Abstract
Introduction
Placental FasL is up-regulated in women with HELLP (hemolysis elevated liver enzyme and low platelet) syndrome and has been proposed to contribute to the liver damage seen in these patients.
Objective
This study aimed to determine if an experimental rodent model of HELLP also had dysregulation of Fas/FasL compared to normal pregnant (NP) rats. We also set out to determine if blockade of the endothelin system regulated Fas/FasL expression in HELLP rats.
Study Design
On gestational day (GD) 12, sEng (7ug/kg) and sFlt-1 (4.7ug/kg) infusion began via mini-osmotic pump into NP rats. On GD19 plasma and tissue were collected and FasL and Fas were measured via enzyme linked immunosorbent assay and gene expression via real-time PCR.
Results
HELLP rats had significantly more circulating and placental FasL compared to NP rats, whereas hepatic FasL was decreased and placental Fas was increased compared to NP rats. Administration of an endothelin A receptor antagonist (ETA) beginning on GD12 significantly decreased placental expression of Fas in HELLP rats. Liver mRNA transcript of Fas was significantly increased in HELLP rats compared to NP rats).
Conclusion
These data suggest that rats in this experimental model of HELLP syndrome have abnormal expression of the Fas/FasL system. Future studies will examine the sources of Fas/FasL dysregulation in this model and if blockade could reduce some of the inflammation and hypertension associated with HELLP syndrome.
Keywords: Endothelin, Fas, Fas Ligand, HELLP Syndrome, Pregnancy
INTRODUCTION
Despite being one of the leading causes of maternal death and a major contributor to maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of HELLP (Hemolysis, Elevated Liver enzymes, Low Platelet) syndrome remain unclear [1, 2]. HELLP syndrome is becoming more recognized as an immune based disease, as studies by our labs and others have reported increased circulating and placental levels of inflammatory cytokines and anti-angiogenic imbalance [3–5]. The Fas receptor and FasL (Fas Ligand) are part of the TNF receptor family and have multiple roles, including regulation of an inflammatory response via activation and proliferation of CD4+ T lymphocytes [6, 7]. Under physiologic conditions, FasL will bind Fas+ cells to induce apoptosis or activate CD4+ T cells. However dysregulation of the Fas/FasL system leads to a decrease in Fas activation and may result in autoimmune and chronic inflammatory diseases [8, 9].
While there is evidence of dysregulation of the Fas/FasL system in pregnancies complicated with hypertension such as HELLP syndrome, the reason or effect of this dysfunction is unclear [10, 11]. Fas/FasL has not been reported to have a direct role in the induction of hypertension, however when this system is left unchecked then apoptosis of inflammatory cytokine secreting leukocytes can become decreased. As leukocytes have been implicated in inducing not only inflammation but also endothelial activation and hypertension [12–14], it is important to determine the role of Fas/FasL in contributing to this phenomenon.
Pharmacological blockade of the endothelin system during pregnancy has been demonstrated to attenuate hypertension [12, 15, 16], which points to the importance of the endothelin system in women who suffer from hypertensive pregnancies. We have recently reported a role of the endothelin system in mediating the hypertension, increased CD4+ T cells and the biochemistry that defines HELLP syndrome in an experimental animal model of HELLP [16]. Interestingly, alteration of the Fas pathway has been shown to lead to endothelial cell activation and vascular dysfunction [17–19]. Therefore in the current study we sought to determine if rats experiencing hypertension, inflammation and endothelin activation in response to a HELLP-like syndrome had alterations in Fas/FasL respective to normal pregnant rats. We also wanted to determine if blockade of the endothelin system altered any HELLP-induced changes in Fas/FasL expression.
MATERIALS AND METHODS
All studies were performed in 230–250g timed-pregnant Sprague Dawley rats (Harlan, Indianapolis, Indiana). Animals were housed in a temperature controlled room with a 12:12 light:dark cycle. All experimental procedures in this study were in accordance with the National Institute of Health guidelines for use and care of animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Experimental Animal Model of HELLP Syndrome
sEng (soluble endoglin) and sFlt-1 (soluble fms-like tyrosine kinase-1; 7 and 4.7 ug/kg respectively, R&D Systems, Minneapolis, MN) were diluted in sterile 0.9% saline to the appropriate concentration. 250μL of the drug solution was loaded into mini-osmotic pumps (model 2002; Alzet Scientific, Cupertino, CA) and surgically inserted into NP (normal pregnant) rats to allow chronic infusion of sFlt-1 and sEng from GD (gestational day) 12 to GD19 to induce HELLP syndrome (n=8) as previously published [16, 20]. NP rats that were subjected to a SHAM surgery but did not receive a mini-osmotic pump served as the control group (n=8). On GD19, whole blood was collected before euthanization via the abdominal aorta and collected for enzyme linked immunosorbent assays (ELISA). Maternal placentas, kidneys (minus the subcapsular layer), and livers were collected, snap frozen and stored at −80°C for the current study.
Inhibition of the ETA receptor in an experimental animal model of HELLP Syndrome
To determine whether attenuation of the endothelin system via the endothelin A receptor (ETA) had an effect on Fas/FasL regulation, rats were treated with an ETA receptor antagonist. Beginning on GD12, NP (n=6) and HELLP rats (n=6) were treated ad libitium (via drinking water) with the ETA receptor antagonist (ABT-627, 5mg/kg) until GD19.
We have previously reported [16] that rats infused with sFlt-1 and sEng to induce HELLP syndrome had significantly increased hemolysis, liver enzymes and a significant decrease in platelets compared to NP rats. Additionally, these HELLP rats were also reported to be hypertensive compared to NP rats, all of these characteristics were attenuated with blockade of the ETA receptor [16]. Samples from these rats were used to conduct the following experiments.
Circulating and local Fas and FasL protein expression
Rat Fas and FasL protein levels were assessed in the circulation, liver and placenta via ELISA (RayBiotech Inc; Norcross, GA) per manufacturer’s instructions. The minimum detectable dose of FasL was 90pg/mL and Fas was 40pg/mL with intra and inter-assay CVs < 10 and 12% respectively for both ELISAs. Livers (section from one maternal lobe) and placentas were homogenized individually on ice in homogenization buffer (10mM Tris Base, 37.22 mg EDTA, pH 7) plus a protease inhibitor cocktail (RPI Inc. Mt. Prospect, IL) and centrifuged at 1600 RPM at 4°C for 10 minutes. Each supernatant was individually filtered via a 100μM cell strainer and the filtrate saved before being assayed. To determine the total protein content in the filtrate, commercially available bicinchoninic acid assays (BCA; ThermoFisher, Waltham, MA) were performed following the manufacturer’s directions. Fas and FasL ELISA protein concentrations were divided by the total protein in each respective sample.
Fas and FasL mRNA transcript expression
To determine if local expression of Fas and/or FasL was altered in response to HELLP syndrome mRNA was extracted from individual liver, placenta, kidney cortex, and kidney medulla as previously described [16]. Briefly, individual tissues (n=4–6/group) were crushed in liquid nitrogen, and mRNA was extracted using a Qiagen kit (Venlo, Netherlands) as outlined in the manufacturer’s instructions followed by treatment with DNAse per manufacturer’s directions (Applied Biosystems, Foster City, CA). cDNA was synthesized from 1μg of RNA with BioRad Iscript cDNA reverse transcriptase kit (Hercules, CA). Real-time polymerase chain reaction was performed using BioRad Sybre Green Supermix with an iCycler. Beta actin Ct (threshold cycle) was used as a housekeeping gene and the 2−ΔΔCt method was used to analyze the results.
delta/delta Ct method was used to assess gene expression. Primer sequences [16, 21] were provided by Life technologies (Carlsbad, CA).
Fas
Forward: TCTAGTTGGAAAGAACCGAAGG
Reverse: CCACAAACGAGATGCAATCAC
FasL
Forward: ATCCCTCTGGAATGGGAAGA
Reverse: CCATATCTGYCCAGTAGTGC
Beta Actin
Forward: GCTCGTCGTCGACAACGGCTCCGGC
Reverse: CAAACATGATCTGGGTCATCTTCTCGCGG
Statistical Analysis
All of the data are reported as mean ± standard error mean. Data was analyzed via two-way analysis of variance (ANOVA). Interactions that equaled p<0.05 were analyzed via Tukey’s multiple comparison test or Student’s T. Graphpad Prism version 5.0 was used as the statistical software program. P<0.05 was considered statistically significant.
RESULTS
Rats with HELLP have increased circulating and placental FasL and decreased hepatic FasL
Rats in this experimental model of HELLP syndrome had significantly more circulating FasL compared to NP rats (p=0.01; Figure 1A). However, there was not a statistically significant main interaction (p=0.31). Placental FasL was significantly increased in HELLP rats compared to NP rats (p<0.01; Figure 1B), however there was not a statistically significant when ETA was administered (p=0.18). Liver FasL levels were significantly decreased in HELLP rats when compared to NP rats (p=0.01; Figure 1C). There was not a statistically significant main interaction (p=0.62).
Figure 1. Rats with HELLP syndrome have increased FasL and Fas.
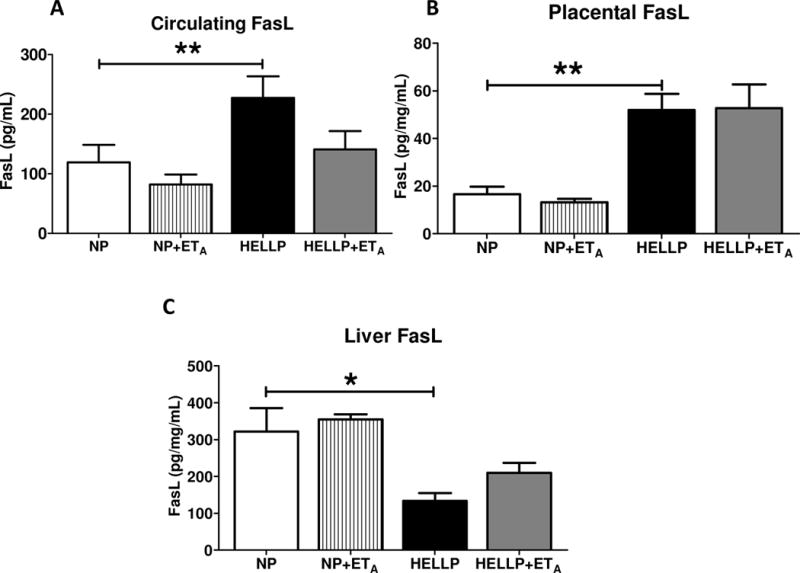
Circulating (A) and placental (B) FasL expression were significantly increased in HELLP rats compared with normal pregnant (NP) rats, while FasL expression was significantly decreased in liver tissues (C) of HELLP rats compared with NP rats. N=6-8/group. *, ** denotes P<0.05, P<0.005 between indicated groups.
Rats with HELLP syndrome have increased placental Fas expression
There was not a statistically significant interaction in circulating Fas (p=0.17; Figure 2A). Placental Fas was significantly increased in HELLP rats compared to NP rats (p<0.05; Figure 2B). Furthermore there was a significant interaction upon administration of ETA where administration of the ETA antagonist significantly decreased placental Fas in HELLP rats (p<0.0005; Figure 2A) and in NP+ETA rats (p<0.01; Figure 2A) compared to untreated HELLP rats. There was not a significant interaction in liver Fas levels (p=0.62; Figure 2C).
Figure 2. Placental Fas is increased in response to HELLP.
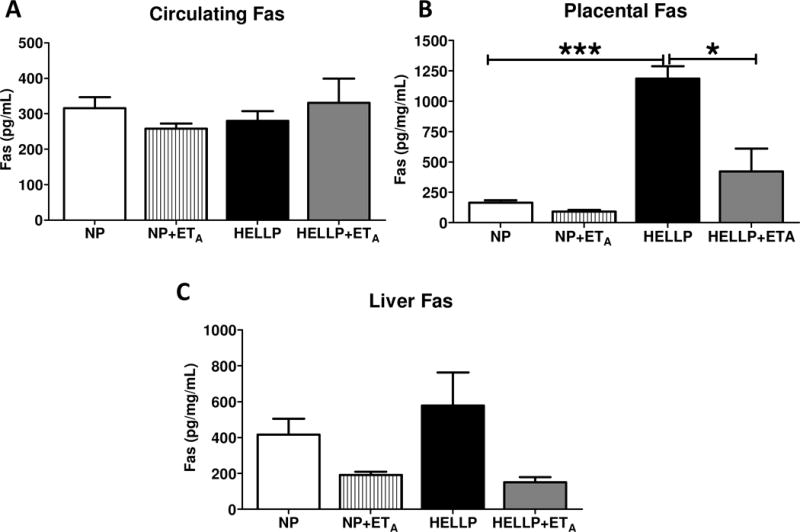
There was no statistically significant difference in circulating levels of Fas (A). Fas protein expression was significantly increased in the placental tissue of HELLP rats compared with NP and HELLP rats treated with the ETA antagonist (B). Fas protein expression in the liver was not statistically significant (C). N=4–5 samples/group. *, *** denotes P<0.05, and P<0.0005 between indicated groups.
Hepatic Fas mRNA transcript expression is downregulated in response to HELLP syndrome
Gene expression of FasL by real-time PCR was undetectable in livers from rats with HELLP syndrome regardless of ETA treatment, but was detectable in livers collected from NP rats. There was no statistically significant difference in FasL transcript expression between NP and NP+ETA treated rats (p=0.69; Table 1). Two-way ANOVA of Fas mRNA transcript expression was found to be statistically significant (p=0.01), with a significant increase in livers of HELLP rats compared to NP rats (p<0.05; Table 1). There was no statistically significant difference in either placental FasL (p=0.32) or placental Fas (p=0.89; Table 1).
Table 1.
Fas or FasL gene expression. The average Fas or FasL — Beta Actin threshold cycle is represented below.
| NP | NP+ETA | HP | HP+ETA | ||
|---|---|---|---|---|---|
| Fas | Liver | 5.07±0.88 | 2.79±0.26 | 2.18±0.61* | 4.02±0.67 |
| Placenta | 2.50±0.2 | 2.30±0.3 | 2.53±0.75 | 2.48±0.47 | |
| Kidney Cortex | 2.+9±0.69 | 2.81±1 | 2.18±0.54 | 4.20±1.24 | |
| Kidney Medulla | 8.87±0.66 | 7.49±0.64 | 9.85±0.68 | 7.61±0.97 | |
| FasL | Liver | 7.94±1.41 | 7.13±1.32 | ND | ND |
| Placenta | 8.69.±0.39 | 7.92±0.53 | 8.09±0.65 | 6.07±0.68 | |
| Kidney Cortex | ND | ND | 3.86±1.87 | 10.36±2.47 | |
| Kidney Medulla | 12.63±0.28 | 10.84±3.07 | 10.86±0.64 | 13.81±0.66 |
ND — Gene expression was not detectable by real-time PCR. This was statistically significant compared to NP.
We also examined FasL and Fas mRNA transcript expression in the kidney cortex and kidney medulla of rats as the kidney has in the etiology of hypertension. As the kidney cortex and kidney medulla have differential expression of FasL and endothelin we analyzed these parts separately [22, 23]. FasL expression was undetectable via real-time PCR in kidney cortices collected from NP rats treated with or without ETA, whereas there was there was no significant difference in FasL mRNA expression in rats with HELLP syndrome treated with or without the ETA antagonist (p=0.08; Table 1). There was no significant expression in cortical Fas expression (p=0.31), in medullary Fas expression (p=0.57) or FasL expression in the medulla (p=0.12; Table 1).
DISCUSSION
Studies have reported that trophoblast debris from ischemic placentas, such as in HELLP syndrome and preeclampsia can lead to the stimulation and activation of the vascular endothelium which results in endothelial dysfunction, inflammation and hypertension [24–26]. The Fas/FasL system has been implicated in contributing to some of the dysfunction present in HELLP syndrome [27] as the increase in FasL-induced apoptosis of trophoblast cells is suggested to be one cause for the excessive release of trophoblast debris from the placenta into the maternal circulation [11, 28, 29].
In the current study we investigated whether an experimental rat model of HELLP syndrome also exhibited alterations in Fas or FasL. We found that circulating and placental levels of FasL are increased in HELLP rats compared to NP rats and hepatic FasL was significantly decreased. These results correlate with data from human studies which have reported that circulating and placental FasL expression is increased in women with HELLP syndrome [10, 11]. Additionally placental derived FasL has been reported to not only be the primary source of FasL in women with HELLP syndrome but to also induce liver apoptosis and cytotoxicity in these women [10]. As the decrease in hepatic FasL in HELLP rats was statistically significant from NP rats, this suggests that indeed the placenta may serve as the primary source of FasL in the circulation.
The lack of significant change in circulating Fas is in contrast to what has been previously reported in the clinical literature, in which studies found that women with HELLP syndrome had increased circulating soluble Fas levels [30, 31]. It could be that as these other studies measured the soluble form of Fas and we did not assay the soluble only portion of Fas, but rather total Fas, we did not have any statistically significant changes in circulating Fas. Placental Fas expression was significantly increased in response to HELLP syndrome. Despite the lack of significant difference in protein levels of hepatic Fas, mRNA transcript of Fas was significantly increased in this model of HELLP syndrome. One might expect to see an increase in Fas levels as there was a statistically significant decrease in FasL protein levels. It’s possible that due to the short nature of disease development (GD12–GD19) in this experimental animal model there was not enough experimental time to allow for statistically significant changes in protein levels of Fas to be expressed. Future experiments will address the protein:mRNA levels of Fas and FasL in rats that have a full gestation.
We have previously reported that attenuation of the endothelin system in this model of HELLP syndrome decreases hypertension, significantly decreases all of the symptoms of HELLP syndrome that were found to be significantly increased in untreated rats infused with sFlt-1 and sEng to mimic HELLP syndrome (i.e. a significant increase in hemolysis, a significant elevation in liver enzymes and a significant decrease in platelets), endothelial activation and cytokine secreting T cells [16]. Indeed, HELLP rats were found to have an average mean arterial pressure (MAP) of 121.5±2.44mmHg compared to 99.5±2.2mmHg in NP rats which was reduced to 100.7±4.8mmHg in HELLP+ETA treated rats [16]. In the current study we used previously collected samples from these same animals to determine if blockade of the endothelin system via the ETA receptor reversed any changes in Fas/FasL expression due to HELLP syndrome. Circulating FasL tended to decrease in response to ETA antagonism in HELLP rats but neither placental nor liver FasL were significantly affected by ETA antagonism. However, ETA blockade in HELLP rats tended to drive both liver FasL and placental Fas levels in the direction of a normal pregnant response. As NP rats treated with the ETA antagonist didn’t exhibit any statistically significant changes in Fas or FasL production compared to the untreated NP rats, we believe that the changes in Fas and FasL are due directly to HELLP syndrome induced by angiogenic imbalance.
Conclusion
In conclusion, rats in this model of HELLP syndrome demonstrated increased placental and circulating FasL and increased placental Fas when compared to NP rats. Administration of an ETA receptor antagonist to HELLP rats tended to decrease circulating FasL and placental Fas, while not affecting placental FasL levels. These results suggest, that there is an additional source of FasL besides the placenta that can be contributing to the increase in circulating FasL and that the endothelin system is not involved in the regulation of FasL in the placenta. In the current study we did not investigate all of the potential sources of FasL, however studies by other labs have indicated that the placenta along with circulating immune cells can potentially serve as a source of FasL [10, 20, 32]. As such future studies, in addition to a closer look at hypertension and vascular function in relation to Fas/FasL will also include examining the role of these immune cells (both circulating and placental) to contribute to the pathogenesis of HELLP syndrome. As the Fas/FasL system can initiate not only apoptosis but also inflammation via immune cell activation, it is important to continue to study the role of this system in contributing to HELLP syndrome where both apoptosis and inflammation are increased.
Highlights.
Angiogenic imbalance during pregnancy is associated with dysregulation of FasL
Placental Fas was increased in response to angiogenic imbalance
Blockade of the ETA receptor prevented an increase in placental Fas
Acknowledgments
Funding.
This work was supported by intramural research support (University of Mississippi Medical Center) to KW and by the Mississippi INBRE, funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103476.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflicts of interest to disclose
References
- 1.Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: Clinical issues and management. A Review. BMC Pregnancy and Childbirth. 2009;9(8) doi: 10.1186/1471-2393-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geary M. The HELLP syndrome. British Journal of Obstetrics and Gynaecology. 1997;104(887–891) doi: 10.1111/j.1471-0528.1997.tb14346.x. [DOI] [PubMed] [Google Scholar]
- 3.Landi B, Tranquilli A. HELLP syndrome and placental inflammatory pathology. Minerva Ginecol. 2008;60(5):389–398. [PubMed] [Google Scholar]
- 4.Tranquilli A, Landi B, Corradetti A, Giannubilo S, Sartini D, Pozzi V, Emanuelli M. Inflammatory cytokines patterns in the placenta of pregnancies complicated by HELLP (hemolysis, elevated liver enzyme, and low platelet) syndrome. Cytokine. 2007;40(2):82–88. doi: 10.1016/j.cyto.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Wallace K, Martin J, Jr, Tam K Tam, Wallukat G, Dechend R, Lamarca B, Owens M. Seeking the Mechanisms of Action for Corticosteroids in HELLP Syndrome: SMASH Study. American Journal of Obstetrics and Gynecology. 2013;208(5):e1–e8. doi: 10.1016/j.ajog.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 6.Paulsen M, Valentin S, Mathew B, Adam-Klages S, Bertsch U, Lavrik I, Krammer P, Kabelitz D, Janssen O. Modulation of CD4+ T-cell activation by CD95 co-stimulation. Cell Death Differ. 2011;18(4):619–631. doi: 10.1038/cdd.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alderson M, Armitage R, Maraskovsky E, Tough T, Roux E, Schooley K, Ramsdell F, Lynch D. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993;178(6):2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 9.Via C, Nguyen P, Shustov A, Drappa J, Elkon K. A major role for the Fas pathway in acute graft-versus-host disease. J Immunol. 1996;157:5387–5393. [PubMed] [Google Scholar]
- 10.Strand S, Strand D, Seufert R, Mann A, Lotz J, Blessing M, Lahn M, Wunsch A, Broering D, Hahn U, Grischke E, Rogiers X, Otto G, Gores G, Galle P. Placenta-derived CD95 Ligand causes liver damage in hemolysis, elevated liver enzymes, and low platelet count syndrome. Gastroenterology. 2004;126:849–858. doi: 10.1053/j.gastro.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 11.Prusac I, Zekic T, Roje D. Apoptosis, proliferation and Fas ligand expression in placental trophoblast from pregnancies complicated by HELLP syndrome for pre-eclampsia. Acta Obstetricia Gynecologica Scandinavica. 2011;90(10):1157–1163. doi: 10.1111/j.1600-0412.2011.01152.x. [DOI] [PubMed] [Google Scholar]
- 12.Wallace K, Novotny S, Heath J, Moseley J, Martin J, Owens M, Lamarca B. Hypertension in response to CD4+ T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol. 2012;303(2):R144–149. doi: 10.1152/ajpregu.00049.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace K, Richards S, Dhillion P, Weimer A, Edholm E, Bengten E, Wilson M, Martin JJ, Lamarca B. CD4+ T Helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57(5):949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsuki M, Hirooka Y, Kishi T, Sunagawa K. Decreased proportion of Foxp3+CD4+regulatory T cells contributes to the development of hypertension in genetically hypertensive rats. J Hypertension. 2015;33(4):773–783. doi: 10.1097/HJH.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 15.Alexander B, Rinewalt A, Cockrell K, Massey M, Bennett W, Granger J. Endothelin type A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37(2):485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 16.Morris R, Spencer S, Kyle P, Williams J, Harris A, Owens M, Wallace K. Hypertension in an animal model of HELLP syndrome is associated with activation of endothelin-1. Reproductive Science. 2016;130(6):409–419. doi: 10.1177/1933719115592707. [DOI] [PubMed] [Google Scholar]
- 17.Yamaoka-Tojo M, Yamaguchi S, Nitobe J, Abe S, Inoue S, Nozaki N, Okuyama M, Sata M, Kubota I, Nakamura H, Tomoike H. Dual response to Fas ligation in human endothelial cells:apoptosis and induction of chemokines, interleukin-8 and monocyte chemoattractant protein-1. Coron Artery Dis. 2003;14(1):89–94. doi: 10.1097/00019501-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Cardier J, Schulte T, Kammer H, Kwak J, Cardier M. Fas (CD95, APO-1) antigen expression and function in murine liver endothelial cells: implications for the regulation of apoptosis in liver endothelial cells. FASEB Journal. 1999;13(14):1950–1960. doi: 10.1096/fasebj.13.14.1950. [DOI] [PubMed] [Google Scholar]
- 19.Agouni A, Ducluzeau P, Benameur T, Faure S, Sladkova M, Duluc L, Leftheriotis G, Pechanova O, Delibegovic M, Martinez M, Andriantsitohaina R. Microparticles from patients with metabolic syndrome induce vascular hyporeactivity via Fas/Fas-ligand pathway in mice. PLos One. 2011;6(1):e27809. doi: 10.1371/journal.pone.0027809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace K, Morris R, Kyle P, Cornelius D, Darby M, Scott J, Moseley J, Chatman K, Lamarca B. Hypertension, inflammation and T lymphocytes are increased in a rat model of HELLP syndrome. Hypertension in Pregnancy. 2014;33(1):41–54. doi: 10.3109/10641955.2013.835820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao K, Zhang L, Yang S, Qian W, Zhang Z. Intervention of selenium on apoptosis and Fas/FasL expressions in the liver of fluoride-exposed rats. Environmental Toxicology and Pharmacology. 2013;36(3):913–920. doi: 10.1016/j.etap.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Pollock D, Opgenorth T. ETA receptor mediated responses to ET-1 in the rat kidney. BRJ Pharmacology. 1994;111:729–732. doi: 10.1111/j.1476-5381.1994.tb14798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazawa K, Suzuki K, Ikeda R, Moriyama M, Ueda Y, Katsuda S. Apoptosis and its related genes in renal epithelial cells of the stone-forming rat. Urol Res. 2005;33(1):31–38. doi: 10.1007/s00240-004-0434-6. [DOI] [PubMed] [Google Scholar]
- 24.Shen F, Wei J, Snowise S, DeSousa J, Stone P, Viall C, Chen Q, Chamley L. Trophoblast debris extruded from preeclamptic placentae activates endothelial cells: A mechanism by which the placenta communicates with the maternal endothelium. Placenta. 2014;35(10):839–847. doi: 10.1016/j.placenta.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Lau S, Barrett C, Guild S, Chamley L. Necrotic trophoblast decris increases blood pressure during pregnancy. J Reprod Immunol. 2013;97(2):175–182. doi: 10.1016/j.jri.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Lamarca B. Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol. 2012;64(4):309–320. [PMC free article] [PubMed] [Google Scholar]
- 27.Miko E, Szereday L, Barakonyi A, Jarkovich A, Varga P, Szekeres-Bartho J. Immunoactivation in preeclampsia: Vdelta2+ and regulator T cells during the inflammatory stage of disease. Journal of Reproductive Immunology. 2009;80:100–108. doi: 10.1016/j.jri.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Abildgaard U, Heimdal K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2013;166(2):117–123. doi: 10.1016/j.ejogrb.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Abumaree M, Chamley L, Badri M, El-Muzaini M. Trophoblast debris modulates the expression of immune proteins in macrophages: a key to maternal tolerance of the fetal allograft? J Reprod Immunol. 2012;94(2):131–141. doi: 10.1016/j.jri.2012.03.488. [DOI] [PubMed] [Google Scholar]
- 30.Kuntz T, Christensen R, Stegner J, Duff P, Koenig J. Fas and Fas Ligand Expression in maternal blood and in umbilical cord blood in preeclampsia. Pediatric Research. 2001;50(6):743–49. doi: 10.1203/00006450-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Harirah H, Donia S, Hsu C. Serum soluble Fas in the syndrome of hemolysis, elevated liver enzymes and low platelets. Obstet Gynecol. 2001;98(2):295–298. doi: 10.1016/s0029-7844(01)01415-6. [DOI] [PubMed] [Google Scholar]
- 32.Steinborn A, Haensch G, Mahnke K, Schmitt E, Toermer A, Meuer S, Sohn C. Distinct subsets of regulatory T cells during pregnancy: Is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clinical Immunology. 2008;129:401–412. doi: 10.1016/j.clim.2008.07.032. [DOI] [PubMed] [Google Scholar]


