Abstract
Objective
The connection between metabolism and flow in the heart, metabolic dilation, is essential for cardiac function. We recently found redox-sensitive Kv1.5 channels play a role in coronary metabolic dilation; however, more than one ion channel likely plays a role in this process since animals null for these channels still showed limited coronary metabolic dilation. Accordingly, we examined the role of another Kv1 family channel, the energetically-linked Kv1.3 channel, in coronary metabolic dilation.
Methods
We measured myocardial blood flow (contrast echocardiography) during norepinephrine-induced increases in cardiac work (heart rate × mean arterial pressure) in wild type mice (WT), WT mice given correolide (preferential Kv1.3 antagonist), and Kv1.3 null mice (Kv1.3−/−). We also measured relaxation of isolated small arteries mounted in a myograph.
Results
During increased cardiac work, myocardial blood flow was attenuated in Kv1.3−/− and in correolide-treated mice. In isolated vessels from Kv1.3−/− mice, relaxation to H2O2 was impaired (vs WT), but responses to adenosine and acetylcholine were equivalent to WT. Correolide reduced dilation to adenosine and acetylcholine in WT and Kv1.3−/−, but had no effect on H2O2-dependent dilation in vessels from Kv1.3−/− mice.
Conclusion
Kv1.3 channels participate in the connection between myocardial blood flow and cardiac metabolism.
Introduction
The heart relies on aerobic metabolism to convert energetic substrates into chemical energy (ATP) to maintain normal cardiac pump function. The maintenance of cardiac function depends on a “balance” between supply of energetic substrates and oxygen, coupled to the demands for oxygen and energy. The principal components in this balance are the coronary circulation, which regulates supply, and the working cardiac myocytes, which dictate demand. The coupling between metabolism and flow is primarily dictated by the production of metabolic vasodilators that are by-products of metabolism1. The production of these vasoactive factors is essential because the primary manner by which the heart increases oxygen delivery is via increased blood flow as oxygen extraction is already of near maximum2, 3. Insufficient delivery of oxygen can impair contractile function of the heart within seconds4. Despite the importance of this connection there is a relative paucity of information regarding the key signaling proteins that transduce the metabolic signals into increases in vascular caliber.
Most previous efforts directed at understanding the links between metabolism and flow in the heart focused on specific metabolites, e.g., adenosine5, 6, which signal through G-protein coupled receptors to produce vasodilation7–10. Although a large body of work supports the role of metabolites such as adenosine in coronary regulation, some observations have challenged this hypothesis11, 12. More recent work is directed at examining the role of ion channels as transducers of the metabolic information9, 13, 14. In this regard, tone of vascular smooth muscle is primarily controlled by the membrane potential15–18, which regulates the open probability of voltage-gated calcium channels. Factors which induce hyperpolarization, such as opening of potassium channels, reduce calcium entry through the voltage-gated calcium channels, producing vasodilatation. In contrast, closure of K+ channels leads to membrane depolarization and causes vasoconstriction19–21.
Coronary blood flow is modulated by metabolic, myogenic and endothelium-dependent vasodilators and constrictors22, 23. These many signals are integrated at the level of the smooth cell with an overall effect on intracellular calcium levels mediated by changes in membrane potential and/or modulation of calcium release or uptake mechanisms in internal stores15, 16, 24, 25. A vasodilator will decrease sarcoplasmic calcium levels; thereby inducing vasodilation. In metabolic hyperemia, blood flow is largely controlled by the production of vasoactive metabolites, which induce vasodilation in order to maintain the match between myocardial metabolism or cardiac work and myocardial blood flow1.
Our previous results have suggested the production of hydrogen peroxide by mitochondria is one of the metabolic mediators contributing to the coupling between metabolism and flow26. We also observed that the dilation produced by H2O2 is mediated by redox-dependent reactions via Kv channels27, 28. Using a murine model of coronary metabolic dilation, we observed that Kv1.5 channels play an important role in connecting flow to metabolism in the heart.14 Mice null for Kv1.5 channels had blunted dilation (compared to wild types) during increases in cardiac work, but it is important to emphasize that some degree of dilation remained. Due to this residual dilation, we hypothesized that additional ion channels are likely involved in coronary metabolic dilation. Based on this speculation, we proposed that Kv1.3 channels play a role in coupling flow to metabolism in the heart, as these channels are thought to be regulated by cell metabolism29–33.
Accordingly, we studied coronary metabolic dilation (changes in myocardial blood flow [MBF] in response to increases in cardiac work) in wild-type mice, mice null for Kv1.3 channels (Kv1.3−/−), and mice acutely treated with the preferential Kv1.3 channel antagonist, correolide. Our results support the idea that Kv1.3 channels play a critical role in connecting myocardial blood flow to cardiac metabolism.
METHODS
Murine Models
Male and Female wild type mice (WT) on the C57Bl/6J background, and Kv 1.3 null mice on the same background (Kv1.3−/−) were used in this study. Kv1.3−/− mice were obtained from Dr. Gary Desir (Yale University), who initially observed that mice null for these channels had altered energy homeostasis34. All procedures were conducted with the approval of the Institutional Animal Care and Use Committee of the Northeastern Ohio Medical University in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996). Mice were housed in a temperature-controlled room with a 12:12-h light-dark cycle and maintained with access to food and water ad libitum. Three groups of male and female mice (4–6 months of age) were studied to establish the role of Kv1.3 channel deletion or blockade on coronary vascular reactions: WT (N=35), Kv1.3−/− (N=20), and WT mice treated with two doses of correolide (80 and 800 ng/g) a preferential Kv1.3 channel antagonist35. Results from male and female mice were combined because no statistically significant differences between genders were observed.
Hemodynamic measurements and estimation of cardiac work
Anesthesia was induced in a small chamber equilibrated with 3% isoflurane with the balance of oxygen. After induction mice were situated on heated surgical table designed for murine surgical procedures and echocardiography. A nose cone was secured to the animal and anesthesia was maintained using isoflurane/oxygen. Body temperature was maintained at 37°C via a heated stage controlled by temperature measurement from a rectal probe. The right jugular vein was cannulated with PE-50 polyethylene tubing containing heparin (50 U/ml) in saline for intravenous drug infusions. The femoral artery was isolated and cannulated with a 1.2F pressure catheter (Scisense Inc.), then connected to a data acquisition system (PowerLab ML820; ADInstruments) through a pressure interface unit (SP200 Pressure System) to measure arterial blood pressure and heart rate (HR). The transducer was advanced into abdominal aorta. All measured variables were continuously recorded and stored on an iMac computer that used the PowerLab system (AD Instruments; Castle Hill, Australia). Blood pressures were collected and analyzed using AD Instruments Chart 7 software. All mice were euthanized following the experimental protocol by exsanguination following high dose of barbiturate or isoflurane.
The product of heart rate (HR) and mean arterial pressure (MAP) (the pressure rate product [PRP]) was used to estimate cardiac metabolic demands. Although this calculation does not account for alterations in stroke volume (SV), we have found that the product of HR × MAP correlates well to the index of triple product (HR × MAP × SV), which is an index of cardiac work.14
Myocardial perfusion imaging by contrast echocardiography
Myocardial contrast echocardiography (MCE) was performed to measure myocardial blood flow (MBF). Contrast imaging was performed with a Sequoia 512 (Siemens Medical Systems) via infusion of contrast (microbubbles; 20 µl/min, 5 × 105 bubbles min−1) into the right jugular vein through a PE-50 catheter. This catheter was also used for drug infusion. Long axis images of the left ventricle were obtained for perfusion imaging. After optimal visualization of the chamber and the ventricular wall, images were collected during a high-energy pulse sequence (used to destroy microbubbles) and for several seconds after destruction to establish refilling of the chamber and ventricular wall. Analysis was done off-line, in which regions of interest (ROI) were positioned within the anterolateral wall in the long axis view. A curve of signal intensity over time was obtained in ROI and fitted to an exponential function: y = A(1 − e−βt), where y is the signal intensity at any given time, A is the signal intensity corresponding to the microvascular cross sectional volume, and β is the initial slope of the curve, which corresponds to the blood volume exchange frequency36, 37. Relative blood volume in the region of interest (RBV) was calculated as the ratio of myocardial to cavity signal intensity (RBV=A/ALV); where, ALV represents the signal intensity of the LV chamber. Myocardial blood flow (MBF) was estimated as the product RBV × β38. Flows were measured in 3–5 different images obtained under the same treatments/conditions. The image acquisition and analyses were performed by operators blinded to the genotype and treatment of the animals. Although measurements of myocardial blood flow using MCE are higher than what are obtained using microspheres, the relative magnitude of the differences would remain and the differences would still exist.
Measurements of myocardial blood flow, heart and arterial pressure were made under basal conditions, following administration of hexamethonium (5 mg/kg) (to eliminate reflexes during changes in arterial pressure), and i.v. doses of norepinephrine (0.5, 1.0, 2.5 and 5.0 µg/kg.min−1) to increase myocardial oxygen demands. In mice treated with correolide, only the two highest doses of norepinephrine were used.
In vitro Relaxation of Small Arteries and Arterioles
Arterioles and small coronary arteries (100–150 µm, id; typically 3rd order branches from the left anterior descending artery) were dissected from the epicardial surface of the left ventricle. After euthanasia, the heart was removed from the mouse, and the left ventricle was visualized under a dissecting microscope in a temperature-controlled dissection dish (4°C) containing a physiological salt solution39. Vessel segments were dissected free from surrounding myocardial tissue and were used for isometric force generation experiments. Two small (30 µm diameter) wires were inserted into the vessel and connected to a force transducer (Living Instruments, Inc). After optimization of resting tension for highest developed force, experiments were conducted to determine constrictor or dilator responses to agonists. For either preparation, pharmacological agents were administered in the bath. Tension was recorded continuously during the course of particular intervention. Vessels were contracted with the thromboxane mimetic U46619 (1 µM) to establish basal tone, and then vasodilation to hydrogen peroxide, adenosine and acetylcholine was assessed. Between agonists, the bath was washed several times and the preparation was contracted to U46619 to develop active tension.
Statistical analyses
All analyses were performed using GraphPad Prism version 4.0 for Windows (GraphPrism Software Inc.). Vasodilation to H2O2, adenosine and acetylcholine were analyzed by repeated measures ANOVA. The, relationships between MBF and Double Product (DP) were assessed by linear regression analysis, and r2 and P values are reported for the regression analyses. A probability of p<0.05 was accepted as statistically significant.
RESULTS
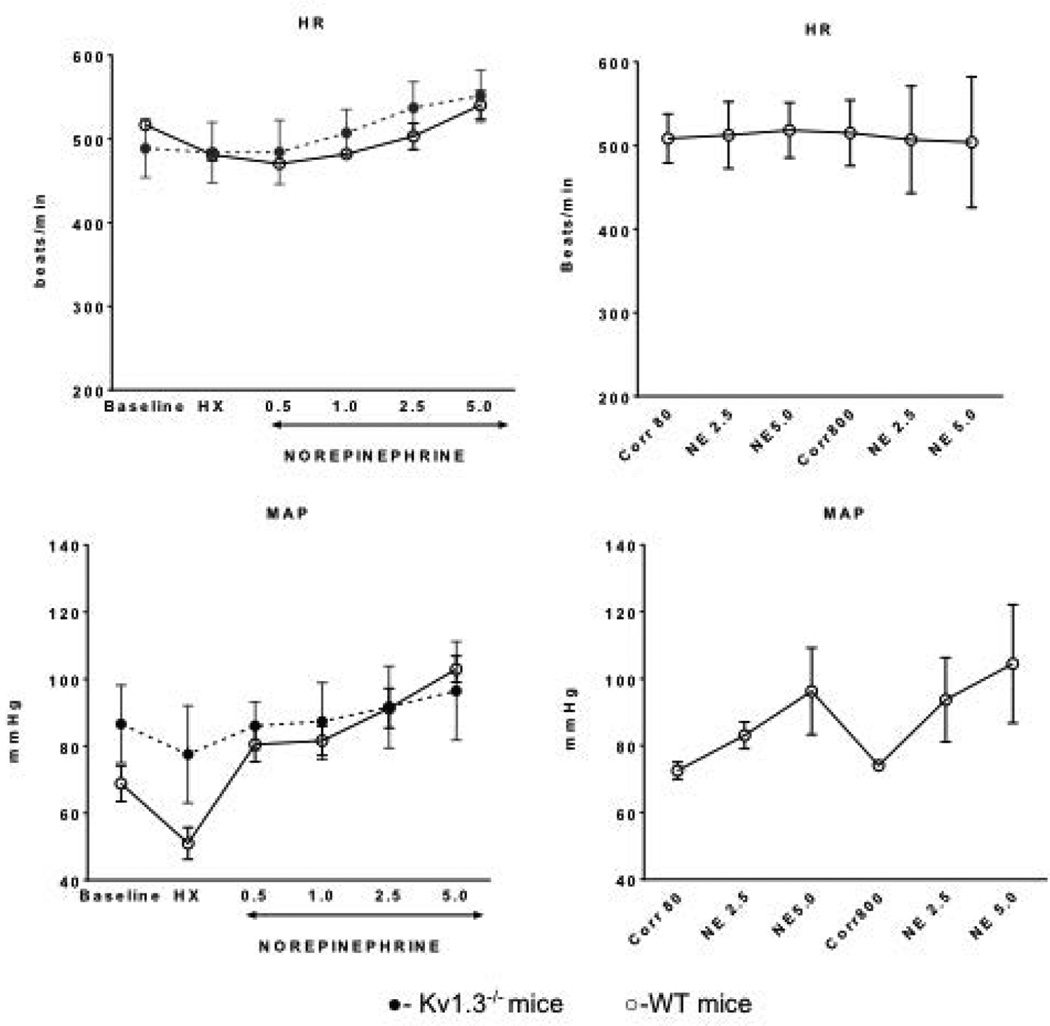
Figure 1 summarizes the hemodynamics (heart rate, arterial pressure) under basal conditions, during administration of hexamethonium, and during infusion of norepinephrine in the various groups. Heart rate and arterial pressure were similar in all groups under basal conditions and during norepinephrine administration. However, the decrement in arterial pressure produced by ganglionic blockade with hexamethonium was greatest in the WT group compared to Kv1.3−/− mice (P<0.05). During administration of norepinephrine, heart rate and arterial pressures were comparable among the groups.
Figure 1.
Left: Hemodynamics (heart rate, mean arterial pressure) in WT (n=14) and Kv.1.3−/− (n=15) mice under basal conditions, during hexamethonium, and during norepinephrine infusion (with hexamethonium). Right: Hemodynamics of WT mice following correolide administration (Corr80 [80 ng/kg, n=6] and Corr800 [800 ng/kg, n=8]) before and during norepinephrine.
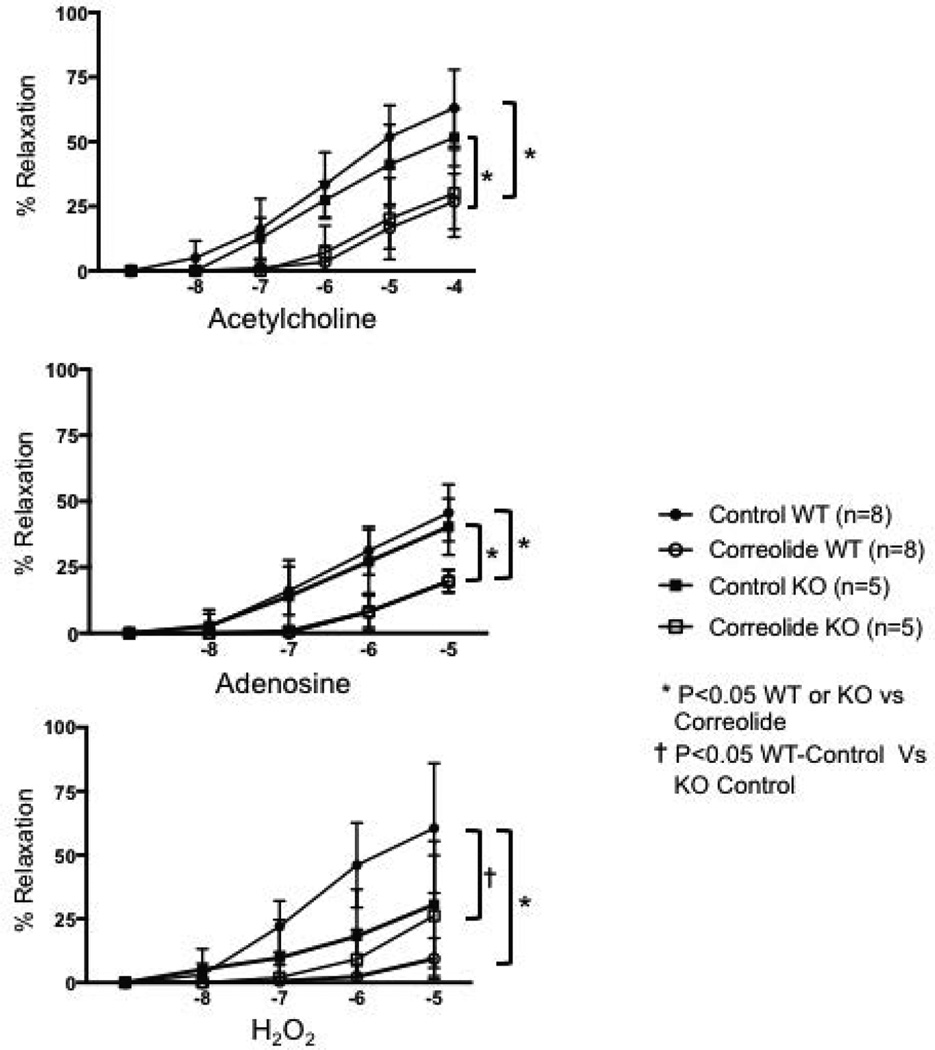
Figure 2 illustrates vasoactive reactions of small coronary arteries (internal diameters in the range of 100–150 µm) to hydrogen peroxide, adenosine, and acetylcholine. Arterioles from the WT and Kv1.3−/− mice exhibited similar vasodilation to adenosine and acetylcholine (NS). In contrast, the responses to H2O2 were decreased in arterioles isolated from Kv1.3−/− mice compared to WT. Administration of correolide blunted vasodilation to Ach and adenosine in vessels from WT and Kv1.3−/− mice. Interestingly, correolide decreased dilation to H2O2 in WT vessels, but not in those isolated from Kv1.3−/− mice.
Figure 2.
Relaxation of isolated small coronary arteries and arterioles to acetylcholine, adenosine, and hydrogen peroxide (H2O2) from wild type (WT) and Kv1.3−/− (KO) mice. Relaxation was assessed under control conditions (without correolide) and after addition of correolide in the WT and KO mice.
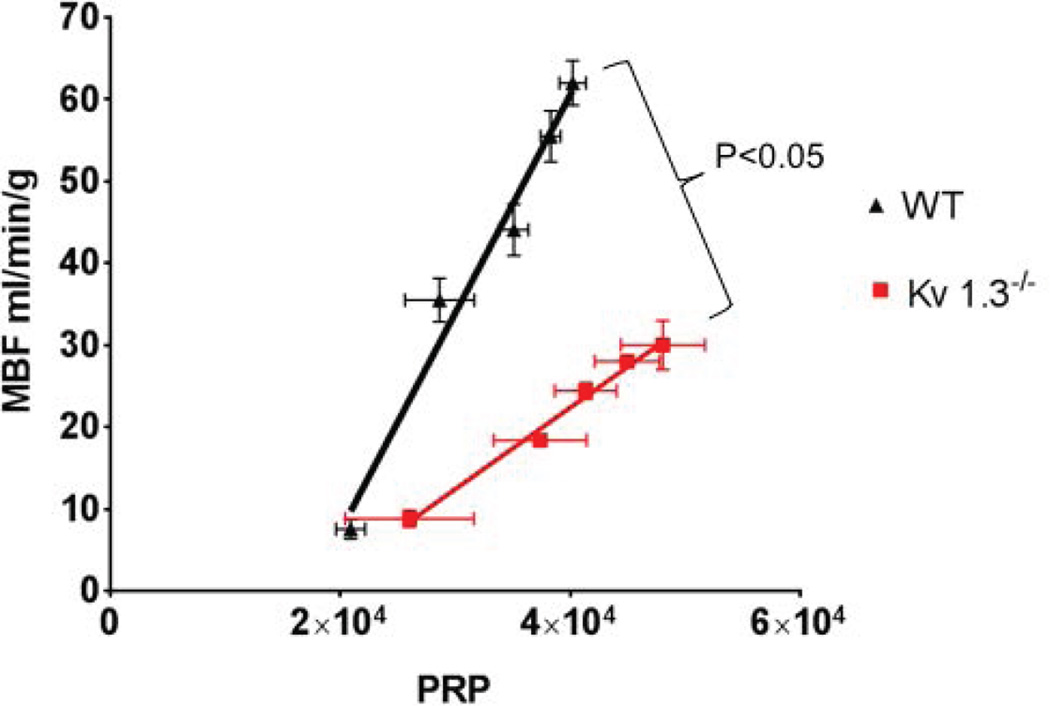
The relationships between metabolic demands (as represented by the pressure rate product [PRP]) and myocardial blood flow for WT and Kv1.3−/− during norepinephrine infusion are shown in Figure 3. Note that the work-flow relationship was significantly reduced in the null mice compared to WT, i.e., MBF in Kv1.3−/− was significantly lower all levels of DP than in the WT group (P<0.05).
Figure 3.
Relationship between Pressure-Rate Product (PRP, mean arterial pressure X heart rate) and myocardial blood flow (MBF). MBF and DP were measured under basal conditions, hexamethonium and norepinephrine (0.5–5.0 µg/kg/min iv; in the presence of hexamethonium). In Kv 1.3−/− mice (n=15) MBF was significantly lower compared to WT mice (n=14) at any level of the PRP.
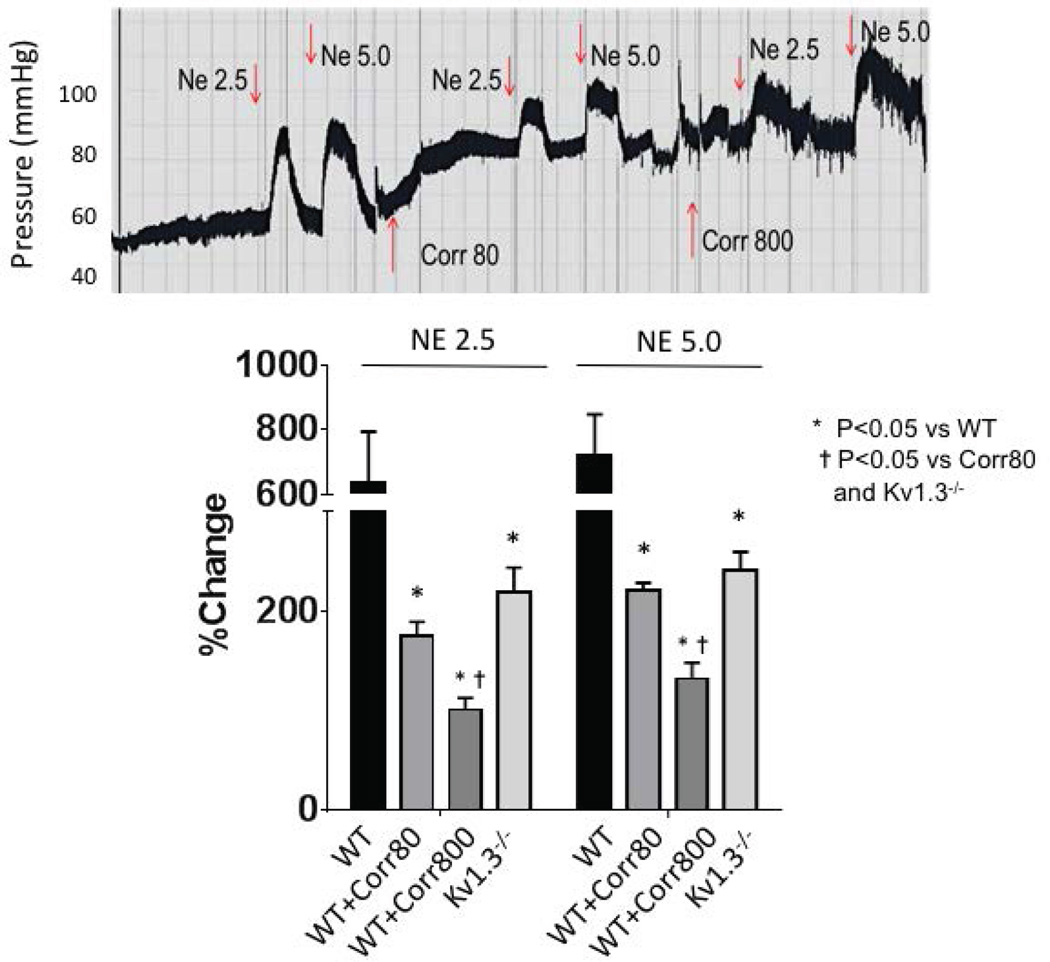
Figure 4 illustrates the percent changes in flow in WT and WT with correolide (WT-Corr80 and -Corr800). Moreover, treatment of WT with the lower dose of correolide (Corr80) reduced the percent increases in MBF to those comparable to Kv1.3−/− but reduced from WT mice. The higher dose of correolide (Corr800) significantly reduced the percent increases in blood flow compared to WT and Corr80.
Figure 4.
Percent increases in myocardial blood flow during norepinephrine infusion in WT, correolide-treated WT (Corr80 [80 ng/g]; Corr800 [800 ng/g]) or Kv1.3−/− groups. Note the increases in blood flow were less (compared to WT) in both Corr and Kv1.3−/− groups. Corr800 suppressed the increase in flow more than Corr 80 and Kv1.3−/−.
DISCUSSION
Our observations support the conclusion that the voltage-gated potassium channel Kv1.3 plays a role in coupling myocardial blood flow to cardiac work. Our data also suggest that Kv1.3 channels contribute to H2O2-induced vasodilation, but these channels do not contribute to vasodilation produced by adenosine or acetylcholine. Our data also indicate that correolide has vasoactive effects beyond its reported “specificity” for Kv1.3 channels, as dilation to acetylcholine and adenosine was decreased even in vessels obtained from Kv1.3 null mice. Cogent to our conclusions are results in the literature that bear upon our findings and implications of our observations in understanding the control of coronary blood flow in health and disease.
Considerations from the literature
The role of potassium channels as metabolic transducers, i.e., connecting blood flow to metabolism has received some attention over the last several years. In the coronary circulation previous studies have focused on KATP40 and BK channels41, but the conclusions that these channels modulate coronary metabolic dilation has been challenged experimentally42, 43 or conceptually because the conclusion drawn about BK channels was based on the use of a fairly non-specific potassium channel antagonist, tetra-ethyl-ammonium. A large hindrance in the in vivo interpretation of results using preferential ion channel antagonists is that the antagonists are preferential and can antagonize many more channels than the target. This invalidates the implicit assumption that the antagonist is targeting only the desired channel. For example, glibenclamide can antagonize the mitochondrial KATP44 and Kv channels45 rendering conclusions that an effect is due to inhibition of sarcolemmal KATP channels as suspect. Although in the present study we employed a reportedly preferential antagonist of Kv1.3 channels (correolide), we observed effects of correolide in small coronary arteries obtained from Kv1.3 null mice, so the drug must have other effects. To overcome the limitation of off-target effects, we also studied mice null for Kv1.3 channels. Importantly, the results from the Kv1.3 null mice and the WT mice treated with correolide suggested a role for Kv1.3 channels, as well as other mechanisms, in coronary metabolic hyperemia. Dick et al.46 found that correolide slightly attenuated adenosine-induced dilation of isolated coronary arterioles. These investigators did not investigate an in vivo effect of the drug and did not examine the effects of H2O2-dependent vasodilation.
The role of Kv1.3 channels in vascular regulation is not well appreciated, and the functions of this channel are most noted in inflammatory cells47, 48, the retina49, 50, and the central nervous system51–54. In the vasculature, Kv1.3 channels have been found in endothelial cells55 and appear to be involved in the phenotype of vascular smooth muscle30, 56–58. The Kv1.3 channel was reported in mitochondria and is proposed to interact with Bax and mediate apoptotic events in lymphocytes59, 60. Our results suggesting that Kv1.3 channels help facilitate the connection between metabolism and flow is new, and has not been previously suggested. However, we would like to offer two caveats that bear upon our interpretations. First, the use of the pharmacological inhibitor of Kv1.3 channels, correolide, must be interpreted in the context that the drug preferentially, but not exclusively blocks Kv1.3 channels. Other Kv1 family channels (e.g., Kv1.2 and Kv1.5) may be antagonized by correolide61, 62 and it is important to note that we previously reported a role for Kv1.5 channels in connecting myocardial blood flow to cardiac work.14 We also used two different doses of correolide in vivo and found the effects were greater with the higher dose. This implies that the higher dose blocked more than Kv1.3 channels, which is consistent with other observations about this antagonist61, 63; however, the lower dose may be more selective. Interestingly Figure 4 may bear upon the specific vs non-specific actions of correolide. Specifically, during the lower dose of correolide, arterial pressure was maintained during norepinephrine infusion; however, at the higher dose, arterial pressure fell during norepinephrine infusion. Previously we observed in Kv1.5 null mice that arterial pressure was not maintained during administration of the highest doses of norepinephrine, because flow was insufficient to match the higher metabolic demands resulting in pump dysfunction64. We believe this result suggests that the higher dose of correolide was blocking other Kv1 family channels in addition to the Kv1.3. Moreover, the higher dose of correolide also compromised the increase in myocardial perfusion to norepinephrine more than we observed at either the lower dose of the antagonist or in the Kv1.3 null mice (Figure 4) implying the higher dose of correolide blocked additional Kv1 family channels. Second, the experimental model, Kv1.3 null mice, has a global knockout of the channel in all tissues. Due to the expression of these channels in many cell types, we believe our conclusion that Kv1.3 channels facilitate coronary metabolic dilation is tenable, but we cannot state with conviction the cell type or types responsible for making this connection. Although it would seem unlikely that inflammatory cells or the central nervous system plays a key role in this connection (especially in view of our use of the ganglionic blocker hexamethonium to prevent autonomic influences), we cannot unequivocally eliminate effects of other cell types in affecting metabolic dilation.
Another caveat to our results regards our experimental approach in that we measured myocardial blood flow using contrast echocardiography. In our previous publication using this technique64, we found that myocardial blood flow measured with this technique was about 40% higher than that using microspheres; however, the correlations between the two techniques were high (r2 value of 0.98). Thus, our measurements of blood flow may be overestimated by contrast echocardiography, but the relative magnitude of the differences in flows would remain.
One puzzling aspect of our observations was that dilation to H2O2 was reduced (vs wild types) in vessels isolated from Kv1.3 null mice and in preparations treated with correolide. The reason we mention this is puzzling is because the Kv1.3 channel is not described as a redox-sensitive ion channel; as opposed to the Kv1.5 which is redox-sensitive63, 65. The reduction in dilation by correolide can be explained by actions of the drug blocking Kv1 family channels in addition to the Kv1.3 channel. However, we cannot readily explain why dilation to H2O2 was compromised in arterioles from the null mice. One possibility for this conundrum are observations of association between Kv1.3 and Kv1.5 channels forming heterotetrameric channels66–68. Although not explicitly tested in the studies, such heterotetrameric channels could have redox sensitivity due to the involvement of the Kv1.5 components, which would help explain why the null animals had reduced vasodilation to H2O2. Another possibility would relate to some actions of H2O2 on vasodilatory pathways that are not considered redox-mediated69.
Implications in coronary microcirculatory physiology and pathophysiology
We previously proposed that H2O2 is a metabolic vasodilator, linking metabolism to flow in the heart70. In the scheme we proposed, the mitochondrial production of H2O2, which is directly linked to the rate of electron transfer by mitochondria, is a feed-forward signal, in that as cardiac metabolism increases, the production of H2O2 also rises. In that paper, we reported that the relationship between coronary blood flow and myocardial oxygen consumption was shifted by the Kv channel antagonist, 4-aminopyridine, resulting in less flow (compared to unblocked animals) for a given level of oxygen consumption. The key role that Kv channels play in this process was also reported by Berwick et al.71 Moreover, Goodwill et al observed that correolide reduced basal coronary blood flow and attenuated both metabolic and ischemic vasodilation.72 The present study builds upon these results by honing into a specific Kv channel, the Kv1.3 channel, in part responsible for the coupling of flow to metabolism. We do not view the present results suggesting a role for Kv1.3 channels as being discrepant from our recent results supporting the concept that Kv1.5 channels were important for coronary metabolic dilation64. With a process as important as coupling blood flow to metabolism, we project that likely there would be redundant mechanisms facilitating the connection.
One limitation in concluding that Kv1.3 channels links metabolism to flow is that we do not have insights into the oxygen balance in the myocardium. Previously we found that mice null for Kv1.5 channels show lower myocardial oxygen tensions at rest (compared to wild type).14 Moreover, during a cardiac stress test with norepinephrine, myocardial oxygen tensions fell considerably in the null mice, but were maintained in wild types. This indicates that in the latter, the balance between oxygen supply and demand (consumption) is maintained in wild types, but the demands for oxygen exceed the supply in the Kv1.5 nulls leading to tissue hypoxia. However, we cannot draw such an unequivocal conclusion in the present study because we did not make the measurements.
We would also like to mention some caveats about interpretations of the results shown in Figure 3. We do not advocate the interpretation that the figure shows that the cardiac efficiency is greater in Kv1.3−/− mice than wild type mice. This interpretation could be drawn from the two relationships, where for a given level of work, flow is less in the null mice than in wild types. The reason we do not believe this to be occurring is due to the nature of the experimental protocol where flow during the stress test is measured during a 30–45 second period after the effects of norepinephrine are first observed, which is often followed by a period of acute cardiac failure (especially at the high doses of norepinephrine). This failure implies insufficient oxygen supply to meet the heightened oxygen needs during the stress test. Evidence for this paradigm is shown in Figure 4 with the reductions in arterial pressure at the two highest doses of norepinephrine (especially at the highest dose of correolide), where pressure starts to decline before the infusion of norepineprhine is stoppled. We interpret this as a insufficient flow to meet the oxygen demands of the heart, which leads to acute pump failure. Importantly, the flow measurements shown in Figure 3 are obtained at the early portions of the stress test when flow is maintained.
The role of the coronary microcirculation in ischemic heart disease has received more attention in the recent past. In part, this appreciation has been the result of large trials and clinical studies that have reported women, without large vessel disease, show symptoms consistent with myocardial ischemia when stressed73–75. In another clinical trial, 30.5% of women with unstable angina and 10.2% of women with STEMI had normal coronary arteries76. Interestingly, women showing symptoms of myocardial ischemia, in the absence of large vessel disease, also have impaired coronary vasodilator reserve77. Perhaps some inherent dysfunction in Kv1.3 channels contributes to the microvascular pathology in patients. At this point it is premature to draw any conclusion, or even speculate about a role of Kv1.3 channels in coronary microvascular disease in patients, because to date genetic studies have not shown any association of this channel with the disorder78.
Conclusions
Our results are consistent with the concept that Kv1.3 channels play a critical role in coronary metabolic dilation, the process that links blood flow to metabolism in the heart.
Perspectives.
Despite the profound importance in the maintenance of normal cardiac pump function via aerobic metabolism, the link between cardiac work and myocardial blood flow remains incompletely understood. Our study has identified that Kv1.3 channels are an effector of this link. The significance of our observation may facilitate a better understanding of the cohort of patients with microvascular angina, that is to say, ischemic heart disease attributed to coronary microvascular pathology.
Acknowledgments
This work was supported by NIH/NHLBI (HL100828Z and HL83366), Fibus Family Foundation, Frances Schermer Charitable Trust & The Lillian Schermer Charitable Trust, and the Joy Cone Company to W.M.C.; NIH/NHLBI (HL115114) to LY, AHA (10POST4360030) to VO, and EB004031 to PK.
References
- 1.Deussen A, Ohanyan V, Jannasch A, Yin L, Chilian W. Mechanisms of metabolic coronary flow regulation. J Mol Cell Cardiol. 2012;52:794–801. doi: 10.1016/j.yjmcc.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Grover RF. Mechanisms augmenting coronary arterial oxygen extraction. Advances in cardiology. 1973;9:89–98. doi: 10.1159/000393428. [DOI] [PubMed] [Google Scholar]
- 3.Wolff CB. Normal cardiac output, oxygen delivery and oxygen extraction. Advances in experimental medicine and biology. 2007;599:169–182. doi: 10.1007/978-0-387-71764-7_23. [DOI] [PubMed] [Google Scholar]
- 4.Tennant R, Wiggers CJ. THE EFFECT OF CORONARY OCCLUSION ON MYOCARDIAL CONTRACTION. 1935 [Google Scholar]
- 5.Berne RM, Rubio R, Dobson JG, Jr, Curnish RR. Adenosine and adenine nucleotides as possible mediators of cardiac and skeletal muscle blood flow regulation. Circ Res. 1971;28(Suppl 1):115+. [PubMed] [Google Scholar]
- 6.Feigl EO. Coronary Physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Sabouni MH, Hussain T, Cushing DJ, Mustafa SJ. G proteins subserve relaxations mediated by adenosine receptors in human coronary artery. Journal of Cardiovascular Pharmacology. 1991;18:696–702. doi: 10.1097/00005344-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Sharifi-Sanjani M, Zhou X, Asano S, Tilley S, Ledent C, Teng B, Dick GM, Mustafa SJ. Interactions between A(2A) adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am J Physiol Heart Circ Physiol. 2013;304:H1294–H1301. doi: 10.1152/ajpheart.00637.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Teng B, Tilley S, Ledent C, Mustafa SJ. Metabolic hyperemia requires ATP-sensitive K+ channels and H2O2 but not adenosine in isolated mouse hearts. Am J Physiol Heart Circ Physiol. 2014;307:H1046–H1055. doi: 10.1152/ajpheart.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Rajamani U, Labazi H, Tilley SL, Ledent C, Teng B, Mustafa SJ. Involvement of NADPH oxidase in A2A adenosine receptor-mediated increase in coronary flow in isolated mouse hearts. Purinergic Signal. 2015;11:263–273. doi: 10.1007/s11302-015-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stepp DW, Kroll K, Feigl EO. K+ATP channels and adenosine are not necessary for coronary autoregulation. Am J Physiol. 1997;273:H1299–H1308. doi: 10.1152/ajpheart.1997.273.3.H1299. [DOI] [PubMed] [Google Scholar]
- 12.Stepp DW, Van Bibber R, Kroll K, Feigl EO. Quantitative relation between interstitial adenosine concentration and coronary blood flow. Circulation Research. 1996;79:601–610. doi: 10.1161/01.res.79.3.601. [DOI] [PubMed] [Google Scholar]
- 13.Guarini G, Ohanyan VA, Kmetz JG, DelloStritto DJ, Thoppil RJ, Thodeti CK, Meszaros JG, Damron DS, Bratz IN. Disruption of TRPV1-mediated coupling of coronary blood flow to cardiac metabolism in diabetic mice: role of nitric oxide and BK channels. Am J Physiol Heart Circ Physiol. 2012;303:H216–H223. doi: 10.1152/ajpheart.00011.2012. [DOI] [PubMed] [Google Scholar]
- 14.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FK, Janota D, Oyewumi MO, Logan S, Lindner JR, Chilian WM. Requisite Role of Kv1.5 Channels in Coronary Metabolic Dilation. Circ Res. 2015;117:612–621. doi: 10.1161/CIRCRESAHA.115.306642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming WW. Membrane potential and vascular smooth muscle sensitivity. Blood vessels. 1987;24:108–112. doi: 10.1159/000158680. [DOI] [PubMed] [Google Scholar]
- 16.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 18.Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand. 1998;164:549–557. doi: 10.1046/j.1365-201X.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu GB, Zhou EX, Qing DX, Li J. Role of potassium channels in regulation of rat coronary arteriole tone. European journal of pharmacology. 2009;620:57–62. doi: 10.1016/j.ejphar.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of Kv1 channels in vasoregulation. Circ Res. 2006;99:53–60. doi: 10.1161/01.RES.0000229654.45090.57. [DOI] [PubMed] [Google Scholar]
- 21.Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. The American journal of physiology. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- 22.Jones CJ, Kuo L, Davis MJ, Chilian WM. Regulation of coronary blood flow: coordination of heterogeneous control mechanisms in vascular microdomains. Cardiovasc Res. 1995;29:585–596. [PubMed] [Google Scholar]
- 23.Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation. 1995;92:518–525. doi: 10.1161/01.cir.92.3.518. [DOI] [PubMed] [Google Scholar]
- 24.Somlyo AP, Somlyo AV. Smooth muscle: excitation-contraction coupling, contractile regulation, and the cross-bridge cycle. Alcoholism: Clinical & Experimental Research. 1994;18:138–143. doi: 10.1111/j.1530-0277.1994.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 25.van Breemen C, Aaronson P, Loutzenhiser R, Meisheri K. Ca2+ movements in smooth muscle. Chest. 1980;78:157–165. doi: 10.1378/chest.78.1_supplement.157. [DOI] [PubMed] [Google Scholar]
- 26.Saitoh S-i, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick G, Swafford A, Chilian WM. Hydrogen Peroxide. A Feed-Forward Dilator That Couples Myocardial Metabolism to Coronary Blood Flow. Arterioscler Thromb Vasc Biol. 2006 doi: 10.1161/01.ATV.0000249408.55796.da. 01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 27.Saitoh S, Kiyooka T, Rocic P, Rogers PA, Zhang C, Swafford A, Dick GM, Viswanathan C, Park Y, Chilian WM. Redox-dependent coronary metabolic dilation. Am J Physiol Heart Circ Physiol. 2007;293:H3720–H3725. doi: 10.1152/ajpheart.00436.2007. [DOI] [PubMed] [Google Scholar]
- 28.Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- 29.Chung I, Schlichter LC. Regulation of native Kv1.3 channels by cAMP-dependent protein phosphorylation. Am J Physiol. 1997;273:C622–C633. doi: 10.1152/ajpcell.1997.273.2.C622. [DOI] [PubMed] [Google Scholar]
- 30.Cidad P, Jimenez-Perez L, Garcia-Arribas D, Miguel-Velado E, Tajada S, Ruiz-McDavitt C, Lopez-Lopez JR, Perez-Garcia MT. Kv1.3 channels can modulate cell proliferation during phenotypic switch by an ion-flux independent mechanism. Arterioscler Thromb Vasc Biol. 2012;32:1299–1307. doi: 10.1161/ATVBAHA.111.242727. [DOI] [PubMed] [Google Scholar]
- 31.Colley B, Tucker K, Fadool DA. Comparison of modulation of Kv1.3 channel by two receptor tyrosine kinases in olfactory bulb neurons of rodents. Receptors Channels. 2004;10:25–36. [PMC free article] [PubMed] [Google Scholar]
- 32.Kosolapov A, Tu L, Wang J, Deutsch C. Structure acquisition of the T1 domain of Kv1.3 during biogenesis. Neuron. 2004;44:295–307. doi: 10.1016/j.neuron.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Xu C, Chen L, Xu P, Xiong H. Involvement of Kv1.3 and p38 MAPK signaling in HIV-1 glycoprotein 120-induced microglia neurotoxicity. Cell Death Dis. 2012;3:e254. doi: 10.1038/cddis.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Koni PA, Wang P, Li G, Kaczmarek L, Wu Y, Li Y, Flavell RA, Desir GV. The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight. Hum Mol Genet. 2003;12:551–559. doi: 10.1093/hmg/ddg049. [DOI] [PubMed] [Google Scholar]
- 35.Vianna-Jorge R, Oliveira CF, Garcia ML, Kaczorowski GJ, Suarez-Kurtz G. Correolide, a nor-triterpenoid blocker of Shaker-type Kv1 channels elicits twitches in guinea-pig ileum by stimulating the enteric nervous system and enhancing neurotransmitter release. Br J Pharmacol. 2000;131:772–778. doi: 10.1038/sj.bjp.0703620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 37.Vogel R, Indermuhle A, Reinhardt J, Meier P, Siegrist PT, Namdar M, Kaufmann PA, Seiler C. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. Journal of the American College of Cardiology. 2005;45:754–762. doi: 10.1016/j.jacc.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 38.Coggins MP, Sklenar J, Le DE, Wei K, Lindner JR, Kaul S. Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow. Circulation. 2001;104:2471–2477. doi: 10.1161/hc4501.098954. [DOI] [PubMed] [Google Scholar]
- 39.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 40.Komaru T, Tanikawa T, Sugimura A, Kumagai T, Sato K, Kanatsuka H, Shirato K. Mechanisms of coronary microvascular dilation induced by the activation of pertussis toxin-sensitive G proteins are vessel-sized dependent: heterogeneous involvement of nitiric oxide pathway and ATP-sensitive K+ channels. Circulation Research. 1997;80:1–10. doi: 10.1161/01.res.80.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. K+Ca-channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.00315.2006. [DOI] [PubMed] [Google Scholar]
- 42.Aversano T, Ouyang P, Silverman H. The ATP-sensitive K+ channel does not modulate metabolic vasodilation. Circulaiton. 1991;84(suppl. II):II 183. [Google Scholar]
- 43.Tune JD, Richmond KN, Gorman MW, Feigl EO. K(ATP)(+) channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2001;280:H868–H875. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- 44.Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem. 1998;273:13578–13582. [PubMed] [Google Scholar]
- 45.Yao X, Chang AY, Boulpaep EL, Segal AS, Desir GV. Molecular cloning of a glibenclamide-sensitive, voltage-gated potassium channel expressed in rabbit kidney. J Clin Invest. 1996;97:2525–2533. doi: 10.1172/JCI118700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol. 2008;294:H2371–H2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- 47.Lintermans LL, Stegeman CA, Heeringa P, Abdulahad WH. T cells in vascular inflammatory diseases. Front Immunol. 2014;5:504. doi: 10.3389/fimmu.2014.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam J, Wulff H. The Lymphocyte Potassium Channels Kv1.3 and KCa3.1 as Targets for Immunosuppression. Drug Dev Res. 2011;72:573–584. doi: 10.1002/ddr.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klumpp DJ, Song EJ, Ito S, Sheng MH, Jan LY, Pinto LH. The Shaker-like potassium channels of the mouse rod bipolar cell and their contributions to the membrane current. J Neurosci. 1995;15:5004–5013. doi: 10.1523/JNEUROSCI.15-07-05004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koeberle PD, Wang Y, Schlichter LC. Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Differ. 2010;17:134–144. doi: 10.1038/cdd.2009.113. [DOI] [PubMed] [Google Scholar]
- 51.Gazula VR, Strumbos JG, Mei X, Chen H, Rahner C, Kaczmarek LK. Localization of Kv1.3 channels in presynaptic terminals of brainstem auditory neurons. J Comp Neurol. 2010;518:3205–3220. doi: 10.1002/cne.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan D, Lee JC, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Expression and biophysical properties of Kv1 channels in supragranular neocortical pyramidal neurones. J Physiol. 2006;571:371–389. doi: 10.1113/jphysiol.2005.097006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson MC, Biju KC, Hoffman J, Fadool DA. Odor enrichment sculpts the abundance of olfactory bulb mitral cells. Neurosci Lett. 2013;541:173–178. doi: 10.1016/j.neulet.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lioudyno MI, Birch AM, Tanaka BS, Sokolov Y, Goldin AL, Chandy KG, Hall JE, Alkire MT. Shaker-related potassium channels in the central medial nucleus of the thalamus are important molecular targets for arousal suppression by volatile general anesthetics. J Neurosci. 2013;33:16310–16322. doi: 10.1523/JNEUROSCI.0344-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millar ID, Wang S, Brown PD, Barrand MA, Hladky SB. Kv1 and Kir2 potassium channels are expressed in rat brain endothelial cells. Pflugers Arch. 2008;456:379–391. doi: 10.1007/s00424-007-0377-1. [DOI] [PubMed] [Google Scholar]
- 56.Olschewski A. Blocking potassium channels: a new principle for treating restenosis? Cardiovasc Res. 2011;89:255–257. doi: 10.1093/cvr/cvq388. [DOI] [PubMed] [Google Scholar]
- 57.Jackson WF. KV1.3: a new therapeutic target to control vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2010;30:1073–1074. doi: 10.1161/ATVBAHA.110.206565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cidad P, Miguel-Velado E, Ruiz-McDavitt C, Alonso E, Jimenez-Perez L, Asuaje A, Carmona Y, Garcia-Arribas D, Lopez J, Marroquin Y, Fernandez M, Roque M, Perez-Garcia MT, Lopez-Lopez JR. Kv1.3 channels modulate human vascular smooth muscle cells proliferation independently of mTOR signaling pathway. Pflugers Arch. 2015;467:1711–1722. doi: 10.1007/s00424-014-1607-y. [DOI] [PubMed] [Google Scholar]
- 59.Gulbins E, Sassi N, Grassme H, Zoratti M, Szabo I. Role of Kv1.3 mitochondrial potassium channel in apoptotic signalling in lymphocytes. Biochim Biophys Acta. 2010;1797:1251–1259. doi: 10.1016/j.bbabio.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Leanza L, Henry B, Sassi N, Zoratti M, Chandy KG, Gulbins E, Szabo I. Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak-independent death of cancer cells. EMBO Mol Med. 2012;4:577–593. doi: 10.1002/emmm.201200235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Remillard CV, Tigno DD, Platoshyn O, Burg ED, Brevnova EE, Conger D, Nicholson A, Rana BK, Channick RN, Rubin LJ, O'Connor D T, Yuan JX. Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2007;292:C1837–C1853. doi: 10.1152/ajpcell.00405.2006. [DOI] [PubMed] [Google Scholar]
- 63.Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 64.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz JG, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FS, Janota D, Oyewumi MO, Logan SJ, Lindner JR, Chilian WM. Requisite Role of Kv1.5 Channels in Coronary Metabolic Dilation. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.306642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Archer SL, Weir EK, Reeve HL, Michelakis E. Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation. Adv Exp Med Biol. 2000;475:219–240. doi: 10.1007/0-306-46825-5_21. [DOI] [PubMed] [Google Scholar]
- 66.Vicente R, Escalada A, Villalonga N, Texido L, Roura-Ferrer M, Martin-Satue M, Lopez-Iglesias C, Soler C, Solsona C, Tamkun MM, Felipe A. Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages. J Biol Chem. 2006;281:37675–37685. doi: 10.1074/jbc.M605617200. [DOI] [PubMed] [Google Scholar]
- 67.Vicente R, Villalonga N, Calvo M, Escalada A, Solsona C, Soler C, Tamkun MM, Felipe A. Kv1.5 association modifies Kv1.3 traffic and membrane localization. J Biol Chem. 2008;283:8756–8764. doi: 10.1074/jbc.M708223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villalonga N, Escalada A, Vicente R, Sanchez-Tillo E, Celada A, Solsona C, Felipe A. Kv1.3/Kv1.5 heteromeric channels compromise pharmacological responses in macrophages. Biochem Biophys Res Commun. 2007;352:913–918. doi: 10.1016/j.bbrc.2006.11.120. [DOI] [PubMed] [Google Scholar]
- 69.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res. 2012;110:471–480. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 71.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52:912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodwill AG, Noblet JN, Sassoon D, Fu L, Kassab GS, Schepers L, Herring BP, Rottgen TS, Tune JD, Dick GM. Critical contribution of KV1 channels to the regulation of coronary blood flow. Basic Res Cardiol. 2016;111:56. doi: 10.1007/s00395-016-0575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J, Sharaf B, Rogers WJ, Mankad S, Forder JR, Kelsey SF, Pohost GM. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:2993–2999. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 74.Marroquin OC, Kip KE, Kelley DE, Johnson BD, Shaw LJ, Bairey Merz CN, Sharaf BL, Pepine CJ, Sopko G, Reis SE. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: a report from the Women's Ischemia Syndrome Evaluation. Circulation. 2004;109:714–721. doi: 10.1161/01.CIR.0000115517.26897.A7. [DOI] [PubMed] [Google Scholar]
- 75.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong PW, Fu Y, Chang WC, Topol EJ, Granger CB, Betriu A, Van de Werf F, Lee KL, Califf RM. Acute coronary syndromes in the GUSTO-IIb trial: prognostic insights and impact of recurrent ischemia. The GUSTO-IIb Investigators. Circulation. 1998;98:1860–1868. doi: 10.1161/01.cir.98.18.1860. [DOI] [PubMed] [Google Scholar]
- 77.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fedele F, Mancone M, Chilian WM, Severino P, Canali E, Logan S, De Marchis ML, Volterrani M, Palmirotta R, Guadagni F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res Cardiol. 2013;108:387. doi: 10.1007/s00395-013-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]