Abstract
Sudden unexpected death in epilepsy (SUDEP) is the leading cause for death in individuals with epilepsy. The frequency of SUDEP correlates with the severity of epilepsies and lack of response to antiepileptic drug treatment, but the underlying mechanisms of SUDEP have not been elucidated fully. GABRG2(Q390X) is a mutation associated with the epileptic encephalopathy Dravet syndrome (DS) and with genetic epilepsy with febrile seizures plus (GEFS+) in patients. The Gabrg2+/Q390X knockin (KI) mouse phenocopies the major features of DS and GEFS+ and has SUDEP throughout life. The Gabrg2+/− knockout (KO) mouse is associated with infrequent absence seizures and represents a model of mild absence epilepsy syndrome without increased mortality. To explore the basis for SUDEP in DS and GEFS+, we compared mutant γ2 subunit and wild-type α1 and β2/3 subunit expression in mice in brainstem nuclei associated with respiratory function including the solitary tract, pre-Botzinger complex and Kolliker-Fuse nuclei. We found that synaptic GABAA receptors were reduced while intracellular nonfunctional γ2(Q390X) subunits were increased in the heterozygous DS and GEFS+ KI mice, but not in the heterozygous absence epilepsy KO mice. Given the critical role of these nuclei in cardiorespiratory function, it is likely the impaired GABAergic transmission and neuronal dysfunction in these brainstem nuclei are involved in the cardiorespiratory collapse in SUDEP. The study provides novel mechanistic insights into cardiorespiratory failure of SUDEP.
Keywords: γ2 subunit, Gabrg2+/Q390X knockin (KI) mice, Gabrg2+/− knockout (KO) mice, epileptic encephalopathy, brainstem, GABAA receptors, protein accumulation
Introduction
Up to 70% of patients with epilepsy are well controlled with existing therapeutic regiments(Schmidt, 2009) and have a full lifespan. About one third of patients with epilepsy are refractory to current treatments and have a high incidence of unexpected death without apparent medical cause, referred to as sudden unexpected death in epilepsy (SUDEP), which is the leading cause for death in individuals with epilepsy(Shorvon and Tomson, 2011). The potential cellular mechanisms underlying SUDEP have not been elucidated fully, although several potential pathomechanisms have been proposed. The leading hypothesis suggests that defects in the cardiorespiratory system contribute to development of SUDEP. The findings from Scn1a+/− knockout (KO) mice suggest that both altered cardiac myocyte excitability(Auerbach et al., 2013) and parasympathetic hyperactivity following tonic-clonic seizures(Kalume et al., 2013) contribute to development of SUDEP. In KV1.1 deficient mice, a mouse model of SUDEP, it has been demonstrated that cardiac dysfunction by autonomic neural mechanisms is independent of cardiac potassium channel expression(Glasscock et al., 2010). A recent exciting study indicated that brainstem spreading depolarization may be a critical threshold event leading to SUDEP during a seizure(Aiba and Noebels, 2015). Additionally, there are other mechanisms associated with the cardiorespiratory collapse during seizures, such as a defect in the serotonergic system(Tupal and Faingold, 2006), ictal adenosine release(Shen et al., 2010) and changes in autonomic output(Druschky et al., 2001).
Epilepsy is a highly heterogeneous disorder, and it is possible that there are multiple pathophysiological factors underlying the cardiorespiratory failure in epilepsy. With genetic advances and availability of deep genetic sequencing data, many genes have been identified that are associated with the severe epileptic encephalopathies. These genes include, but are not limited to, SCN1A, SCN2A, SCN1B, KCNQ2, GABRG2, GABRA1, GABRB3, STXBP1, CDKL5 and ARX (Deprez et al., 2009). GABAA receptor subunit mutation is one of major causes for epilepsy, and a few GABAA receptor subunit genes like GABRG2, GABRA1 and GABRB3 are associated with epileptic encephalopathies such as Dravet syndrome and Lennox-Gastaut syndrome. To date, there is no demonstration that impaired GABAergic synaptic transmission is associated with the pathophysiology of SUDEP. This is largely because there has been no available animal model of GABAergic dysfunction and SUDEP. Additionally, studies of SUDEP have focused on cardiac electrophysiology and seizures in the cortex; there is little evidence available during SUDEP about the status of neurons in the brainstem, which mediate central autonomic function including cardio-respiratory function. It is also unclear why SUDEP is not associated with mild epilepsy syndromes like generalized absence epilepsy.
Here we compared GABAA receptor expression and mortality of two genetically modified mice, Gabrg2+/Q390X knockin (KI)(Kang et al., 2015) and Gabrg2+/− KO mice associated with different human epilepsy syndromes. The KI mouse is associated with a severe epileptic encephalopathy(Kang et al., 2015), while the KO mouse has been reported to be associated with a mild epilepsy only in mice with a seizure-prone genetic background(Crestani et al., 1999;Reid et al., 2013). We have recently demonstrated that the heterozygous KO mice in a seizure-resistant background (C57BL/6J) also had increased frequency and duration of spike (Warner et al., in revision) wave discharges (SWDs), suggesting that the KO mice could serve as a mild epilepsy absence model (Warner et al., in revision). In this study, both KI and KO mice were bred in the most seizure-resistant genetic background, C57BL/6J. We demonstrated that in the C57BL/6J background, KI mice had increased mortality while KO mice had normal survival compared with their wild-type littermates. Important for cardiorespiratory function and SUDEP, GABAA receptor expression was different in the brainstem deep nuclei of KI and KO mice.
Methods
Mice
The Gabrg2+/Q390X KI and Gabrg2+/− KO mouse lines in C57BL/6J were recently developed or bred as reported in the previous study(Kang et al., 2015). Mice in both the mixed and congenic C57BL/6J backgrounds were included for the study of postnatal day 0 mortality. Mixed background represents a mixture of S129/SVJ and C57BL/6J genetic backgrounds. The C57BL/6J mice had a pure congenic genetic background, and these mice have been bred into a pure C57BL/6J background for at least 8–10 generations. The mice used for immunohistochemistry studies were between 2–4 months old and had a congenic C57BL/6J background.
Brain sectioning and immunohistochemistry
The brains were prepared with short fixation11 and sectioned at 30 μm on cryostat. The brainstems were cut coronally, and the sections around the levels of interaural 5.3 mm and Bregma 14.4 mm and of interaural 2.3 mm and Bregma 11.3 mm were taken for staining. The staining procedures, antibodies and methods for quantification were described in our previous study11.
Statistics
The fluorescence intensity values were quantified by using ImageJ, and the fluorescence intensity raw values were measured. Data were expressed as mean ± S.E.M values and analyzed with Graphpad Prism 5.0 software. All analyses used an alpha level of 0.05 to determine statistical significance by a Student’s unpaired t test.
Results
SUDEP is generally associated with intractable epilepsies, like epileptic encephalopathies, and not associated with mild epilepsy syndromes, like absence epilepsy. Since the KI and KO mice are associated with epilepsy syndromes with different severities, we first determined the survival of wild-type, heterozygous and homozygous KI and KO mice at postnatal day 1 (P0). At P0, all homozygous KI and homozygous KO mice in the C57/B6 background died within a few hours. Only one out of 45 homozygous KO mice in a mixed background survived for 2 weeks; the surviving homozygous mouse was undersized and had impaired grooming and an uncoordinated gait. Autopsy on KI pups (n = 3) did not identify major defects in cardiorespiratory systems, but pulmonary edema was found. The KI pups often were bluish indicating reduced oxygenation. For the heterozygous mice, however, only the KI mice had increased mortality at P0 for both mixed (12 ± 3%) and congenic C57BL/6J (15 ± 4%) backgrounds (Figure 1A), while the heterozygous KO mice had a survival rate similar to their wild-type littermates (Figure 1B).
Figure 1. Heterozygous Gabrg2+/Q390X knockin mice but not Gabrg2+/− knockout mice had increased mortality, and neither homozygous mouse was viable.
(A, B) The percent death on the first postnatal day was plotted for Gabrg2+/Q390X knockin (KI) and Gabrg2+/− knockout (KO) mice in mixed C57Bl/6J/129Svj or pure C57Bl/6J background. (C) Heterozygous KI mice had spontaneous death throughout the first 30 postnatal weeks. (D) Heterozygous KO mice had a normal life span that was similar to that of their wild-type littermates.
Next we determined the survival rate of wild type and heterozygous KI and KO mice for 30 weeks; we did not determine the survival rate of homozygous mice because virtually none of them were viable. About 27% of KI mice had spontaneous death within 30 weeks without apparent cause (Figure 1C). In contrast, the heterozygous KO mice had a normal survival rate (Figure 1D). We hypothesized that the spontaneous deaths were associated with seizures, because we have observed several mice that died after generalized tonic clonic seizures during routine handling. Although we did not determine if any mice died during seizures, the mice did not have any other cause of death. We have performed autopsies on two of the dead mice, and no gross abnormalities were identified except pulmonary edema. We therefore conclude that the increased mortality in the KI mice was due to SUDEP.
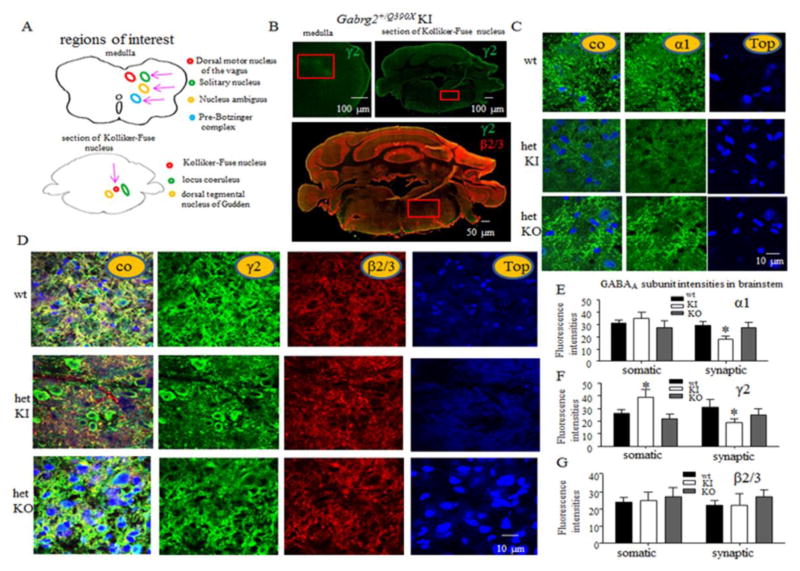
What is the basis for the increased mortality in KI, but not KO, mice? The γ2 subunit is ubiquitously expressed in the brain including the brainstem, the center for cardiorespiratory function, and is critical for GABAA receptor clustering(Alldred et al., 2005). We have previously determined the expression of remaining wild-type γ2 subunits and α1 subunits were reduced in multiple brain regions including cortex, hippocampus, cerebellum and thalamus (Kang et al., 2015). Here we determined expression of GABAA receptor subunits in the brainstem of wild-type and heterozygous KI and KO mice. Respiratory neurons are concentrated in the nucleus of the solitary tract (NTS), nucleus ambiguus, pre-Botzinger complex (preBotC), retrotrapezoid nucleus, Kolliker-Fuse nucleus and other regions of the lower brainstem(Feldman et al., 2003) (Figure 2A). We could not identify each specific nucleus but did identify neurons in some of the related regions including solitary nucleus, preBotC and Kolliker-Fuse nucleus (Figure 2B). We found that the GABAA receptor subunit expression pattern was similar in the region of the medulla containing the solitary nucleus and in the region containing the Kolliker-Fuse nucleus. Thus only GABAA receptor subunit expression in medulla was reported. Our data indicated that synaptic α1 (p = 0.01), and γ2 subunits were reduced (p = 0.03), but somatic γ2 subunits were increased (p = 0.04) in heterozygous KI, but not in KO, mice while there was no change in the expression of β2/3 subunits in either somatic or synaptic region in the KI or KO mouse brainstem. As we have previously demonstrated, the mutant γ2 subunits were retained in the endoplasmic reticulum and were nonfunctional. The increased presence of the intracellular, nonfunctional γ2 subunits suggests more reduction of GABAergic neurotransmission in KI, than in KO, mice.
Figure 2. Both Gabrg2+/Q390X KI and Gabrg2+/− KO mice had a loss-of-function allele, but only heterozygous KI mice had nonfunctional GABAA receptor γ2 subunit accumulation and reduced GABAA receptor expression in brainstem neurons critical for respiration.
(A) Cartoons illustrate the deep brainstem nuclei, which are critical for respiration, and adjacent brain structures. Pink arrows point to the structures critical for respiration that were inspected in the study. (B) Representative images of the medulla (right side of the brainstem) and a brainstem section containing the Kolliker-Fuse nucleus from a two-month-old heterozygous KI mouse stained with rabbit anti-γ2 subunit antibody without or with mouse anti-β2/3 subunit antibody. Red box represents the inspected areas in the medulla and the brainstem section containing Kolliker-Fuse nucleus. (C–G) Images presented in C and D were obtained from the boxed region in the medulla shown in B. Freshly prepared brain sections from 2 month-old wild-type KI and KO mice were immunostained with antibody to α1 subunit (C) or γ2 and β2/3 subunits (D) and visualized with Alexa-488 (green) and Rhodamine (red) fluorophores under confocal microscopy. The nuclei were stained with To-pro-3 (blue). Fluorescence intensities of α1 (E), γ2 (F) and β2/3 (G) subunits in medulla from wild-type and heterozygous KI and KO mice were quantified (unpaired t test; wt versus het for 2 months old; *p < 0.05 vs wt, (n = 8 independent immunostaining experiments for 6 pairs of mice; error bars represent S.E.M.).
Discussion
The pathophysiology of SUDEP has been elusive, in part because it is multifactorial involving intractable seizures, changes in autonomic tone, respiratory dysregulation and cardiac arrhythmias(Shorvon and Tomson, 2011). Comparison of the cellular changes in neurons mediating cardiorespiratory function in the brainstem in the severe epilepsy and mild epilepsy syndromes that are associated with the same gene may provide novel insights into the pathophysiology of SUDEP because it is comorbid with severe, but not mild, epilepsy syndromes. It is very likely that the spontaneous death observed in the Gabrg2+/Q390X KI mice is SUDEP. The death of the mice was sudden and unexpected. There was no traumatic injury or any other obvious medical conditions that could cause death. In several cases, we have noticed generalized tonic seizures during routine handlings and the mice died afterwards.
We first compared the survival rates of mice with severe (heterozygous Gabrg2+/Q390X KI mice) and mild (heterozygous Gabrg2+/− KO mice) epilepsy syndromes from 1 day to 30 weeks postnatal. The KI heterozygous mice represented severe epilepsies, because it is associated with Dravet syndrome, while the KO heterozygous mice represented mild epilepsies because it is associated with absence seizures. We demonstrated that increased mortality was associated only with the severe epilepsy KI mice but not with the mild absence epilepsy KO mice, although both mice had impaired γ2 subunit function. Neither homozygous KI nor KO mice survived, dying soon after birth. In contrast, heterozygous KO mice had a normal survival rate throughout life, and heterozygous KI mice associated with epileptic encephalopathy had reduced life span, with about 27% of mice dying during the first 30 weeks of life.
The findings from the homozygous pups suggested that the γ2 subunit is essential for the function of neurons controlling breathing as most of the pups were cyanotic and all of the homozygous pups failed to survive. We demonstrated reduced functional GABAA receptor expression in the brainstem nuclei of heterozygous KI, but not KO, mice. This may be why heterozygous KI mice are associated with SUDEP and suggests cardiorespiratory failure during seizures in KI, but not KO, mice. The sites of normal respiratory control are in the medulla and pons. The neurons that generate and shape the respiratory motor output are concentrated in the nucleus of the solitary tract (NTS), nucleus ambiguous, preBotC complex, retrotrapezoid nucleus, Kolliker-Fuse nucleus, and the other regions of the lower brainstem(Feldman et al., 2003). Our findings indicated that there is impaired GABAA receptor expression and accumulation of nonfunctional mutant γ2 subunits inside neurons that are critical for respiration in the KI, but not KO, mice. This may lead to dysfunction of neurons upon stress or aversive external stimulus and may explain why heterozygous KI mice are more likely to suffer from SUDEP than heterozygous KO mice.
Acknowledgments
This work was supported by NIH grant NS082635 to J.Q.K and grants from Citizen United for Research in Epilepsy (CURE), Dravet syndrome foundation (DSF), Dravet.org, Vanderbilt Clinical and Translation Science Award to J.Q.K. and NS 51590 to R.L.M. Many thanks to Wangzhen Shen, Joe Liu and Kelienne Verdier for their excellent assistance with mouse colony maintenance and genotyping. Special thanks to Dr. Ariel Deutch for consultation on brainstem anatomy. The authors have no conflicts of interest to report.
Footnotes
Suggested referees:
Franck Kalume (an expert in SUDEP) Departments of Pharmacology and Neurosurgery, University of Washington, fkalume@uw.edu,
Carolyn Hauser (an expert for GABAA receptor and epilepsy mouse models) David Geffen school of medicine at UCLA, Department of Neurobiology, houser@mednet.ucla.edu
Reference List
- Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med. 2015;7:282ra46. doi: 10.1126/scitranslmed.aaa4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach DS, Jones J, Clawson BC, Offord J, Lenk GM, Ogiwara I, Yamakawa K, Meisler MH, Parent JM, Isom LL. Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS One. 2013;8:e77843. doi: 10.1371/journal.pone.0077843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Deprez L, Jansen A, De JP. Genetics of epilepsy syndromes starting in the first year of life. Neurology. 2009;72:273–281. doi: 10.1212/01.wnl.0000339494.76377.d6. [DOI] [PubMed] [Google Scholar]
- Druschky A, Hilz MJ, Hopp P, Platsch G, Radespiel-Troger M, Druschky K, Kuwert T, Stefan H, Neundorfer B. Interictal cardiac autonomic dysfunction in temporal lobe epilepsy demonstrated by [(123)I]metaiodobenzylguanidine-SPECT. Brain. 2001;124:2372–2382. doi: 10.1093/brain/124.12.2372. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–5175. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 2013;123:1798–1808. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Shen W, Zhou C, Xu D, Macdonald RL. The human epilepsy mutation GABRG2(Q390X) causes chronic subunit accumulation and neurodegeneration. Nat Neurosci. 2015 doi: 10.1038/nn.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Kim T, Phillips AM, Low J, Berkovic SF, Luscher B, Petrou S. Multiple molecular mechanisms for a single GABAA mutation in epilepsy. Neurology. 2013;80:1003–1008. doi: 10.1212/WNL.0b013e3182872867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D. Drug treatment of epilepsy: options and limitations. Epilepsy Behav. 2009;15:56–65. doi: 10.1016/j.yebeh.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Shen HY, Li T, Boison D. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia. 2010;51:465–468. doi: 10.1111/j.1528-1167.2009.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011;378:2028–2038. doi: 10.1016/S0140-6736(11)60176-1. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–26. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- Warner TA, Shen W, Huang X, Liu Z, Macdonald RL, Kang JQ. Molecular Basis for Phenotypic Heterogeneity of Human Epilepsy Syndromes with Different Severities: Insights from GABRG2 Loss-of-function Mutations. J Neurosci. (in revision) [Google Scholar]