Abstract
Background
Reduced triglyceride clearance due to impaired lipoprotein lipase (LPL)-mediated lipolysis contributes to severe hypertriglyceridemia in lipodystrophy. Angiopoietin-like protein 3 (ANGPTL3) and 4 (ANGPTL4) impair clearance of triglycerides by inhibiting LPL. Whether circulating ANGPTL3/4 levels are altered in lipodystrophy and the effects of leptin replacement on these ANGPTLs are unknown.
Objective
To examine if ANGPTL3/4 levels are elevated in patients with generalized lipodystrophy and assess the effects of leptin replacement on these ANGPTLs.
Methods
Pre-leptin treatment plasma levels of ANGPTLs in patients with generalized lipodystrophy (n=22) were compared with healthy controls (n=39) using a post-hoc case-control study design. In a prospective, open-label study, we studied the effects of metreleptin therapy (16–32 weeks) on plasma ANGPTL3/4 in patients with generalized lipodystrophy.
Results
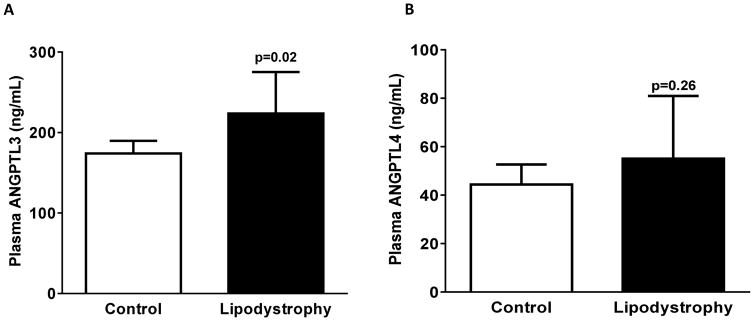
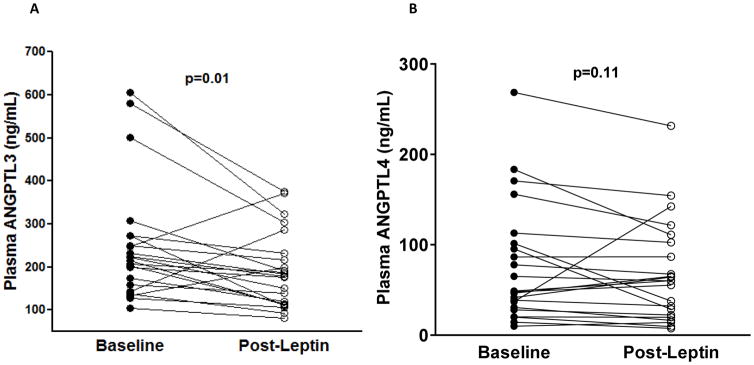
Plasma ANGPTL3 (geometric mean [95% CI]) (223 ng/mL [182,275] vs. 174 [160,189], p=0.02) but not ANGPTL4 levels (55 ng/mL [37,81] vs. 44 [37,52], p=0.26) were higher in patients with lipodystrophy compared with healthy controls. There was a significant decrease in total cholesterol, triglycerides, and glycosylated hemoglobin (A1C) levels following metreleptin therapy. Post-metreleptin, ANGPTL3 concentrations decreased significantly (223 ng/mL [182,275] vs. 175 [144,214], p=0.01) with no change in ANGPTL4 (55 ng/mL [37,81] vs. 48 [32,73], p=0.11).
Conclusions
These findings suggest that elevated plasma levels of ANGPTL3 in leptin-deficient states is attenuated with leptin therapy.
Keywords: Leptin, Angiopoietin-like protein 3, and Lipodystrophy
Introduction
Lipodystrophy, a rare disorder characterized by hypoleptinemia and partial or complete (generalized) absence of adipose tissue, is associated with insulin resistance and hypertriglyceridemia 1. Inefficient storage of circulating triglycerides due to the lack of adipose tissue, increased lipolysis, elevated hepatic fat synthesis, reduced activity of lipoprotein lipase (LPL), and impaired clearance of chylomicrons have all been proposed to play a causal role in hypertriglyceridemia2, 3. In fact, hypertriglyceridemia is a frequent and consistent (>70%) manifestation in patients with generalized lipodystrophy 4. In these patients, leptin replacement with recombinant human methionyl leptin (metreleptin), in addition to improving glycemia, causes an impressive reduction in triglycerides (> 70%) 4–6. However, the mechanisms mediating the beneficial effects of leptin are not well understood.
Circulating angiopoietin-like proteins 3 (ANGPTL3) and 4 (ANGPTL4) are secreted glycoproteins that modulate triglyceride metabolism by directly inhibiting LPL 7–9. In humans, ANGPTL3 is predominantly expressed in the liver, while ANGPTL4 is expressed in both liver and adipose tissue 10. Elegant studies in mice and genetic studies in humans confirm the important role of these proteins in triglyceride metabolism. Mice deficient in ANGPTL3 or -4 have reduced atherosclerosis and a favorable lipid phenotype 11, 12. In humans, nonsense mutations in ANGPTL3 13 and ANGPTL4 14 are associated with lower triglyceride levels and reduced risk for cardiovascular disease.
Circulating levels and hepatic expression of ANGPTL3 are increased in leptin-resistant db/db mice and leptin-deficient ob/ob mice 15. Administration of leptin to ob/ob mice decreases hepatic ANGPTL3 mRNA expression and plasma ANGPTL3 levels 15. Based on these studies and the key role played by these ANGPTLs in triglyceride metabolism, we hypothesized that ANGPTL3 and 4 would be elevated in patients with lipodystrophy prior to metreleptin replacement compared to healthy controls and that ANGPTL3 and 4 would decrease upon metreleptin replacement. To that end, in this study, we examined the levels of plasma ANGPTL3 and ANGPTL4 in healthy controls and patients with lipodystrophy prior to and during metreleptin replacement therapy.
Subjects and Methods
Study Design and Study Subjects
The study was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases and conducted at the Clinical Center of the National Institutes of Health, Bethesda, Maryland. Written informed consent/assent was obtained from all participants/guardians. We used a cross-sectional case-control design to compare plasma levels of ANGPTL 3 and 4 in healthy volunteers recruited from a study of the phenotype of overweight and obese adults (ClinicalTrials.gov identifier NCT00428987) with patients with generalized lipodystrophy enrolled in a study evaluating the effects of metreleptin therapy in patients with lipodystrophy (ClinicalTrials.gov identifier NCT00025883). The study design, rationale, and inclusion criteria for this study have been described previously 5. Patients with HIV-associated lipodystrophy were not studied in this protocol. The effects of leptin replacement on plasma ANGPTL3 and 4 levels as an exploratory outcome was examined in generalized lipodystrophy patients (n=22) treated with metreleptin (Bristol Myers Squibb and Astra Zeneca). These patients received self-administered subcutaneous metreleptin injections.
Study procedures
In healthy and lipodystrophic patients at baseline and 16–32 weeks after initiation of metreleptin, fasting blood samples were obtained to measure ANGPTL3, ANGPTL4, insulin, glucose, A1C, lipid panel, liver and renal function tests. A 75-g oral glucose tolerance test (OGTT) after an overnight fast was performed in patients with lipodystrophy. Samples for determination of plasma glucose, insulin, C-peptide at 0, 30, 60, 90, 120, and 180 min after the oral glucose load were collected. Oral Glucose Insulin Sensitivity (OGIS), a surrogate marker for insulin sensitivity was derived as previously described 16. Body composition was measured by dual energy x-ray absorptiometry (DXA) using a total body scanner (Lunar iDXA, GE Healthcare, Madison WI). Plasma ANGPTL3 and ANGPTL4 were measured by commercially-available ELISA (BioVendor, Asheville, NC). Leptin was measured by RIA using a commercial kit (Linco Research). All other measurements were done in the NIH Clinical Center laboratory using standard methodology.
Statistical analysis
After testing for normality, data were logarithm transformed where appropriate. Data are presented as means ± SD for normally distributed variables or median (interquartile range [IQR]) for non-symmetric distributions. Comparison of various parameters were performed using unpaired or paired Student’s t test for parameters with normal distribution and non-parametric equivalent of Student’s t tests for those with non-normal distribution. In tests comparing plasma ANGPTL3 and 4 levels, values are presented as geometric mean and 95% confidence intervals [95% CI]. P < 0.05 was considered to represent statistical significance. The statistical software JMP, Version 8.1 (SAS Institute, Inc., Cary, NC), was used for data analysis.
Results
Clinical Characteristics of Study Subjects
The clinical characteristics of the healthy controls and patients with lipodystrophy are presented in Table 1. In this cohort, mutations in 3 genes were identified as the cause for CGL [n=11, AGPAT2 (acylglycerol phosphate acyltransferase, isoform 2); n=6, BSCL2 (Berardinelli-Seip congenital lipodystrophy 2); and 1 homozygous recessive mutation in LMNA (lamin A/C)]. In this group of patients, lipodystrophy was generalized and either congenital (n=18) or acquired (n=4). The median baseline age of lipodystrophic patients was 17 years with a range of 4–51 years. Mean serum leptin prior to initiation of metreleptin therapy was 1.33 (1.81) ng/mL. Participants in the healthy control group were older and had a higher BMI and body fat content than patients with lipodystrophy (Table 1). As expected, patients with lipodystrophy had diabetes, dyslipidemia, and higher levels of plasma ALT and AST consistent with hepatic steatosis (Table 1). The median fasting triglyceride levels in patients with lipodystrophy was 5.5 mmol/L with a range of 1.8–62.4 mmol/L. At baseline, patients were on metformin (n=12), thiazolidinedione (n=1), sulfonylurea (n=1), dipeptidyl peptidase-4 inhibitor (n=1), and insulin (n=14). Dyslipidemia was treated with lipid-lowering agents (fenofibrate, n=8, statin, n=7, and omega-3-acid ethyl esters, n=3).
Table 1.
Clinical characteristics and metabolic parameters in patients with lipodystrophy and healthy controls
| Clinical Parameters | Lipodystrophy (n=22) | Controls (n=39) | P-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 19 ± 10 | 37 ± 12 | <0.001 |
| Sex (n=F/M) | 16/6 | 24/15 | 0.36 |
| Race (n=White/Black/Asian) | 24/12/3 | 14/6/2 | |
| BMI, kg/m2 | 21.9 ± 3.3 | 26.8 ± 4.9 | <0.001 |
| Body fat, % | 8.9 ± 2.9 | 33.0 ± 9.7 | <0.001 |
| Lipids | |||
| Total Cholesterol, mmol/L | 5.5 ± 2.3 | 4.5 ± 0.9 | 0.02 |
| LDL, mmol/L | 2.3 ± 1.3 | 2.7 ± 0.7 | 0.13 |
| ApoB 100, g/L | 0.47 (0.17) | 0.47 (0.23) | 0.98 |
| HDL, mmol/L | 0.7 ± 0.2 | 1.3 ± 0.4 | <0.001 |
| Triglycerides, mmol/L | 5.5 (8.2) | 1.1 (0.7) | <0.001 |
| Free Fatty Acids, mmol/L | 0.33 (0.54) | 0.49 (0.26) | 0.10 |
| Glucose Metabolism | |||
| Fasting glucose, mmol/L | 10.9 ± 5.1 | 4.9 ± 0.6 | <0.001 |
| Hemoglobin A1c, % | 9.1 ± 2.2 | 5.3 ± 0.4 | <0.001 |
| Fasting insulin, pmol/L | 352 (365) | 29 (43) | <0.001 |
| Hepatic enzymes | |||
| ALT, U/L | 77 (51) | 22 (12) | 0.003 |
| AST, U/L | 50 (49) | 23 (9) | 0.002 |
| Renal function | |||
| Creatinine, μmol/L | 46 ± 16 | 73 ± 18 | <0.001 |
| Urea nitrogen, mmol/L | 4.1 ± 1.4 | 4.4 ± 1.3 | 0.50 |
Values shown are unadjusted means ± SD or median (IQR). P values indicate significance between groups using unpaired Student’s t test for parameters with normal distribution and non-parametric equivalent of Student’s t tests for those with non-normal distribution. P < 0.05 was considered to represent statistical significance.
ANGPTL3 and 4 levels
In healthy controls, ANGPTL3, but not ANGPTL4 levels were lower in men [median (IQR), 151 (45) ng/mL] than in women [200 (63) ng/mL, p=0.02]. Plasma levels of ANGPTL3 were higher in lipodystrophic patients when compared with healthy controls (unadjusted geometric mean [95% CI]) (223 ng/mL [182, 275] vs. 174 [160, 189], p=0.02) (Figure 1). This difference remained significant even after adjustment for age, sex, and BMI. In contrast, unadjusted and adjusted ANGPTL4 levels were not significantly different between the groups (55 ng/mL [37, 81] vs. 44 [37, 52], p=0.26) (Figure 1). ANGPTL3 levels did not vary by sex in the lipodystrophy group (data not shown). At baseline, plasma ANGPTL3 and ANGPTL4 levels were unrelated to age, percent body fat, BMI, fasting glucose, insulin, total cholesterol, triglyceride levels, or QUICKI in either group. However, plasma HDL was positively correlated (r=0.33, p=0.03) with ANGPTL3 (but not ANGPTL4) levels in control subjects but not in the lipodystrophy group.
Figure 1.
Plasma Angiopoietin-like protein 3 (ANGPTL3) and 4 (ANGPTL4) concentrations in generalized lipodystrophy and healthy controls. Bars and error bars represent unadjusted geometric mean with 95% CI. A Mann-Whitney t test was used for group comparisons and a p <0.05 was considered statistically significant.
Effects of Metreleptin Replacement on Metabolic Parameters and ANGPTL3 and 4 levels
The starting dose of metreleptin was based on age, sex, and weight and subsequent dosing was titrated based on changes in metabolic parameters. The average daily dose of metreleptin at the end of 16–32 wk was (0.07 ± 0.02 mg/kg/day). Plasma leptin levels increased with therapy (Table 2). Metreleptin replacement was associated with a significant decrease in total cholesterol, triglyceride, and ApoB levels with no change in HDL or free fatty acid (FFA) levels (Table 2). Metreleptin therapy decreased A1C levels significantly and was accompanied by a decrease in fasting glucose levels. Insulin sensitivity was assessed by measuring the oral glucose insulin sensitivity (OGIS) index, a robust surrogate measure of insulin sensitivity derived from an oral glucose tolerance test (OGTT). Treatment with metreleptin was not associated with significant change in OGIS (pre- vs. post-treatment: 255 ± 68 vs. 282 ± 98 ml/min/m2, p=0.33). The average number of anti-diabetic medications including insulin (pre: 1.32 ± 0.72, post: 1.09 ± 0.68), lipid-lowering agents (pre: 0.86 ± 0.94, post: 0.81 ± 0.90), and anti-hypertensive medications (pre: 0.45 ± 0.59, post: 0.45 ± 0.59) did not significantly change among the lipodystrophy patients at baseline and follow-up. The percentage of lipodystrophy patients that used insulin at baseline (63%) and follow-up (50%) also did not significantly change.
Table 2.
Clinical characteristics and metabolic parameters before and following leptin replacement (16–32 weeks) in patients with lipodystrophy
| Clinical Parameters | Baseline (n=22) | Post-Metreleptin (n=22) | P-value |
|---|---|---|---|
| Serum leptin, ng/mL | 1.33 (1.81) | 18.70 (42.11) | <0.001 |
| Total daily leptin dose, mg/d | - | 4.10 (2.20) | - |
| Body composition | |||
| BMI, kg/m2 | 21.9 ± 3.3 | 20.9 ± 2.8 | <0.001 |
| Weight, kg | 58.6 ± 16.9 | 55.7 ± 14.3 | <0.001 |
| Body fat, % | 8.9 ± 2.9 | 7.7 ± 2.1 | 0.24 |
| Lipids | |||
| Total Cholesterol, mmol/L | 5.5 ± 2.3 | 4.0 ± 1.9 | 0.005 |
| LDL, mmol/L | 2.3 ± 1.3 | 1.8 ± 1.1 | 0.15 |
| ApoB 100, g/L | 0.47 (0.17) | 0.40 (0.31) | 0.05 |
| HDL, mmol/L | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.30 |
| Triglycerides, mmol/L | 5.5 (8.2) | 2.6 (2.2) | <0.001 |
| Free Fatty Acids, mmol/L | 0.33 (0.54) | 0.49 (0.26) | 0.10 |
| Glucose metabolism | |||
| Fasting glucose, mmol/L | 10.9 ± 5.1 | 8.1 ± 3.8 | 0.06 |
| Hemoglobin A1c, % | 9.1 ± 2.2 | 7.3 ± 2.1 | <0.001 |
| Fasting insulin, pmol/L | 352 (365) | 233 (428) | 0.60 |
| Hepatic enzymes | |||
| ALT, U/L | 77 (51) | 48 (32) | 0.007 |
| AST, U/L | 50 (49) | 29 (16) | 0.03 |
| Renal function | |||
| Creatinine, μmol/L | 46 ± 16 | 43 ± 14 | 0.10 |
| Urea nitrogen, mmol/L | 4.1 ± 1.4 | 3.7 ± 1.3 | 0.11 |
Leptin values shown reflect trough levels. P values indicate significance of changes in measures (post vs. pre-leptin) using paired Student’s t test for parameters with normal distribution and non-parametric equivalent of Student’s t tests for those with non-normal distribution. P < 0.05 was considered to represent statistical significance.
Post-metreleptin therapy, plasma ANGPTL3 concentrations decreased significantly (223 ng/mL [182, 275] vs.175 ng/mL [144, 214], p=0.01) with no change in ANGPTL4 (55 ng/mL [37, 81] vs. 48 [32, 73]) levels, p=0.11. Metreleptin associated changes in ANGPTL3 levels were not correlated with changes in BMI, triglyceride, total cholesterol, A1C, fasting glucose levels, or OGIS. In a Post-hoc analyses we examined the effect of metreleptin therapy on ANGPTL3 levels in acquired (n=4) and congenital generalized lipodystrophy (n=18) separately. ANGPTL3 levels were significantly lower in both acquired (269 ng/mL [137, 526] vs. 205 ng/mL [134, 311], p=0.04) and congenital generalized lipodystrophy (215 ng/mL [169, 273] vs.170 ng/mL [133, 315], p=0.02) patients following metreleptin administration.
Discussion
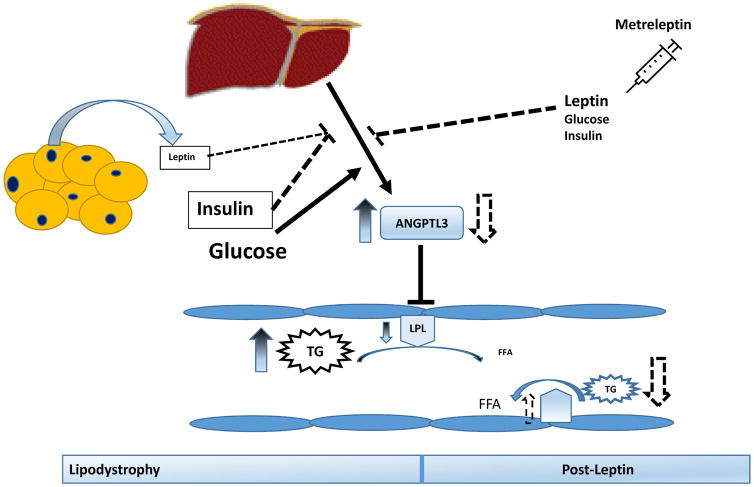
The causal mechanisms that mediate hypertriglyceridemia in lipodystrophy are unclear. Increased energy intake and ectopic lipid deposition, particularly in the liver, leading to enhanced very low density lipoprotein secretion have been proposed as primary mechanisms leading to hypertriglyceridemia. Although few small studies have suggested diminished clearance of triglyceride-rich lipoproteins in this population, the factors mediating this effect have not been previously identified. Furthermore, the effects of leptin replacement on these putative factors are also unknown. In the present study, we show that patients with generalized lipodystrophy have higher plasma concentrations of ANGPTL3 compared to healthy controls and metreleptin therapy significantly attenuates the elevated ANGPTL3 levels. Leptin administration to leptin-deficient ob/ob mice reverses the higher plasma ANGPTL3 levels 15. These findings in humans and rodents suggest that circulating ANGPTL3 is upregulated in leptin-deficient states and responsive to leptin replacement (Fig. 3). Although functionally similar to ANGPTL3, ANGPTL4 levels were not different in lipodystrophic patients and were unaltered with metreleptin therapy.
Figure 3.
Schematic of the effects of Metreleptin replacement on plasma Angiopoietin-like protein 3 (ANGPTL3) in patients with generalized lipodystrophy. ANGPTL3 inhibits lipoprotein lipase (LPL) activity. Leptin and insulin inhibits, while glucose stimulates hepatic ANGPTL3 expression. Lipodystrophy is characterized by low leptin levels, hyperinsulinemia, insulin resistance, hyperglycemia, hypertriglyceridemia, and reduced LPL activity. Metreleptin therapy increases leptin, reduces hyperglycemia, lowers triglycerides (TG), and attenuates ANGPTL3 levels.
LPL activity is a critical determinant of triglyceride clearance and plays a major role in triglyceride metabolism 7, 17. LPL activity not only varies with the fast-fed cycle, but is tissue-specific and regulated in a reciprocal fashion in the white adipose tissue and skeletal muscle. In the fed state, LPL activity is lower in skeletal muscle and higher in adipose tissue, thereby selectively increasing FFAs uptake and storage in adipose tissue 7, 18. In the fasting state, the reverse occurs and triglycerides are directed to skeletal muscle. Fasting upregulates the expression of ANGPTL4 in adipose tissue and reduces ANGPTL3 activity in skeletal muscle; these changes are reversed with refeeding 18. Thus, ANGPTL3 and 4, inhibitors of LPL play important roles in triglyceride trafficking 18.
In a small study of patients with generalized lipodystrophy (n=5), peripheral LPL activity was significantly reduced 19. LPL-mediated chylomicron clearance (measured using intravenously injected chylomicron-like emulsions labeled with 3H-triglycerides) was reduced by ~ 50–90% compared to controls. In another study of patients with congenital generalized lipodystrophy (n=4), LPL activity in the plasma was significantly lower when compared with controls (2.3±1.1 vs. 8.9±1.3 nM/100μl/h) 17. Interestingly, LPL activity in omental adipose tissue from one patient with lipodystrophy was undetectable. In this study, serum from patients with generalized lipodystrophy or healthy controls was incubated with LPL isolated from normal adipose tissue and LPL activity measured. LPL activity was significantly reduced (~50%) when co-incubated with serum from generalized lipodystrophy patients. These results suggest presence of an inhibitory factor modulating LPL activity in patients with lipodystrophy. Although, ANGPTL 3/4 levels were not measured in either of these two studies, the finding of elevated ANGPTL3 levels in lipodystrophic patients in our study raises the hypothesis that ANGPTL3 may have been the serum factor inhibiting LPL activity.
In our cohort of lipodystrophic patients, as expected, the use of glucose and lipid-lowering medications was higher compared to healthy controls. ANGPTL3 and ANGPTL4 are modulated by liver X receptor (LXR) and peroxisome-activated receptor (PPARα and γ) ligands, respectively 20, 21. Insulin is known to down regulate ANGPTL3 expression in the liver and decrease circulating levels in humans 10, 15. Thus, despite higher insulin use, which suggests severe insulin resistance lipodystrophy patients have higher ANGPTL3 levels. Similarly, TZDs and fibrates are known to upregulate ANGPTL4 levels. However, ANGPTL4 levels were not significantly different in patients with lipodystrophy compared with healthy controls. Although not demonstrated in humans, it is plausible that patients with lipodystrophy are resistant to ANGPTL3-lowering effects of insulin. As in other studies of healthy individuals, for unclear reasons, ANGPTL3 levels are lower in men when compared with women 22. Sexual dimorphism in the severity of metabolic complications has been observed in lipodystrophy with earlier onset and more severe diabetes and hypertriglyceridemia in females 1, 23. However, the lack of sexual differences in ANGPTL3 levels in patients with lipodystrophy may be due to the small sample size and over-representation of women in our cohort.
Metreleptin is approved for the treatment of generalized lipodystrophy in US and in Japan, but not in Europe. Consistent with prior reports, metreleptin therapy improves glycemia and lowers triglyceride levels in patients with lipodystrophy 4–6. Leptin replacement was associated with lowering of ANGPTL3 levels. The average number of anti-diabetic, lipid-lowering, and anti-hypertensive medications did not significantly change among the lipodystrophy patients post-metreleptin therapy. Despite this, our study design precludes us from concluding that metreleptin directly inhibits ANGPTL3 expression. It is certainly possible that improvements in insulin sensitivity may augment the inhibitory effects of insulin on ANGPTL3. However, consistent with prior reports 24, insulin sensitivity was not significantly altered by leptin replacement in the current study. In animal studies and in vitro experiments, leptin directly decreases hepatic ANGPTL3 expression and plasma levels. In leptin deficient ob/ob mice, plasma ANGPTL3 levels were elevated ~ 1.5 fold and leptin administration for 12 days decreased ANGPTL levels by 50% 15. In human hepatoma HepG2 cells, leptin exposure for 24 hours decreased ANGPTL3 mRNA expression by 61% relative to control 15. Taking these findings together, direct effects on hepatic expression may have contributed to the lower levels of ANGPTL3 post-metreleptin therapy. The change in triglyceride levels following metreleptin therapy was unrelated to change in ANGPTL3 levels. Even at baseline, we did not observe any relationship between ANGPTL3 and 4 levels and triglyceride levels in healthy or lipodystrophic patients. This is consistent with findings from other studies in healthy subjects 22, 25,26. The reasons for the lack of association are not clear. However, ANGPTL levels in these studies, as in ours, were measured in plasma samples after an overnight fast, while ANGPTL3 levels respond to re-feeding 18. These results suggest that additional factors may play a role in ANGPTL3 mediated effects on TG metabolism. ANGPTL8 is known to interact and cleave ANGPTL3, resulting in the release of the N-terminal domain which then potently inhibits LPL 18. In fact, plasma ANGPTL8 levels are positively associated with TG levels 27. Similar to ANGPTL3, ANGPTL4 expression in adipose tissue is elevated in ob/ob mice and is negatively modulated by leptin 28, 29. However, we did not see changes in ANGPTL4 levels in leptin-deficient or replete states.
We recently examined another negative modulator of LPL activity, apolipoprotein CIII patients with lipodystrophy 30. Similar to ANGPTL3, apoCIII levels are elevated in lipodystrophic patients, but unlike ANGPTL3, apoCIII levels are unaffected by leptin replacement. Thus, it appears that among the key negative modulators of LPL activity (apoCIII, ANGPTL3 and 4), only ANGPTL3 levels are responsive to leptin therapy. Whether elevated ANGPTL3 levels are causally related to hypertriglyceridemia cannot be ascertained in this study. In healthy humans, ANGPTL3 inhibition using antisense oligonucleotide technology has been shown to reduce ANGPTL3 levels by ~ 80% 31. Future studies examining the effects of anti-sense inhibition of ANGPTL3 on hypertriglyceridemia may be warranted to address the causality of elevated ANGPTL3 in the hypertriglyceridemia of lipodystrophy.
Generalized lipodystrophy is a rare disorder (1 in 10 million), therefore notwithstanding the small sample size, our findings are novel and provides insights into the pathogenesis of severe hypertriglyceridemia in this severe form of insulin resistance. These insights can be useful in understanding dyslipidemia observed in obesity-associated insulin resistance. The nature of the study population and severity of metabolic dysfunction precludes us from using the more robust, randomized, double-blind study with a control group.
In summary, we report that ANGPTL3, but not ANGPTL4, levels are elevated in lipodystrophy and that short-term leptin replacement lowers ANGPTL3 levels. Whether ANGPTL3 lowering could be a potential therapeutic target strategy to treat severe hypertriglyceridemia frequently observed in lipodystrophy is a topic of active investigation.
Figure 2.
Plasma Angiopoietin-like protein 3 (ANGPTL3) and 4 (ANGPTL4) concentrations in patients with generalized lipodystrophy before and after leptin replacement. Bars and error bars represent unadjusted geometric mean with 95% CI. Wilcoxon matched pairs signed rank test was used to test the significance of changes in plasma concentrations of ANGPTL3 (panel A) and ANGPTL4 (panel B). p <0.05 was considered statistically significant.
Highlights.
Lipodystrophy, a leptin-deficient state is associated with high triglyceride levels.
Lipoprotein-lipase mediated lipolysis is impaired in lipodystrophy.
Plasma ANGPTL3, an inhibitor of lipoprotein lipase is elevated in lipodystrophy.
Metreleptin therapy reduces ANGPTL3 levels in lipodystrophy.
Acknowledgments
This work was supported by the Intramural Research Program of NIDDK
Footnotes
ClinicalTrials.gov Identifier: NCT00025883
Disclosure Summary: The authors have nothing to declare.
Contributions: R.M. participated in the design of the study, collection of data, data analysis, and writing of the manuscript and approved the final manuscript. R.J.B., M.C.S., and E.K.C. participated in the design, conduct of the study and collection of data and approved the final manuscript. B.S.A., A.A., and M.A.W and ATR participated in the collection of data and writing of the manuscript and approved the final manuscript. P.G. participated in the design of the study, writing of the manuscript, and approved the final manuscript. R.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moon HS, Dalamaga M, Kim SY, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocrine reviews. 2013;34:377–412. doi: 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joseph J, Shamburek RD, Cochran EK, Gorden P, Brown RJ. Lipid regulation in lipodystrophy versus the obesity-associated metabolic syndrome: the dissociation of HDL-C and triglycerides. The Journal of clinical endocrinology and metabolism. 2015;99:E1676–1680. doi: 10.1210/jc.2014-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simha V, Garg A. Inherited lipodystrophies and hypertriglyceridemia. Current opinion in lipidology. 2009;20:300–308. doi: 10.1097/MOL.0b013e32832d4a33. [DOI] [PubMed] [Google Scholar]
- 4.Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. The Journal of clinical endocrinology and metabolism. 2015;100:1802–1810. doi: 10.1210/jc.2014-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17:922–932. doi: 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 7.Kersten S. Physiological regulation of lipoprotein lipase. Biochimica et biophysica acta. 2014;1841:919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Dijk W, Kersten S. Regulation of lipoprotein lipase by Angptl4. Trends in endocrinology and metabolism: TEM. 2014;25:146–155. doi: 10.1016/j.tem.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Koster A, Chao YB, Mosior M, et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 10.Nidhina Haridas PA, Soronen J, Sadevirta S, et al. Regulation of Angiopoietin-Like Proteins (ANGPTLs) 3 and 8 by Insulin. The Journal of clinical endocrinology and metabolism. 2015;100:E1299–1307. doi: 10.1210/jc.2015-1254. [DOI] [PubMed] [Google Scholar]
- 11.Adachi H, Fujiwara Y, Kondo T, et al. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochemical and biophysical research communications. 2009;379:806–811. doi: 10.1016/j.bbrc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Ando Y, Shimizugawa T, Takeshita S, et al. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. Journal of lipid research. 2003;44:1216–1223. doi: 10.1194/jlr.M300031-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. The New England journal of medicine. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewey FE, Gusarova V, O’Dushlaine C, et al. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. The New England journal of medicine. 2016;374:1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimamura M, Matsuda M, Ando Y, et al. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochemical and biophysical research communications. 2004;322:1080–1085. doi: 10.1016/j.bbrc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes care. 2001;24:539–548. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 17.Enzi G, Digito M, Baldo-Enzi G, et al. Lipid metabolism in lipoatrophic diabetes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1988;20:587–591. doi: 10.1055/s-2007-1010891. [DOI] [PubMed] [Google Scholar]
- 18.Dijk W, Kersten S. Regulation of lipid metabolism by angiopoietin-like proteins. Current opinion in lipidology. 2016;27:249–256. doi: 10.1097/MOL.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 19.Wajchenberg BL, Amancio RF, Santomauro AT, Maranhao RC. Metabolism of chylomicrons in patients with congenital lipoatrophic diabetes: a study with emulsion models of chylomicrons. Clinical endocrinology. 2004;61:347–352. doi: 10.1111/j.1365-2265.2004.02103.x. [DOI] [PubMed] [Google Scholar]
- 20.Ge H, Cha JY, Gopal H, et al. Differential regulation and properties of angiopoietin-like proteins 3 and 4. Journal of lipid research. 2005;46:1484–1490. doi: 10.1194/jlr.M500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan R, Zhang T, Hernandez M, et al. Regulation of the angiopoietin-like protein 3 gene by LXR. Journal of lipid research. 2003;44:136–143. doi: 10.1194/jlr.m200367-jlr200. [DOI] [PubMed] [Google Scholar]
- 22.Mehta N, Qamar A, Qu L, et al. Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1057–1063. doi: 10.1161/ATVBAHA.113.302802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown WV, Garg A, Gorden P, Shamburek R. JCL roundtable: Diagnosis and clinical management of lipodystrophy. Journal of clinical lipidology. 2016;10:728–736. doi: 10.1016/j.jacl.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Muniyappa R, Brown RJ, Mari A, et al. Effects of leptin replacement therapy on pancreatic beta-cell function in patients with lipodystrophy. Diabetes care. 2014;37:1101–1107. doi: 10.2337/dc13-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robciuc MR, Tahvanainen E, Jauhiainen M, Ehnholm C. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population sample. Journal of lipid research. 2010;51:824–831. doi: 10.1194/jlr.M002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung HS, Lee MJ, Hwang SY, et al. Circulating angiopoietin-like protein 8 (ANGPTL8) and ANGPTL3 concentrations in relation to anthropometric and metabolic profiles in Korean children: a prospective cohort study. Cardiovascular diabetology. 2016;15:1. doi: 10.1186/s12933-015-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao T, Jin K, Chen P, et al. Circulating Betatrophin Correlates with Triglycerides and Postprandial Glucose among Different Glucose Tolerance Statuses--A Case-Control Study. PloS one. 2015;10:e0133640. doi: 10.1371/journal.pone.0133640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon JC, Chickering TW, Rosen ED, et al. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Molecular and cellular biology. 2000;20:5343–5349. doi: 10.1128/mcb.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imran SA, Brown RE, Wilkinson M. Tissue-specific effects of valsartan on rstn and fiaf gene expression in the ob/ob mouse. Diabetes & vascular disease research. 2010;7:231–238. doi: 10.1177/1479164110369848. [DOI] [PubMed] [Google Scholar]
- 30.Kassai A, Muniyappa R, Levenson AE, et al. Effect of Leptin Administration on Circulating Apolipoprotein CIII levels in Patients With Lipodystrophy. The Journal of clinical endocrinology and metabolism. 2016;101:1790–1797. doi: 10.1210/jc.2015-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt TA, Lee RG, Digenio A, et al. ISIS-ANGPTL3RX, an antisense inhibitor to angiopoietin-like 3, reduces plasma lipid levels in mouse models and in healthy human volunteers. Atherosclerosis. 2015;241:e30–e31. [Google Scholar]