzed for statistical significance by ANOVA and Tukey HSD post hoc (p < 0.05). In a separate series of experiments, the mold for specimen preparation was filled with water and exposed to the cure lamp for 20 s. This was repeated for a total of six portions of water, and the initial and final temperatures were determined in each case using a digital thermometer (TC309 K-type, Dostmann Electronic GmbH, Germany).
Results
Results for the two glass-ionomers are shown in Tables 1 and 2 for Fuji Equia and Ketac Molar Aplicap respectively. In both cases results were similar, but not identical. The specimens’ stored uncoated in water gave the lowest flexural strength in both cases. Coated specimens stored in water were significantly stronger than uncoated specimens, and specimens stored in either artificial saliva or lactic acid were also stronger. Specimens coated with varnish and light-cured were the strongest of all for both brands, and their flexural strengths did not differ significantly.
Table 1.
Mean flexural strength values for Fuji Equia with various coating treatments, stored in different media (standard deviations in parentheses).
| Storage medium | Uncoated | Coated with petroleum jelly | Coated with EquiaCoat + 20 s cure |
|---|---|---|---|
| Water | 21.8 (13.9)a | 36.9 (7.8)b | 69.7 (13.4)c |
| Artificial saliva | 37.4 (22.3)b | 33.7 (6.4)b | 53.5 (27.2)c |
| Lactic acid | 36.5 (10.9)b | 37.4 (14.8)b | 66.3 (15.8)c |
a, b, c show groups which do not differ significantly from each other. Means for groups a, b and c differ significantly (p < 0.05).
Table 2.
Mean flexural strength values for Ketac Molar Aplicap with various coating treatments, stored in different media (standard deviations in parentheses).
| Storage medium | Uncoated | Coated with petroleum jelly | Coated with EquiaCoat + 20 s cure |
|---|---|---|---|
| Water | 10.0 (3.3)a | 17.3 (4.7)c | 59.3 (19.8)d |
| Artificial saliva | 36.6 (15.4)b | 14.8 (5.0)a,c | 62.7 (16.6)d |
| Lactic acid | 31.4 (12.1)b | 35.4 (10.2)b | 60.0 (22.4)d |
a, b, c and d show groups which do not differ significantly from each other. Means for groups a, b, c and d differ significantly (p < 0.05).
For Fuji Equia, specimens coated with petroleum jelly formed a set of specimens whose flexural strength did not differ significantly. For specimens stored in artificial saliva or in lactic acid, there was no significant difference between uncoated specimens and those coated with petroleum jelly. For Ketac Molar Aplicap, by contrast, the specimens coated with petroleum jelly and stored in artificial saliva were significantly weaker in flexure than those stored in artificial saliva without being coated. There was thus some evidence that, for these specimens, coating with petroleum jelly if anything has an adverse effect on mechanical strength.
Results for the exposure of the water in the cylinder to the cure lamp are shown in Table 3. The mean final temperature was 4.9 °C higher than the mean initial temperature at 20 s, showing that the lamp exhibited a significant heating effect.
Table 3.
Mean temperatures of water samples in the cylinder before and after application of the cure lamp for 20 s (standard deviation in parentheses).
| Time/s | Temperature (°C) |
|---|---|
| 0 | 21.9 (0.9) |
| 20 | 26.8 (1.0) |
Discussion
The results show that the flexural strengths of the particular brands of glass-ionomer studied are improved by coating with varnish, followed by curing. There is also a general improvement by coating in petroleum jelly, and by storing in aqueous solutions rather than pure water.
Under the conditions used in our experiments, water loss from the specimens is not a problem. Coating is therefore not of any effect on appearance, i.e. does not prevent the phenomenon of ‘chalking’ which damages the appearance of glass-ionomers under dry conditions.[1] Rather, the effects all relate to the ability of cement-forming ions to be retained within the cement. It has been shown experimentally that ions are released from glass-ionomers into aqueous solution, and these ions are of the type that eventually become incorporated into the cement matrix and thus contribute to the final structure.[13] They include Ca2+, Al3+, silicate and phosphate.[13]
The ability of such ions to dissolve depends on the quantity of ionic species already in solution. In other words, dissolution into pure water is more extensive than into artificial saliva, which already contains dissolved ions. This suggests that, for both materials, dissolution into artificial saliva would be hindered, and more of the structure-forming ions retained by the cement. This, in turn, suggests why the resulting materials are found to be stronger. The surface undergoing tensile extension during flexure has greater structural integrity than the equivalent surface stored in pure water, and thus resists fracture to a greater extent.
The finding for specimens stored in lactic acid is less easily explained. Previous studies have shown that greater amounts of ion-release occur in lactic acid solution.[13] Against that, studies have also shown that there is an initial increase in mass of specimens of glass-ionomer cement stored in lactic acid,[17] and also that ionomer glasses are capable of forming insoluble cements with lactic acid under appropriate conditions.[18] It might be that lactic acid is able to provide additional cement-forming capability under the conditions of our experiments, and thus for specimens to develop higher flexural strengths. Whatever the explanation, the results are clear: storage in lactic acid gives specimens with improved flexural strength compared with storage in water.
Coating with petroleum jelly and varnish has known effects on ion release, having been shown to reduce it.[10] This means that, once again structure-forming ions can be retained by the cement and contribute to the strength rather than being lost by dissolution into the surrounding aqueous medium and diffusing away. In both cases, coating with petroleum jelly gave specimens with higher flexural strengths than those in pure water, though there was a surprising reduction for the jelly-coated specimens stored in artificial saliva, which we are unable to explain.
The flexural strength for specimens coated with varnish and cured is greater still, exceeding the strength of both the uncoated specimens and those coated in petroleum jelly. This may be due as much to the effect of the cure process as to the coating. The dental cure lamp used has a nominal power output of 1200 mW cm− 2, and as such generates a reasonable amount of heat, as shown by the mean increase in temperature of 4.9 °C (Table 3). Such heat would be expected to accelerate the setting reaction in the surface layers of the specimens, at least to an extent, and thus contribute to strength and structure.[19] Whether the heating effect really does contribute to the extra strength needs further investigation, but whatever the cause, coating specimens with varnish and light-curing them results in specimens with markedly improved flexural strengths.
The adverse effects of the surrounding environment on the properties of glass-ionomer cements have been known for many years. For this reason, the use of coatings, including petroleum jelly and varnishes, has long been recommended in clinical practice.[1] Our results suggest that these protective steps have the additional advantage of improving the mechanical properties of the cements, which in turn is likely to improve their long-term performance and durability. Although there is a reduction in early fluoride release as a result of coating the cements,[10] the overall effects of coating appear beneficial. The current clinical practice of protecting newly placed glass-ionomers is thus sound, and it is especially recommended that light-curable varnish be used, preferably in association with a cure lamp having high powder output and capable of causing a significant temperature rise in adjacent specimens.
Conclusion
The flexural strength of glass-ionomers is affected by both the storage medium of the specimens, and by whether or not they were coated and exposed to a dental cure lamp. Lowest values of flexural strength were obtained for specimens allowed to self-cure then stored in pure water, conditions which are known to allow relatively free dissolution of a small proportion of cement-forming ions from the surface of cements. Changing the storage medium, either to artificial saliva or to lactic acid, leads to an increase in flexural strength. In the case of artificial saliva, this can be attributed to reduced dissolution of structure-forming ions into the surrounding medium; the reason for the effect in lactic acid is unclear.
Coating specimens with petroleum jelly protects them from loss of these ions, and results in cements that are generally slightly stronger in flexure. Coating with varnish followed by light curing with a high power cure lamp leads to still greater improvement in strength, which we attribute to the combined effects of reduced loss of structure-forming ions and acceleration of curing due to heat from the lamp. However, further work is necessary to confirm that this effect is due to heating.
Overall the observed increases in flexural strength are likely to lead to improved durability in clinical service, and these findings confirm the soundness of the current clinical practice of coating freshly placed glass-ionomers immediately after placement. It also suggests that coating with light-curable varnish followed by the application of a high power output cure lamp for 20 s is of particular clinical benefit.
Disclosure statement
The authors have no conflict of interest arising from the information presented in this paper. They alone are responsible for the content and for the writing of this article.
References
- 1. Mount GJ. Color atlas of glass ionomer cement. 3rd ed. London: Mosby; 2002. [Google Scholar]
- 2. Nicholson JW, Abiden F.. Changes in compressive strength on ageing in glass polyalkenoate (“glass-ionomer”) cements prepared from acrylic/maleic acid copolymers. Biomaterials. 1997;18:59–62. [DOI] [PubMed] [Google Scholar]
- 3. Nicholson JW. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19:485–494. [DOI] [PubMed] [Google Scholar]
- 4. Wasson EA, Nicholson JW.. New aspects of the setting of glass-ionomer cements. J Dent Res. 1993;72:481–483. [DOI] [PubMed] [Google Scholar]
- 5. Shahid S, Billington RW, Pearson GJ. The role of glass composition in the behaviour of glass acetic acid and glass lactic acid cements. J Mater Sci Mater Med. 2008;19:541–545. [DOI] [PubMed] [Google Scholar]
- 6. Brito CR, Velasco LG, Bonini GACV, et al. Glass ionomer cement hardness after different materials for surface protection. J Biomed Mater Res. 2010;93:243–246. [DOI] [PubMed] [Google Scholar]
- 7. Earl MSA, Mount GJ, Hume WR.. The effect of varnishes and other surface treatments on water movement across the glass-ionomer cement surface. Aust Dent J. 1989;34:326–329. [DOI] [PubMed] [Google Scholar]
- 8. Frencken JE, Wolke J. Clinical and SEM assessment of ART high-viscosity glass-ionomer sealants after 8-13 years in 4 teeth . J Dent. 2010;38:59–64. [DOI] [PubMed] [Google Scholar]
- 9. Booth SE, Deacon AD, Coleman NJ.. Properties of glass-ionomer cements sealed with petroleum jelly or wax. World Acad Sci Eng Technol. 2012;6:886–889. [Google Scholar]
- 10. Forsten L. Fluoride release from a glass ionomer cement. Scand J Dent Res. 1977;85:503–504. [DOI] [PubMed] [Google Scholar]
- 11. Nicholson JW, Czarnecka B.. Kinetic studies of the effect of varnish on water loss by glass-ionomer cements. Dent Mater. 2007;23:1549–1552. [DOI] [PubMed] [Google Scholar]
- 12. Algera TJ, Kleverlaan CJ, Prahl-Andersen B, et al. The influence of environmental conditions on the material properties of setting glass-ionomer cements. Dent Mater. 2006;22:852–856. [DOI] [PubMed] [Google Scholar]
- 13. Czarnecka B, Limanowska-Shaw Nicholson JW.. Buffering and ion-release by a glass-ionomer cement under near-neutral and acidic conditions. Biomaterials. 2002;23:2783–2788. [DOI] [PubMed] [Google Scholar]
- 14. Okada K, Tosaki S, Hirota K, et al. Surface hardness change of restorative filling materials stored in saliva. Dent Mater. 2001;17:34–39. [DOI] [PubMed] [Google Scholar]
- 15. Nicholson JW, McKenzie MA, Goodridge R, et al. Variations in the compressive strength of dental cements stored in ionic or acidic solutions. J Mater Sci Mater Med. 2001;12:647–652. [DOI] [PubMed] [Google Scholar]
- 16. Ellakuria J, Triana R, Minguez N, et al. Effect of one-year water storage on the surface microhardness of resn-modifed versus conventional glass ionomer cements. Dent Mater. 2003;19:286–290. [DOI] [PubMed] [Google Scholar]
- 17. Nicholson JW, Amiri MA.. The interaction of dental cements with aqueous solutions of varying pH. J Mater Sci Mater Med. 1998;9: 549–554. [DOI] [PubMed] [Google Scholar]
- 18. Nicholson JW, Tawfik H, Czarnecka B. A study of cements formed by aqueous lactic acid and aluminosilicate glass. J Mater Sci Mater Med. 2002;13:417–419. [DOI] [PubMed] [Google Scholar]
- 19. Kleverlaan C, van Duinen RN, Feilzer AJ.. Mechanical properties of glass ionomer cements affected by curing methods. Dent Mater. 2004;20:45–50. [DOI] [PubMed] [Google Scholar]


