Abstract
The role of gadolinium (Gd)-based contrast agents (GBCAs) in the pathophysiology of nephrogenic systemic fibrosis (NSF) is now uncontested. While the definitive mechanism has not been established, the association with weaker GBCA ligands and with reduced renal clearance supports a hypothesis that Gd release from the GBCAs is a key process in precipitating the disease. Prevention strategies often include the use of more stable GBCA ligands in patients with reduced kidney function, but animal models and some clinical data suggest better patient outcomes can be achieved when excess ligand is administered with weaker GBCAs; this is particularly significant for OptiMARK®, which contains a nonionic, linear ligand similar to gadodiamide, the active ingredient in Omniscan®, but contains twice the amount of excess ligand. Here we review evidence regarding the use of OptiMARK® over Omniscan® for prevention of NSF, and perform a pharmacokinetic-based simulation to determine if the presented evidence is consistent with the established kinetics of GBCAs and Gd.
Keywords: gadolinium, nephrogenic systemic fibrosis, gadodiamide, gadoversetamide, kinetics
Introduction
The use of gadolinium (Gd)-based contrast agents (GBCAs) for magnetic resonance imaging has recently been associated with several clinical controversies, all of which relate to the chemical and pharmacological kinetics of these agents. In patients with normal renal function, Gd has been found deposited in the brain1, bone2 and skin3,4 and to be present in urine collections years after the GBCA should have been completely cleared, sometimes associated with painful and disorienting symptoms5,6. Similar skin retention has been observed in patients with severely reduced renal function, but to a greater extent and strongly associated with nephrogenic systemic fibrosis7,8. Retention and its outcomes in all patients is independently associated with the thermodynamic and kinetic stability of GBCAs9,10,11, resulting in a common hypothesis that the outcomes are associated with the release of Gd1010. Since GBCAs are cleared by the kidneys12, the obvious result of slowed clearance is greater residence time of the GBCAs in patients with renal dysfunction, and thus facilitating greater release of Gd.
In terms of both thermodynamic and kinetic stability of GBCAs, the nonionic, linear contrast agents (e.g., gadodiamide) rank lowest, and macrocyclic contrast agents (e.g., gadoterate) rank highest13,14. This demonstrates a theoretical benefit of macrocyclic agents, which is corroborated by clinical outcomes data15. Thus, policies seeking to reduce the incidence of NSF in populations focus on using more stable agents in patients with renal dysfunction16. But are there other approaches that do not eliminate the role of nonionic formulations such as Omniscan® (gadodiamide with 5 mol% caldiamide)17 and OptiMARK® (gadoversetamide with 10 mol% calversetamide)18? Are both agents associated with equally high risk, or does the 5% difference in the amount of excess calcium-associated ligand make a clinically relevant difference? Here we review in vitro, preclinical and clinical evidence for or against the use of OptiMARK® over Omniscan® in patients with reduced renal function, and present a simulation-based analysis to determine if the physiological and chemical kinetics of GBCAs can explain any observed differences in OptiMARK® and Omniscan® outcomes.
In vitro data
All kinetic studies are based on measurable rates, and the chemical kinetics of GBCA stability can be defined by the rates of two reactions: dissociation and association of Gd and ligand. The rate of association is first order with respect to Gd and ligand concentrations, and the rate of dissociation is first order with respect to GBCA concentration. Thus, when a GBCA is present in solution with no other ligand or Gd, dissociation is the favored reaction; any excess ligand in solution slows dissociation by increasing the rate of the reverse reaction. Both Omniscan® and OptiMARK® are formulated with excess ligand, but at only 5% and 10% the molar concentration, respectively, which would presumably have a marginal effect on release of Gd in vivo. Based on early literature from the inventors of Omniscan®, the decision to include excess caldiamide was based on a trend of improving LD50 values as the mol% excess ligand increased, which was hypothesized to be the result of reduced Gd release19; computationally, it was expected that even 1% free ligand reduced Gd release by 80%, 5% excess reduced release by a further 85% (97% total reduction), and following their trend 10% excess would result in an over 99% reduction in Gd release compared to gadodiamide without excess. An in vitro analysis of Gd release from various GBCAs in human serum corroborated the reaction rate hypothesis in that Gd dissociated from OptiMARK® and Omniscan® dissociated slower than gadodiamide and gadoversetamide, but OptiMARK® and Omniscan® did not release significantly different amounts of Gd14. When release was stimulated by excess phosphate, release rates and amounts were approximately the same between OptiMARK®, Omniscan®, gadodiamide and gadoversetamide. Thus, in vitro kinetic analyses do not support a conclusion that 10% excess is any different from 5% excess, and that strong competing reactions for Gd neutralize any effect from excess.
Fibroblast stimulation has been developed as an important in vitro model to assess how GBCAs contribute to the pathology of NSF20. One study compared the proliferation of fibroblasts from control and NSF patients incubated in different classes of GBCAs, including gadodiamide with or without excess caldiamide, and gadoversetamide without excess calversetamide21. In control patients, there was more proliferation with gadodiamide+caldiamide and gadoversetamide alone than gadodiamide alone, but in NSF patients gadodiamide alone was more proliferative than gadoversetamide, and both more so than gadodiamide+caldiamide. In general, the more stable ionic, linear GBCAs induced more fibroblast proliferation than the nonionic, linear GBCAs, which induced more proliferation than the most stable macrocyclic GBCAs in control patients; a more intuitive trend of more proliferation with decreasing stability was observed in NSF fibroblasts. The observed differences between gadodiamide and gadoversetamide, and the inversion of excess ligand trend in control and NSF fibroblasts suggest thermodynamic and kinetic stability would not be the exclusive cause of clinical outcome differences, and may perhaps be a minor factor.
Preclinical models
Bayer Schering Pharma, the manufacturer of the linear, ionic GBCA Magnevist® (gadopentetate with 0.2% molar excess of DTPA), Eovist® (gadoxetate) and the macrocyclic, nonionic GBCA Gadavist® (gadobutrol) has performed some of the most extensive rodent studies of NSF-induction from different GBCA classes and formulations22. Most of their work used healthy rats administered high daily doses of Gd formulations over a prolonged period to model the slowed clearance in patients with renal impairment23,24,25,26, but observed similar trends in nephrectomized rats27. In each study, they report macroscopic and microscopic skin lesion trends, and skin concentrations of Gd, which in all cases demonstrate a positive correlation (i.e., the greater skin concentration of Gd, the more lesions observed). In several studies, Omniscan® was compared to gadodiamide alone23,26, or OptiMARK®24,25,26,27, and in those cases there was an intuitive inverse correlation between excess ligand and skin concentration of Gd. In a key analysis, gadodiamide and gadoversetamide with 0, 5 and 10 mol% calcium-ligand were administered26. No significant differences were observed between the skin retention of each agent at the same amount of excess ligand, and the number of lesions was essentially the same (6/6 for 0 mol%, 3–4/6 for 5 mol% and 0/6 for 10 mol%). This work supports a conclusion that excess ligand independently reduces retention in some compartments, but the mechanism is uncertain based on the in vitro work from Bayer which demonstrated no difference in OptiMARK® and Omniscan® Gd release rate and amount14. This suggests that the pharmacokinetics of GBCAs, Gd and ligand are responsible for observed differences, but is the magnitude of difference expected to be the same in clinical situations? The rats were given ~5x the body surface area-based human-animal dose conversion recommended by the FDA (0.6 mmol/kg)28 daily for 4 weeks. If the hypothesized mechanism is reducing Gd release with excess ligand, administering an excessively high dose (which was used to improve detection) would poorly simulate a normal dose, because Gd acetate pharmacokinetics in some compartments are known to be dose-dependent29. Additionally, there was no assessment to determine if the rats continued to have normal renal function despite the fact that GBCAs have been associated with nephrotoxicity (though the association is stronger for ionic agents)30; it is also known that high doses of Gd acetate in male rats is associated with a biomarker of renal dysfunction (blood urea nitrogen)31, which implies that release from GBCAs would magnify any observed retention in male rats because it would itself reduce clearance, thus making these high, sequential dosing models overpowered for minor differences.
Clinical evidence
There is minimal clinical evidence directly comparing the different outcomes in human patients given Omniscan® or OptiMARK®. In clinical trials for a variety of indications, Omniscan® is associated with adverse events in 8.4% of patients32,33,34,35,36, while adverse events occur in 28.3% of OptiMARK® patients37,38. This trend is also observed in non-NSF adverse event reports submitted to the FDA, in which 8.3 events per million doses have been reported for OptiMARK®, but only 4.7 events per million doses have been reported for Omniscan®39. Despite the trends observed in common adverse events in clinical trials and reported post-marketing adverse events, we have found in our own investigation into FDA reports and a legal database that that Omniscan® is associated with 2–5-fold more reported NSF per dose than OptiMARK® (unpublished data). The known limitations of analyzing reported errors notwithstanding, if the relative rate of NSF is accepted as higher in Omniscan® compared to OptiMARK®, then the relative risk of more common adverse events in the general population with OptiMARK® must be accepted based not only on equally compelling FDA reports but also on clinical trial evidence. It may be that the etiology of NSF relates more to released Gd than more common adverse events, but whether or not the magnitude of OptiMARK® benefit is up to the level observed in error report analysis remains to be determined with stronger evidence.
Simulation
Methods
We have developed a biokinetic model of GBCAs based on a multi-compartmental model for Gd40, and the modeling of DTPA for plutonium decorporation using the Coordinated Network on RAdiation Dosimetry (CONRAD) approach41. The Gd biokinetic model and DTPA decorporation model were selected because they were built empirically from data in mammals (including human data when available) and adequately reproduced observed pharmacokinetic measurements (e.g., urinary excretion or plasma levels). The combined model in the present analysis considers the first-order, linear transport of Gd, intact GBCA and ligand across defined compartments, and the first-order, reversible binding between Gd and ligand in any compartment in which both can enter (Figure 1). The transfer constants for GBCAs were calculated from data used in a previous meta-analysis of GBCA pharmacokinetics42, and the association and dissociation rate constants were calculated from a previous analysis of GBCA dissociation in human serum14. All analyses modeled gadodiamide formulations with 0, 5 or 10 mol% excess ligand. Kidney dysfunction was modeled by reducing the blood-to-urine and blood-to-kidney elimination constants for Gd and GBCAs by the fraction of normal renal function used in the simulation (1%, 5%, 10%, 25%, 50% and 100%); excess ligand was modeled by declaring initial conditions with the administered dose and excess ligand in the blood compartment. The development and validation of this model in normal renal function will be the subject of a future report, but the present analysis includes simulated concentration-time profiles alongside those observed in patients43,44,45 for the purposes of partial validation.
Figure 1.

Diagram of the biokinetic model for gadolinium (Gd) as delivered from Gd-based contrast agents (GBCAs). The model is adapted from Taylor and Leggett (40) and the CONRAD model for DTPA for decorporation of radionuclides (41). Compartments labeled with an asterisk are permeable to free ligand and intact GBCA, and the dashed arrows were reduced by the simulated fraction of normal kidney function. GBCA compartments also allow dissociation of the intact Gd-ligand complex and association between Gd and ligand.
The simulation results were analyzed statistically by comparing the influence of excess ligand on percent Gd retained after 3 days; this time frame was selected to represent a reasonable interval between GBCA administration and hemodialysis, which was not included in the model and would confound longer-term comparisons with extremely low renal function. The percent Gd retained at day 3 was fit to a general linear model considering renal function (indexed as percent of normal multiplied by 120 mL/min; a polynomial term was also included to account for curvature), excess ligand (indexed as 0, 5 of 10), the presence of end-stage renal disease (i.e., renal function indexed at < 15 mL/min) and the interaction of ESRD and renal function (including the polynomial term) and excess percentage ligand.
Results
The concentration-time profile of Gd in plasma and amount excreted correlated well with clinical data. In plasma, excess ligand slowed apparent elimination of Gd from the plasma by retaining Gd in the blood and rapid-turnover soft tissue compartments, preventing “elimination” into other compartments (Figure 2). Over 5 days, excess ligand had a strong effect on the amount of Gd from a GBCA dose retained; the release of Gd when chelation is modeled as a reversible reaction causes curvature in the semi-logarithmic scale that is not observed when only the kinetics of intact GBCAs are considered (Figure 3). In the simulation, once the plateau is reached in the amount-excreted data, all remaining Gd is dissociated from the GBCA.
Figure 2.
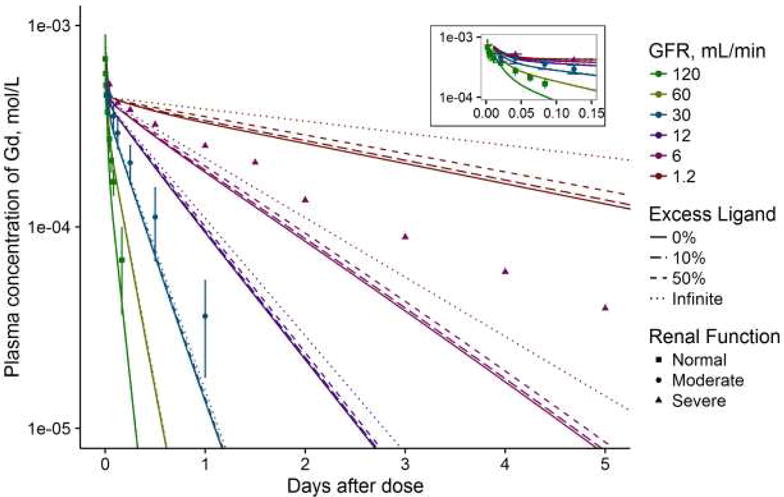
The plasma concentration-time profile of GBCAs as modeled by the simulation. Patient data from clinical trials are plotted as points with different shapes, and error bars represent the standard deviations if they were reported. Points are colored to match the approximate matching renal function in the simulation; the normal renal function data were extracted from (43) with patients whose creatinine clearances (CrCl) were reported as normal (with minimum cited as 62 mL/min), the moderate renal function data were from (44) in patients who had an average CrCl of 39 mL/min (SD 8, range 25–50) and the severe renal function data were from (45) in patients with CrCl in the range of 0.6–13.5 mL/min. The line for “infinite” excess ligand is equivalent to the model without reversible chelation (i.e., if Gd could not dissociate). The inset presents the concentration-time curve for the first ~3 hours to demonstrate early model fit.
Figure 3.

The Gd retention profile of GBCAs as modeled by the simulation. See Figure 2 for common legend description. (A) presents the complete simulation with human data, (B) is presented in log-scale to illustrate curvature in the simulation with or without Gd dissociation and (C) demonstrates how much of retained Gd is unchelated according to the simulation.
The general linear model regression demonstrated that the percent of Gd retained is more influenced by renal function than excess ligand, and that ESRD classification alters the effects of independent variables (Table 1). For up to 50% excess ligand (the maximum included in the simulation), each percentage point in associated with a 0.017 percentage point reduction in percent retained for non-ESRD patients, and a 0.063 point reduction for ESRD patients.
Table 1.
Regression coefficients for the influence of excess ligand on percent of Gd retained after three days. The coefficients are listed as estimate (standard error). The intercepts for the linear regression model (i.e., the base value altered by excess and GFR) are provided for completeness. The dependent variable is the percentage points for the retained fraction, excess is indexed as the percentage points of excess ligand in each simulation, and GFR is indexed in mL/min. The Akaike information criterion for the regression fit is 34.03.
| Variable | Non-ESRD | With ESRD-interaction | Significance of interaction term |
| (Intercept) | 8.53 (0.70) | 88.6 (1.0) | P < 0.0001 |
| Excess | –0.017 (0.006) | –0.063 (0.010) | P < 0.0001 |
| GFR | –0.13 (0.02) | –15.0 (0.1) | P < 0.0001 |
| GFR2 | 0.0006 (0.0001) | 0.716 (0.009) | P < 0.0001 |
Discussion
The simulation analysis revealed that while excess ligand helps maintain Gd in plasma and rapid-turnover soft tissue, it has a minimal effect on overall Gd retention. To extrapolate from the findings, it can be calculated that in an ESRD patient, the percent of Gd from a GBCA dose retained after 3 days will be reduced by 0.3 percentage points with 5% excess ligand (i.e., Omniscan®) and 0.6 percentage points with 10% excess ligand (i.e., OptiMARK®). Since a patient in this category with a GFR of 10 mL/min would be expected to retain ~10.2% of a dose of gadodiamide or gadoversetamide without excess ligand, 10% excess ligand would still retain 97% of what is retained with 5% excess ligand. While the magnitude of reduction in percent retained is greater in ESRD patients, the lower percent retained in non-ESRD means that if a patient with normal renal function were given 10% excess ligand, they would retain 94% of the amount they would retain with 5% excess ligand after 3 days. Nevertheless, reductions in Gd retention in all patients modeled by the simulation were not clinically significant, and in patients at risk for NSF (those with ESRD) the answer to “what difference of 5% make?” appears to be a relatively low “3%.”
Conclusions
The current evidence supports inconsistent conclusions on whether 10% excess ligand in OptiMARK® provides an absolute benefit over the 5% in Omniscan®. Chemical kinetics and pharmacokinetics fail to demonstrate an appreciable difference between the two entities, but physiological, preclinical and clinical outcomes data all find differences. The majority of methodologies support a benefit, but with notable limitations that reduce clinical relevance; the pharmacokinetic model suggesting no benefit has not been prospectively validated, and may not adequately reflect outcomes in real patients. Despite limitations of the discussed findings, there is sufficient data to encourage further investigation into these differences. However, much of the positive outcome data and in vitro work continues to support stronger linear GBCAs (e.g., gadopentetate), those with alternative clearance pathways (e.g., gadoxetate) or macrocyclic GBCAs (e.g., gadoterate) over OptiMARK® (and thus also Omniscan®). Thus, while future work should attempt to collect stronger clinical data comparing Omniscan® and OptiMARK®, its practical clinical implications may be minimal, as better strategies for gadolinium agent safety exist.
Clinical Summary.
In vitro cell culture work, animal models and FDA error reports suggest OptiMARK® is safer than Omniscan® with regard to induction of NSF, but these findings suggest highly variable magnitudes of effect (i.e., true reduced risk of NSF in using OptiMARK® rather than Omniscan®) and are limited by methodologies.
Chemical kinetics do not support a difference between Omniscan® and OptiMARK®.
A multi-compartmental model of GBCAs and Gd allowing reversible chelation can simulate observations in patients well, and suggest that Gd release from the GBCAs dictates the long-term retention in patients.
The simulation predicts that excess ligand can reduce Gd retention, but the reduction is not clinically significant, not as impactful as kidney function and of more relative importance in non-ESRD patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology. 2015 Jul;276(1):228–32. doi: 10.1148/radiol.2015142690. [DOI] [PubMed] [Google Scholar]
- 2.Darrah TH, Prutsman-Pfeiffer JJ, et al. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics. 2009 Nov;1(6):479–88. doi: 10.1039/b905145g. [DOI] [PubMed] [Google Scholar]
- 3.Roberts DR, Lindhorst SM, Welsh CT, et al. High Levels of Gadolinium Deposition in the Skin of a Patient with Normal Renal Function. Invest Radiol. 2016 May;51(5):280–9. doi: 10.1097/RLI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 4.Murata N, Murata K, Gonzalez-Cuyar LF, Maravilla KR. Gadolinium tissue deposition in brain and bone. Magn Reson Imaging. 2016 Dec;34(10):1359–1365. doi: 10.1016/j.mri.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Semelka RC, Commander CW, Jay M, Burke LM, Ramalho M. Presumed Gadolinium Toxicity in Subjects with Normal Renal Function: A Report of 4 Cases. Invest Radiol. 2016 Oct;51(10):661–5. doi: 10.1097/RLI.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 6.Burke LM, Ramalho M, AlObaidy M, Chang E, Jay M, Semelka RC. Self-reported gadolinium toxicity: A survey of patients with chronic symptoms. Magn Reson Imaging. 2016 Oct;34(8):1078–80. doi: 10.1016/j.mri.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Schäd SG, Heitland P, Kühn-Velten WN, Gross GE, Jonas L. Time-dependent decrement of dermal gadolinium deposits and significant improvement of skin symptoms in a patient with nephrogenic systemic fibrosis after temporary renal failure. J Cutan Pathol. 2013 Nov;40(11):935–44. doi: 10.1111/cup.12214. [DOI] [PubMed] [Google Scholar]
- 8.Christensen KN, Lee CU, Hanley MM, Leung N, Moyer TP, Pittelkow MR. Quantification of gadolinium in fresh skin and serum samples from patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2011 Jan;64(1):91–6. doi: 10.1016/j.jaad.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 9.Radbruch A, Weberling LD, Kieslich PJ, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015 Jun;275(3):783–91. doi: 10.1148/radiol.2015150337. [DOI] [PubMed] [Google Scholar]
- 10.Idée JM, Fretellier N, Robic C, Corot C. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: A critical update. Crit Rev Toxicol. 2014 Nov;44(10):895–913. doi: 10.3109/10408444.2014.955568. [DOI] [PubMed] [Google Scholar]
- 11.Runge VM. Safety of the Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging, Focusing in Part on Their Accumulation in the Brain and Especially the Dentate Nucleus. Invest Radiol. 2016 May;51(5):273–9. doi: 10.1097/RLI.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 12.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009 Dec;30(6):1259–67. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedeking P, Kumar K, Tweedle MF. Dissociation of gadolinium chelates in mice: relationship to chemical characteristics. Magn Reson Imaging. 1992;10(4):641–8. doi: 10.1016/0730-725x(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 14.Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann HJ. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008 Dec;43(12):817–28. doi: 10.1097/RLI.0b013e3181852171. [DOI] [PubMed] [Google Scholar]
- 15.Elmholdt TR, Pedersen M, Jørgensen B, et al. Nephrogenic systemic fibrosis is found only among gadolinium-exposed patients with renal insufficiency: a case-control study from Denmark. Br J Dermatol. 2011 Oct;165(4):828–36. doi: 10.1111/j.1365-2133.2011.10465.x. [DOI] [PubMed] [Google Scholar]
- 16.Altun E, Martin DR, Wertman R, Lugo-Somolinos A, Fuller ER, 3rd, Semelka RC. Nephrogenic systemic fibrosis: change in incidence following a switch in gadolinium agents and adoption of a gadolinium policy--report from two U.S. universities. Radiology. 2009 Dec;253(3):689–96. doi: 10.1148/radiol.2533090649. [DOI] [PubMed] [Google Scholar]
- 17.Omniscan (gadodiamide) injection. Princeton, NJ; GE Healthcare Inc.; 2012. Feb, Package insert. Distributed by GE Healthcare Inc. [Google Scholar]
- 18.OptiMARK (gadoversetamide) injection. Raleigh, NC: Liebel-Flarsheim Company LLC; 2016. Sep, Package inset. Distributed by Liebel-Flarsheim Company LLC. [Google Scholar]
- 19.Cacheris WP, Quay SC, Rocklage SM. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging. 1990;8(4):467–81. doi: 10.1016/0730-725x(90)90055-7. [DOI] [PubMed] [Google Scholar]
- 20.Edward M, Quinn JA, Mukherjee S, et al. Gadodiamide contrast agent ‘activates’ fibroblasts: a possible cause of nephrogenic systemic fibrosis. J Pathol. 2008 Apr;214(5):584–93. doi: 10.1002/path.2311. [DOI] [PubMed] [Google Scholar]
- 21.Edward M, Quinn JA, Burden AD, Newton BB, Jardine AG. Effect of different classes of gadolinium-based contrast agents on control and nephrogenic systemic fibrosis-derived fibroblast proliferation. Radiology. 2010 Sep;256(3):735–43. doi: 10.1148/radiol.10091131. [DOI] [PubMed] [Google Scholar]
- 22.Sieber MA, Steger-Hartmann T, Lengsfeld P, Pietsch H. Gadolinium-based contrast agents and NSF: evidence from animal experience. J Magn Reson Imaging. 2009 Dec;30(6):1268–76. doi: 10.1002/jmri.21971. [DOI] [PubMed] [Google Scholar]
- 23.Sieber MA, Pietsch H, Walter J, Haider W, Frenzel T, Weinmann HJ. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol. 2008 Jan;43(1):65–75. doi: 10.1097/RLI.0b013e31815e6277. [DOI] [PubMed] [Google Scholar]
- 24.Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008 Oct;18(10):2164–73. doi: 10.1007/s00330-008-0977-y. [DOI] [PubMed] [Google Scholar]
- 25.Pietsch H, Lengsfeld P, Jost G, Frenzel T, Hutter J, Sieber MA. Long-term retention of gadolinium in the skin of rodents following the administration of gadolinium-based contrast agents. Eur Radiol. 2009 Jun;19(6):1417–24. doi: 10.1007/s00330-008-1259-4. [DOI] [PubMed] [Google Scholar]
- 26.Sieber MA, Lengsfeld P, Walter J, et al. Gadolinium-based contrast agents and their potential role in the pathogenesis of nephrogenic systemic fibrosis: the role of excess ligand. J Magn Reson Imaging. 2008 May;27(5):955–62. doi: 10.1002/jmri.21368. [DOI] [PubMed] [Google Scholar]
- 27.Pietsch H, Lengsfeld P, Steger-Hartmann T, et al. Impact of renal impairment on long-term retention of gadolinium in the rodent skin following the administration of gadolinium-based contrast agents. Invest Radiol. 2009 Apr;44(4):226–33. doi: 10.1097/RLI.0b013e3181998eb7. [DOI] [PubMed] [Google Scholar]
- 28.CfDEaR UFaDA. Guidance for industry. FDA, editor. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf Accessed Jan 01, 2017.
- 29.Wedeking P, Kumar K, Tweedle MF. Dose-dependent biodistribution of [153Gd]Gd(acetate)n in mice. Nucl Med Biol. 1993 Jul;20(5):679–91. doi: 10.1016/0969-8051(93)90039-w. [DOI] [PubMed] [Google Scholar]
- 30.Perazella MA. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Curr Drug Saf. 2008 Jan;3(1):67–75. doi: 10.2174/157488608783333989. [DOI] [PubMed] [Google Scholar]
- 31.Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E. adolinium chloride toxicity in the rat. Toxicol Pathol. 1997 May-Jun;25(3):245–55. doi: 10.1177/019262339702500301. [DOI] [PubMed] [Google Scholar]
- 32.Runge VM, Armstrong MR, Barr RG, et al. A clinical comparison of the safety and efficacy of MultiHance (gadobenate dimeglumine) and Omniscan (Gadodiamide) in magnetic resonance imaging in patients with central nervous system pathology. Invest Radiol. 2001 Feb.36(2):65–71. doi: 10.1097/00004424-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Sze G, Brant-Zawadzki M, McNamara MT, et al. Use of the magnetic resonance contrast agent gadodiamide in the central nervous system. Results of a multicenter trial. Invest Radiol. 1993 Mar.28(Suppl 1):S49–S55. doi: 10.1097/00004424-199303001-00006. [DOI] [PubMed] [Google Scholar]
- 34.Aslanian V, Lemaignen H, Bunouf P, Svaland MG, Borseth A, Lundby B. Evaluation of the clinical safety of gadodiamide injection, a new nonionic MRI contrast medium for the central nervous system: a European perspective. Neuroradiology. 1996 Aug.38(6):537–41. doi: 10.1007/BF00626092. [DOI] [PubMed] [Google Scholar]
- 35.Sze G, Brant-Zawadzki M, Haughton VM, et al. ulticenter study of gadodiamide injection as a contrast agent in MR imaging of the brain and spine. adiology. 1991 Dec.181(3):693–9. doi: 10.1148/radiology.181.3.1947084. [DOI] [PubMed] [Google Scholar]
- 36.Ekholm S, Jonsson E, Sandvik L, et al. Tolerance and efficacy of Omniscan (gadodiamide injection) in MR imaging of the central nervous system. Acta Radiol. 1996 Mar.37(2):223–8. doi: 10.1177/02841851960371P146. [DOI] [PubMed] [Google Scholar]
- 37.Myhr G, Rinck PA, Borseth A. Gadodiamide injection and gadopentetate dimeglumine. A double-blind study in MR imaging of the CNS. Acta Radiol. 1992 Sep.33(5):405–9. [PubMed] [Google Scholar]
- 38.Huber S, Muthupillai R, Cheong B, et al. Safety of gadoversetamide in patients with acute and chronic myocardial infarction. J Magn Reson Imaging. 2008 Dec.28(6):1368–78. doi: 10.1002/jmri.21502. [DOI] [PubMed] [Google Scholar]
- 39.Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011 Feb;196(2):W138–43. doi: 10.2214/AJR.10.4885. [DOI] [PubMed] [Google Scholar]
- 40.Taylor DM, Leggett RW. A generic biokinetic model for predicting the behaviour of the lanthanide elements in the human body. Radiat Prot Dosimetry. 2003;105(1–4):193–8. doi: 10.1093/oxfordjournals.rpd.a006222. [DOI] [PubMed] [Google Scholar]
- 41.Breustedt B, Blanchardon E, Berard P, et al. Biokinetic modelling of DTPA decorporation therapy: the CONRAD approach. Radiat Prot Dosimetry. 2009 Feb;134(1):38–48. doi: 10.1093/rpd/ncp058. [DOI] [PubMed] [Google Scholar]
- 42.Lancelot E. Revisiting the Pharmacokinetic Profiles of Gadolinium-Based Contrast Agents: Differences in Long-Term Biodistribution and Excretion. Invest Radiol. 2016 Nov;51(11):691–700. doi: 10.1097/RLI.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 43.VanWagoner M, O’Toole M, Worah D, Leese PT, Quay SC. A phase I clinical trial with gadodiamide injection, a nonionic magnetic resonance imaging enhancement agent. Invest Radiol. 1991 Nov;26(11):980–6. doi: 10.1097/00004424-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Berg KJ, Lundby B, Reinton V, Nordal KP, Rootwelt K, Smith HJ. Gadodiamide in renal transplant patients: effects on kidney function and usefulness as a glomerular filtration rate marker. Nephron. 1996;72(2):212–7. doi: 10.1159/000188844. [DOI] [PubMed] [Google Scholar]
- 45.Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol. 1998 Jul;5(7):491–502. doi: 10.1016/s1076-6332(98)80191-8. [DOI] [PubMed] [Google Scholar]


