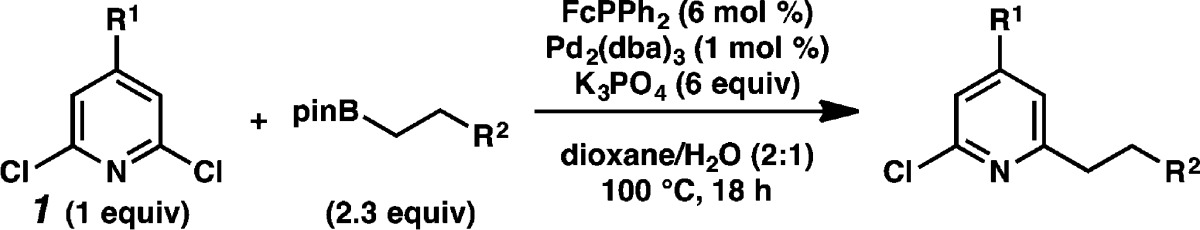Abstract

Among cross-coupling reactions, the Suzuki–Miyaura transformation stands out because of its practical advantages, including the commercial availability and low toxicity of the required reagents, mild reaction conditions, and functional group compatibility. Nevertheless, few conditions can be used to cross-couple alkyl boronic acids or esters with aryl halides, especially 2-pyridyl halides. Herein, we describe two novel Suzuki–Miyaura protocols that enable selective conversion of polychlorinated aromatics, with a focus on reactions to convert 2,6-dichloropyridines to 2-chloro-6-alkylpyridines or 2-aryl-6-alkylpyridines.
The 2-alkylpyridyl motif is critically important within natural products, pharmaceutical agents, fragrances, ligands for catalysis, molecular electronic devices, and biomimetic scaffolds (Figure 1).1 Several powerful strategies have been reported recently to form new C(sp2)–C(sp3) bonds in order to access substituted pyrdines.2−5 In terms of cross-coupling reactions, access to 2-pyridyl organometallic reagents6,7 is imperfect,8 as most remain difficult to isolate or have limited stability. Additionally, many of the associated reactions require toxic reagents or prove ineffective with primary alkyl electrophiles. By contrast, heteroaryl chlorides are readily accessible, but have remained challenging substrates9,10 particularly in Suzuki–Miyaura10,11 reactions. Herein, we disclose systems for the reaction of bench stable alkyl pinacol boronic esters with aryl chlorides, with a focus on polyhalogenated 2-pyridyl chlorides. Polyhalogenated six-membered heteroarenes12 react with predictable selectivity (Scheme 1, eq 1) and undergo successive cross-coupling reactions with distinct organoboron compounds to generate two new carbon–carbon bonds serially in a single reaction vessel (eq 2).
Figure 1.
Alkylpyridine motifs are critical. Glc = d-glucose.1
Scheme 1. Selective and Serial Reactions.
Owing to their relatively low cost and broad commercial availability, heteroaryl chlorides offer practical value over heteroaryl bromides and iodides as substrates in Suzuki–Miyaura reactions. Nevertheless, this transformation is challenging due to (1) the strength of aryl chloride bonds relative to aryl iodide or bromide bonds13 and (2) the ability of heteroatoms to coordinate to and inhibit the reactivity of transition metal catalysts. Alkylboron reagents add to these challenges as they can contribute to formation of undesired byproducts through protodeboronation, protodehalogenation, oxidation, and homocoupling.
To overcome these limitations, we chose to focus on the reaction of 2,6-dichloropyridine (1a) with heptyl pinacol boronic ester (2a). We sought conditions to generate 2-chloro-6-alkylpyridine 3a selectively (Table 1). The optimal conditions would not furnish exhaustively cross-coupled 2,6-dialkylpyridine 4a.
Table 1. Reaction Optimization.

Determined by 1H NMR.
5 mol % Pd.
80 °C.
110 °C.
1 mol % Pd.
2-Heptylpyridine (14% yield) was detected by 1H NMR.
The targeted bond-forming reaction proceeds with poor efficiency using many of the cutting-edge protocols designed to couple aryl halides with organoboron compounds (entries 1–4).14 We anticipated that use of sterically encumbered bidentate phosphine ligands would promote the desired reaction.15 Indeed, some bidentate ligands furnish 2-chloro-6-heptylpyridine (3a, entries 5–7).
The denticity of these bidentate ligands is not critical to reaction efficiency. When a monodentate analogue of DPEPhos, (o-MeOC6H4)PPh2, is employed, high catalyst turnover numbers are achieved, though reaction selectivity erodes (entry 8). Under the optimal conditions, a monodentate analogue of dppf, FcPPh2, is used as a ligand. To the best of our knowledge FcPPh2 has been described only once as a ligand for Suzuki–Miyaura reactions,16 though more sterically encumbered analogues are in common use.17 Under the optimal conditions, 2,6-dichloropyridines react with heptyl boronic pinacol ester in 2:1 dioxane/H2O at 100 °C with K3PO4 as a base, 1 mol % Pd2(dba)3, and 6 mol % FcPPh2 to afford desired 2-chloro-6-heptylpyridine (3a) in excellent selectivity and 74% isolated yield (entry 9).18
FcPPh2 proves to be more effective in this reaction than many standard ligands, including PPh3 (entry 10). Relative to a phenyl group, the ferrocenyl group is more sterically encumbering19 and better able to stabilize adjacent partial positive charge.20 These features have great effect, as PPh3 furnishes desired 3a in only 21% 1H NMR yield.
Under these selective conditions, chloropyridines react in good yields with alkyl pinacol boronic esters that are saturated, or incorporate distal functional groups including an olefin, an acetal masked aldehyde, and a primary alkyl chloride (Table 2). These conditions are tolerant of nitro and ketone functional groups, transforming electron-deficient chlorobenzene analogues efficiently (Table 3, entries 1, 2). Electronic perturbations influence reactivity: electron-rich 4-chloroanisole does not react under these conditions (Table 3, entry 3).
Table 2. Tolerated Functional Group Variations.

General reaction conditions: 1.0 equiv of aryl chloride, 0.13 M dioxane/H2O (2:1), 2.3 equiv of R2(CH2)2Bpin, 1 mol % Pd2(dba)3, 6 mol % FcPPh2, 6.0 equiv of K3PO4, 18 h, 100 °C.
Isolated yield.
48 h.
Isolated after reaction with trifluoroacetic acid.
1.5 equiv of R2(CH2)2Bpin, 3.0 equiv of K3PO4.
Table 3. Selectivity Based on Electronic Bias.

General reaction conditions: 1.0 equiv of aryl chloride, 0.13 M dioxane/H2O (2:1), 2.3 equiv of R2(CH2)2Bpin, 1 mol % Pd2(dba)3, 6 mol % FcPPh2, 6.0 equiv of K3PO4, 18–20 h, 100 °C.
Isolated yield.
1.5 equiv of R2(CH2)2Bpin, 3.0 equiv of K3PO4.
Not detected by 1H NMR.
Exhaustively coupled product (6% yield) was detected by crude 1H NMR.
48 h.
Isolated after reaction with trifluoroacetic acid.
2 mol % Pd2(dba)3, 12 mol % FcPPh2.
These conditions face expected limitations in terms of substrate scope. Aromatic compounds that are sensitive to the combination of water and base, such as 2,4- and 2,5-dichloropyrimidine, degrade under the reaction conditions.
The reaction proceeds selectively to generate the 2-chloro-6-alkylpyridine rather than the 2,6-dialkylpyridine. In principle, the selectivity of the reaction may arise from the 6-alkyl substituent performing the following roles: (a) hindering precoordination of the catalyst by sterically shielding the pyridine nitrogen, (b) raising the transition state barrier for oxidative addition based on sterically induced strain,21 (c) disfavoring transmetalation by sterically encumbering the reactive aryl–Pd species, or (d) increasing the barrier to oxidative addition or transmetalation electronically. To evaluate these possibilities, we probed the differences in substrate reactivity (Table 3). If the steric size of the alkyl substituent were the principal determinant of selectivity, we would expect to see statistical mixtures of coupled products for substrates with distal chlorides, yet these substrates couple selectively (entries 4–6).
Furthermore, these conditions affect the chemoselective reaction of 1,3-dichloroisoquinoline at the more sterically encumbered C(1) position (entry 7). The observed selectivity complements known reactions to install an aryl group at C(1).22 Moreover, this selectivity has been predicted21,23,24 and rationalized by comparing the expected bond dissociation energies of 1,3-dichloroisoquinoline at C(1) and C(3).21 This same logic predicts that 2,8-dichloroquinoline will undergo cross-coupling at C(2), as is observed (entry 8).
As selectivity is not principally steric in origin, electron donation by the new alkyl substituent must increase the barrier either to oxidative addition or to transmetalation. This hypothesis is consistent with the remarkably efficient transformation of 2-chloro-6-tert-butoxypyridine, which incorporates an inductively withdrawing group meta- to the activated C–Cl bond (entry 9), and the diminished reactivity of 5-chloro-2-methoxypyridine, which includes a resonance donating group para- to the targeted C–Cl bond (entry 10).
The power of this cross-coupling method can be demonstrated through the combination of our method with Burke’s conditions6d to transform 2,6-dichloropyridine to an antimycoplasmal agent25 (Scheme 2) via 2-chloro-6-butylpyridine (Table 2, entry 4).
Scheme 2. Serial Cross-Coupling Transformations.
We sought to merge the selective method with a compatible reaction, which would obviate the need to isolate the 2-chloro-6-alkylpyridine intermediate. The resultant one-pot serial reaction relies on the rapid rate of transmetalation of arylboron compounds relative to alkylboron reagents (Table 4). These conditions transform a wide range of arylboron compounds, including arylboronic acids and esters, trifluoroborate salts,26 and N-methyl-iminodiacetic acid (MIDA) boronate esters27 (entries 1–4). The reaction can convert furyl, thiophenyl, and pyridyl boron compounds, as well as an estrone derivative (entries 6–9) to furnish high value differently substituted pyridines efficiently.
Table 4. Iterative Cross-Coupling Reactions.


General reaction conditions: 1.0 equiv of aryl chloride, 0.13 M dioxane/H2O (2:1), 2.3 equiv of R2(CH2)2Bpin, 1 mol % Pd2(dba)3, 6 mol % FcPPh2, 6.0 equiv of K3PO4, 18 h, 100 °C then 2.0 equiv of organoboron reagent, 18–20 h unless noted.
Isolated yield.
5 h.
The disclosed methods couple inexpensive and readily available chloroaryl subunits with bench and air stable pinacol boronic esters. A chemoselective transformation mediated by FcPPh2/Pd2(dba)3 predictably and selectively converts polychlorinated pyridine or benzene starting materials to monoalkylated products. These conditions allow efficient access to an antimycoplasmal agent. Additionally, a one-pot sequential Suzuki–Miyaura reaction of alkyl- and then arylboron compounds has been developed to access differentially substituted pharmacologically relevant motifs.
Acknowledgments
This project was funded by the American Chemical Society Petroleum Research Fund Doctoral New Investigator Program (PRF# 54824-DNI1) and by Duke University. J.M.B. was supported by the National Institute of General Medical Sciences (T32GM007105-41). Characterization data were obtained on instrumentation secured by funding from the NSF (CHE-0923097, ESI-MS, George Dubay, the Duke Dept. of Chemistry Instrument Center), or the NSF, the NIH, HHMI, the North Carolina Biotechnology Center and Duke (Duke Magnetic Resonance Spectroscopy Center). EI-MS data were obtained by M. Walla at the Univ. of South Carolina Chemistry and Biochemistry Mass Spectrometry Center. Jacob Timmerman, Prof. S. Malcolmson and Prof. R. Widenhoefer of Duke University are thanked for their insights.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.6b02323.
Full experimental details, copies of NMR spectra (PDF)
Author Present Address
† Department of Chemistry and Chemical Biology, IUPUI, Indianapolis, IN 46202.
The authors declare no competing financial interest.
Supplementary Material
References
- a Liu J.; Nakamura S.; Matsuda H.; Yoshikawa M. Tetrahedron Lett. 2013, 54, 32–34. 10.1016/j.tetlet.2012.10.052. [DOI] [Google Scholar]; b Schinz H.; Ruzicka L.; Geyer U.; Prelog V. Helv. Chim. Acta 1946, 29, 1524–1528. [Google Scholar]; c Zhang Y.-H.; Shi B.-F.; Yu J.-Q. J. Am. Chem. Soc. 2009, 131, 5072–5074. 10.1021/ja900327e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Davis J. M.; Truong A.; Hamilton A. D. Org. Lett. 2005, 7, 5405–5408. 10.1021/ol0521228. [DOI] [PubMed] [Google Scholar]
- Goetz A. E.; Garg N. K. Nat. Chem. 2013, 5, 54–60. 10.1038/nchem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For select examples that involve distinct bond-forming and deprotonation steps, see:; a Jeffrey J. L.; Sarpong R. Org. Lett. 2012, 14, 5400–5403. 10.1021/ol3024117. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chen Q.; León T.; Knochel P. Angew. Chem., Int. Ed. 2014, 53, 8746–8750. 10.1002/anie.201400750. [DOI] [PubMed] [Google Scholar]; c Hyodo I.; Tobisu M.; Chatani N. Chem. - Asian J. 2012, 7, 1357–1365. 10.1002/asia.201100971. [DOI] [PubMed] [Google Scholar]
- a Lewis J. C.; Bergman R. G.; Ellman J. A. J. Am. Chem. Soc. 2007, 129, 5332–5333. 10.1021/ja070388z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nakao Y.; Yamada Y.; Kashihara N.; Hiyama T. J. Am. Chem. Soc. 2010, 132, 13666–13668. 10.1021/ja106514b. [DOI] [PubMed] [Google Scholar]
- For select Minisci-type pathways, see:; a Jin J.; MacMillan D. W. C. Nature 2015, 525, 87–90. 10.1038/nature14885. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gianatassio R.; Kawamura S.; Eprile C. L.; Foo K.; Ge J.; Burns A. C.; Collins M. R.; Baran P. S. Angew. Chem., Int. Ed. 2014, 53, 9851–9855. 10.1002/anie.201406622. [DOI] [PMC free article] [PubMed] [Google Scholar]; c O’Hara F.; Blackmond D. G.; Baran P. S. J. Am. Chem. Soc. 2013, 135, 12122–12134. 10.1021/ja406223k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For select 2-pyridyl zinc reagents, see:; a Cornella J.; Edwards J. T.; Qin T.; Kawamura S.; Wang J.; Pan C.-M.; Gianatassio R.; Schmidt M.; Eastgate M. D.; Baran P. S. J. Am. Chem. Soc. 2016, 138, 2174–2177. 10.1021/jacs.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Colombe J. R.; Bernhardt S.; Stathakis C.; Buchwald S. L.; Knochel P. Org. Lett. 2013, 15, 5754–5757. 10.1021/ol402798z. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Luzung M. R.; Patel J. S.; Yin J. J. Org. Chem. 2010, 75, 8330–8332. 10.1021/jo1018798. [DOI] [PubMed] [Google Scholar]; For select 2-pyridyl boron reagents, see:; d Dick G. R.; Woerly E. M.; Burke M. D. Angew. Chem., Int. Ed. 2012, 51, 2667–2672. 10.1002/anie.201108608. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Sakashita S.; Takizawa M.; Sugai J.; Ito H.; Yamamoto Y. Org. Lett. 2013, 15, 4308–4311. 10.1021/ol402268g. [DOI] [PubMed] [Google Scholar]; f Billingsley K. L.; Buchwald S. L. Angew. Chem., Int. Ed. 2008, 47, 4695–4698. 10.1002/anie.200801465. [DOI] [PMC free article] [PubMed] [Google Scholar]; For 2-pyridyl tin reagents, see:; g Littke A. F.; Schwarz L.; Fu G. C. J. Am. Chem. Soc. 2002, 124, 6343–6348. 10.1021/ja020012f. [DOI] [PubMed] [Google Scholar]; h Bailey T. R. Tetrahedron Lett. 1986, 27, 4407–4410. 10.1016/S0040-4039(00)84964-3. [DOI] [Google Scholar]; For 2-pyridyl aluminum reagents, see:; i Chen X.; Zhou L.; Li Y.; Xie T.; Zhou S. J. Org. Chem. 2014, 79, 230–239. 10.1021/jo4024123. [DOI] [PubMed] [Google Scholar]
- For a select coupling reaction, see:Llaveria J.; Leonori D.; Aggarwal V. K. J. Am. Chem. Soc. 2015, 137, 10958–10961. 10.1021/jacs.5b07842. [DOI] [PubMed] [Google Scholar]
- a Old D. W.; Wolfe J. P.; Buchwald S. L. J. Am. Chem. Soc. 1998, 120, 9722–9723. 10.1021/ja982250+. [DOI] [Google Scholar]; b Wolfe J. P.; Singer R. A.; Yang B. H.; Buchwald S. L. J. Am. Chem. Soc. 1999, 121, 9550–9561. 10.1021/ja992130h. [DOI] [Google Scholar]
- For select Negishi and Kumada reactions, see:; a Malhotra S.; Seng P. S.; Koenig S. G.; Deese A. J.; Ford K. A. Org. Lett. 2013, 15, 3698–3701. 10.1021/ol401508u. [DOI] [PubMed] [Google Scholar]; b Fürstner A.; Leitner A. Angew. Chem., Int. Ed. 2003, 42, 308–311. 10.1002/anie.200390103. [DOI] [PubMed] [Google Scholar]; c Getmanenko Y. A.; Twieg R. J. J. Org. Chem. 2008, 73, 830–839. 10.1021/jo701812t. [DOI] [PubMed] [Google Scholar]; For cross-electrophile reactions, see:; d Everson D. A.; Buonomo J. A.; Weix D. J. Synlett 2014, 25, 233–238. 10.1055/s-0033-1340151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Martin R.; Buchwald S. L. Acc. Chem. Res. 2008, 41, 1461–1473. 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Billingsley K.; Anderson K. W.; Buchwald S. L. Angew. Chem., Int. Ed. 2006, 45, 3484–3488. 10.1002/anie.200600493. [DOI] [PubMed] [Google Scholar]; c Li L.; Zhao S.; Joshi-Pangu A.; Diane M.; Biscoe M. R. J. Am. Chem. Soc. 2014, 136, 14027–14033. 10.1021/ja508815w. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Jouffroy M.; Primer D. N.; Molander G. A. J. Am. Chem. Soc. 2016, 138, 475–478. 10.1021/jacs.5b10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Miyaura N.; Suzuki A. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]; b Lennox A. J. J.; Lloyd-Jones G. C. Chem. Soc. Rev. 2014, 43, 412–443. 10.1039/C3CS60197H. [DOI] [PubMed] [Google Scholar]
- a Rossi R.; Bellina F.; Lessi M. Adv. Synth. Catal. 2012, 354, 1181–1255. 10.1002/adsc.201100942. [DOI] [Google Scholar]; b Fairlamb I. Chem. Soc. Rev. 2007, 36, 1036–1045. 10.1039/b611177g. [DOI] [PubMed] [Google Scholar]
- a Bedford R. B.; Cazin C. S. J.; Holder D. Coord. Chem. Rev. 2004, 248, 2283–2321. 10.1016/j.ccr.2004.06.012. [DOI] [Google Scholar]; b Littke A. F.; Fu G. C. Angew. Chem., Int. Ed. 2002, 41, 4176–4211. . [DOI] [PubMed] [Google Scholar]
- a Li Q.; Liskey C. W.; Hartwig J. F. J. Am. Chem. Soc. 2014, 136, 8755–8765. 10.1021/ja503676d. [DOI] [PubMed] [Google Scholar]; b Yang C. T.; Zhang Z. Q.; Tajuddin H.; Wu C. C.; Liang J.; Liu J. H.; Fu Y.; Czyzewska M.; Steel P. G.; Marder T. B.; Liu L. Angew. Chem., Int. Ed. 2012, 51, 528–532. 10.1002/anie.201106299. [DOI] [PubMed] [Google Scholar]; c Fleury-Bregeot N.; Presset M.; Beaumard F.; Colombel V.; Oehlrich D.; Rombouts F.; Molander G. A. J. Org. Chem. 2012, 77, 10399–10408. 10.1021/jo3021665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. F. Synlett 2006, 2006, 1283–1294. 10.1055/s-2006-939728. [DOI] [Google Scholar]
- Milde B.; Lohan M.; Schreiner C.; Ruffer T.; Lang H. Eur. J. Inorg. Chem. 2011, 2011, 5437–5449. 10.1002/ejic.201100842. [DOI] [Google Scholar]
- a Pickett T. E.; Roca F. X.; Richards C. J. J. Org. Chem. 2003, 68, 2592–2599. 10.1021/jo0265479. [DOI] [PubMed] [Google Scholar]; b Liu S.-Y.; Choi M. J.; Fu G. C. Chem. Commun. 2001, 2408–2409. 10.1039/b107888g. [DOI] [PubMed] [Google Scholar]
- The mass balance for this reaction is principally accounted for by starting material and the desired product.
- Otto S. J. Chem. Crystallogr. 2001, 31, 185–190. 10.1023/A:1014339015004. [DOI] [Google Scholar]
- Allenmark S. Tetrahedron Lett. 1974, 15, 371–374. 10.1016/S0040-4039(01)82217-6. [DOI] [Google Scholar]
- Legault C. Y.; Garcia Y.; Merlic C. A.; Houk K. N. J. Am. Chem. Soc. 2007, 129, 12664–12665. and references therein. 10.1021/ja075785o. [DOI] [PubMed] [Google Scholar]
- Ford A.; Sinn E.; Woodward S. J. Chem. Soc., Perkin Trans. 1 1997, 927–934. 10.1039/a605827b. [DOI] [Google Scholar]
- Handy S. T.; Zhang Y. Chem. Commun. 2006, 299–301. 10.1039/B512948F. [DOI] [PubMed] [Google Scholar]
- Garcia Y.; Schoenebeck F.; Legault C. Y.; Merlic C. A.; Houk K. N. J. Am. Chem. Soc. 2009, 131, 6632–6639. 10.1021/ja9004927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijper P. J.; Van der Goot H.; Timmerman H.; Nauta W. T. Eur. J. Med. Chem. 1984, 19, 399–404. [Google Scholar]
- Molander G. A. J. Org. Chem. 2015, 80, 7837–7848. 10.1021/acs.joc.5b00981. [DOI] [PubMed] [Google Scholar]
- a Mancilla T.; Contreras R.; Wrackmeyer B. J. Organomet. Chem. 1986, 307, 1–6. 10.1016/0022-328X(86)80169-3. [DOI] [Google Scholar]; b Li J.; Grillo A. S.; Burke M. D. Acc. Chem. Res. 2015, 48, 2297–2307. 10.1021/acs.accounts.5b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.