Abstract
Background & objectives:
The process of human placentation is complex and still not well understood. This study was aimed to examine the relationship between clinical features of pre-eclampsia and degree of trophoblastic invasion after its immunohistochemical visualization in the context of possible alterations in the number of natural killer (NK) cells and macrophages in the decidua.
Methods:
This prospective study included a study group comprising 30 pregnant women with pre-eclampsia delivered by caesarean section and a control group comprising 20 healthy pregnant women also delivered by caesarean section. Samples of placental bed obtained during caesarean section were analyzed after immunohistochemical labelling CD56+ NK cells, CD68+ macrophages and cytokeratin 7 trophoblastic cells.
Results:
In pre-eclampsia, there was a significantly lower number of CD56+ NK cells in the decidua (P<0.001) and a higher number of CD68+ macrophages (P<0.001) compared to control group. In the subgroup of pre-eclampsia with intrauterine growth retardation (IUGR), a significantly greater number of NK cells (P<0.05) was recorded, as well as an increased number of macrophages, but not significantly compared to pre-eclampsia without IUGR. There was no significant difference in the distribution of these cells in the decidua in relation to the severity of pre-eclampsia. CD56+ NK cells were significantly less (P<0.05) and macrophages were more (P<0.05) in the group with poor trophoblastic invasion.
Interpretation & conclusions:
Alterations in the number of immune cells in relation to the degree of trophoblastic invasion indicated their role in aetiopathogenesis of pre-eclampsia, while the direct association between their number and severity of pre-eclampsia was not confirmed.
Keywords: Decidua, macrophages, natural killer cells, preeclampsia, trophoblast
Inadequate placental invasion is associated with reproductive complications such as pre-eclampsia, intrauterine growth retardation (IUGR) and recurrent pregnancy loss. Initial changes in the uterine spiral arteries begin before cellular interaction with cytotrophoblasts1 including generalized changes in the wall of these arteries such as endothelial basophilia, vacuolization and disorganization of smooth muscle cell layer. The main event of later stages of remodelling is dilatation and elongation of blood vessels associated with endovascular trophoblastic cells that come in direct contact with maternal blood2, as well as the impact on the remodelling of spiral arteries by increasing the diameter of blood vessels and formation of the zones of low resistance with high blood flow. Increased blood flow is essential for embryonic development, and the lack of modification of spiral arteries is the basis of a pregnancy complication such as pre-eclampsia3.
During human pregnancy, trophoblastic invasion and vascular remodelling are synchronized with the expansion of the activated population of maternal uterine natural killer (uNK) cells in the decidua. These decidual NK cells get hemoattraction from peripheral blood and then were differentiated in situ4. It has been shown that the uNK cells are present in large numbers in the early decidua and accumulated, especially at the site of implantation, where these can closely contact the invading trophoblastic placental cells5. Previous studies have emphasized the role of trophoblast in preserving the stability of blood vessels, as well as in the modification of spiral arteries, but it has also been shown that uNK cells regulate the key developmental processes in the human foeto-maternal interface such as the control of extravillous trophoblastic invasion (EVT) in the decidua, myometrium and vascular development6,7,8.
Macrophages also participate in implantation and decidualization and play a very important role in the synchronization of the elements of the immune system. During human pregnancy, the majority of decidual macrophages are near the invading EVT indicating their role in mediating both normal and pathological placentation, as well as the modulation of placental response to infection. Macrophages are also involved in the degradation of the extracellular matrix of local tissue contributing to EVT invasion9. Furthermore, macrophages perform phagocytosis of apoptotic cells in the decidua during EVT invasion and remodelling process10. Ingestion of these apoptotic cells promotes Th2 cytokine secretion by macrophages, which plays an important role in the initiation of immune tolerance. This accelerates further invasion of EVT. However, incomplete removal of apoptotic cells leads to the release of intracellular contents from apoptotic bodies and into the induction of proinflammatory responses, which can cause further damage of the tissue11. Hence, if the macrophages are dysregulated, these contribute to poor placentation. Macrophages play a dual role: the promotion of normal pregnancy (defence against pathogens, removal of apoptotic cells during trophoblastic invasion and vascular remodelling, secretion of angiogenic factors and contribution to angiogenesis and promoting foetomaternal tolerance through Th2 cytokine production), as well as the development of pathological pregnancy - pre-eclampsia (aberrant macrophage infiltration of the decidua, trophoblastic invasion inhibition, induction of trophoblast apoptosis under the influence of pro-inflammatory stimuli).
This study was undertaken to examine the association between the clinical features of pre-eclampsia as a major manifestation of pregnancy on the basis of altered placentation, the degree of trophoblast invasion after its immunohistochemical visualization in the context of possible alterations in the number of NK cells and macrophages in the decidua.
Material & Methods
At the Clinic of Gynecology and Obstetrics and the Pathology and Pathological Anatomy Center, Clinical Center Nis, Serbia, a prospective, case-control, observational study was conducted from 2009 to 2014. The examined group consisted of 30 pregnant women with pregnancies complicated by pre-eclampsia, ended by caesarean section, singletons, with no foetal anomalies and preexisting clinical disorders in pregnant women. The sample size was restricted because of the time and cost involved in the procedures. Criteria for diagnosis of pre-eclampsia were new-onset arterial hypertension, or diastolic pressure ≥90 mmHg and systolic pressure ≥140 mmHg, measured in two separate occasions within 24 h, with a gap of more than 6 h and proteinuria ≥300 mg of protein in 24 h urine which were developed after the 20th gestational week in previously normotensive women12. The examined study group was divided into two subgroups based on the presence of IUGR where the criterion for setting the diagnosis was the birth weight of neonates below the 10th percentile for a given gestational age. There was also a division of the examined group into two subgroups based on the severity of pre-eclampsia where the criterion for the diagnosis of severe pre-eclampsia was the presence of one of the following: systolic blood pressure ≥160 mmHg or diastolic ≥110 mmHg, proteinuria ≥2 g/24 h, increased serum creatinine, persistent headache or cerebro-visual disorders, persistent epigastric pain, platelet count <100,000/μl and/or findings of microangiopathic haemolytic anaemia (with increased lactate-dehydrogenase)13. The control group consisted of 20 healthy pregnant women with singleton pregnancies, no foetal anomalies, delivered by elective caesarean section due to other obstetric indications that cannot be associated with the aetiopathogenesis of tested disorders. The examined and the control groups consisted of the pregnant Caucasian women hospitalized in the High-risk Pregnancies Department at the University Clinic of Gynecology and Obstetrics, Clinical Center Nis. The research protocol was approved by the Ethical Committee of Medical Faculty University of Nis and with the informed written consent of involved participants.
Decidual tissue was obtained during the caesarean section from the place of placental bed by using large curette after the birth of placenta. After routine fixation, processing and moulding, 4 mm thick sections of decidua tissue were stained with haematoxylin-eosin (H-E) stain, quality control of the sampled tissues was performed first, and only those samples containing extravillous trophoblast (decidua basalis) were selected and included in further analysis (Fig. 1). Following the selection of appropriate sections, immunohistochemical analysis was conducted using EnVision + System – horseradish peroxidase method. We used monoclonal antibodies manufactured by Dako - Agilent Technologies (Glostrup, Denmark): monoclonal mouse anti-human CD56 clone 123C3 antibody, NK cells marker; monoclonal mouse anti-human CD68 clone PG-M1 antibody, a macrophage marker and monoclonal mouse anti-human cytokeratin 7 (CK7) clone OV-TL 12/30 antibody, a trophoblastic cell marker.
Fig. 1.
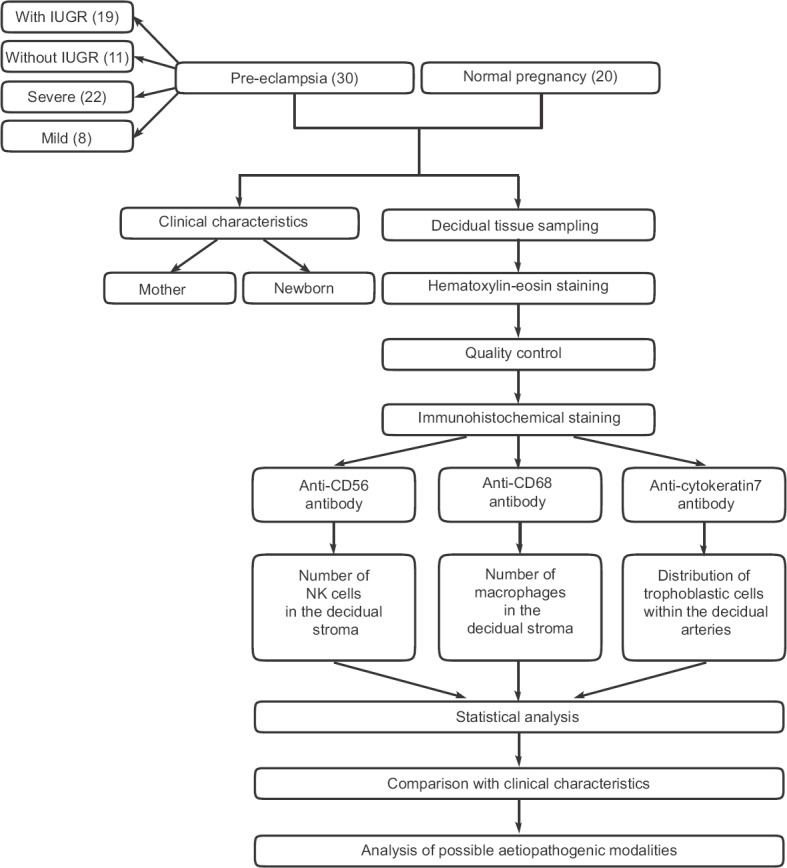
Flowchart of study design. Number of patients in each category is given in parentheses. IUGR, intrauterine growth retardation; NK, natural killer cells.
In brief, after standard pretreatment, blocking of endogenous peroxidase activity and thorough washing in the buffer and incubation with the primary antibody were performed at room temperature for 1 h. The visualization of marked antigens was obtained by chromogenic substance di-amino-benzidine-tetra-hydrochloride. Specifically marked morphological elements, using planimetric methods, were quantified by counting each type of immunohistochemically labelled cells in the decidual stroma on every section. The number of cells positive for specific marker (CD56 and CD68) was determined by counting all stained cells in 10 representative high-power fields, while the trophoblastic cells labelled by anti-CK7 antibody were determined by their distribution within the circumference of the decidual arteries as a visually estimated predominant finding: (i) Adequate trophoblastic invasion and transformation of spiral arteries (the trophoblastic cells are present in more than a half of the circumference of the vessel as a visually estimated predominant finding and spiral arteries are transformed - the vessel wall lacks leio-muscular cells, and there is fibrinoid deposition); and (ii) Inadequate trophoblastic invasion and poor transformation of spiral arteries (the trophoblastic cells are present in less than a half of the circumference of the vessel and spiral arteries are untransformed as a visually estimated predominant finding).
Statistical analysis: Statistical analysis was performed in SPSS version 15.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as mean values, standard deviations, median and interquartile range, while the qualitative ones were presented by their frequency and percentage. Determination of the normality of distribution of continuous variables was performed by Shapiro-Wilk test. If the distribution of continuous data was normal, comparison of arithmetic means of two independent samples was performed by Student's t test for independent samples, and if it was not, Mann-Whitney U-test was performed. Comparison of absolute frequencies of categorical variables was performed by Chi-square test and its variants in relation to the size of samples.
Results
Table I shows the clinical characteristics of pregnancies in the study group complicated by pre-eclampsia compared to the control group. The mean age of patients with pre-eclampsia was 31.3±5.9 yr (range: 19-41 yr) and was slightly higher than in the control group (29.5±4.5, range: 22-40 yr), but there was no significant difference in the age of pregnant women. Parity of pregnant women was significantly lower in the study group compared to the control group (P<0.01). The average gestational age in pre-eclampsia at the time of delivery was significantly lower than in the control group (P<0.001). Severe pre-eclampsia in the study group was reported in 73.3 per cent of patients, while in 63.3 per cent, pre-eclampsia was associated with IUGR. The average birth weight of neonates in pre-eclampsia was lower, and the perinatal outcome was poorer. All newborns were livebirths.
Table I.
Clinical characteristics of pregnancies in the study group complicated by pre-eclampsia compared to the control group
In pre-eclampsia group, there was significantly lower number of CD56+ NK cells in the decidual stroma (P<0.001) and a significantly higher number of CD68+ macrophages (P<0.001) compared to control group (Table II). Fig. 2 represents macrophages labelled by anti-CD68 antibody (Fig. 2A) and NK cells labelled by anti-CD56 antibody (Fig. 2B) in decidual stroma. Within the group of pre-eclampsia, the subset of pre-eclampsia associated with IUGR reported a significantly higher number of NK cells (P<0.05) and also the higher number of macrophages in the decidual stroma, compared to the subgroup of pre-eclampsia without IUGR. There was no significant difference in the distribution of these immune cells in the decidua in relation to the severity of pre-eclampsia. After immunohistochemical visualization of trophoblastic cells in whole sample (study and control groups), there was 24 cases of adequate (17 cases in control and 7 cases in study groups) and 26 cases of inadequate trophoblastic invasion (23 cases in study and 3 cases in control groups). In a group of pre-eclampsia, CD56+ NK cells were significantly (P<0.05) less frequent and the number of CD68+ macrophages was significantly (P<0.05) higher in the group with inadequate trophoblastic invasion compared to the group with adequate invasion (Table III).
Table II.
Number of natural killer (NK) cells and macrophages in the decidual stroma within the group of pre-eclampsia, control group, subgroups of pre-eclampsia with or without intrauterine growth retardation (IUGR) and subgroups of pre-eclampsia in relation to the severity of pre-eclampsia
Fig. 2.
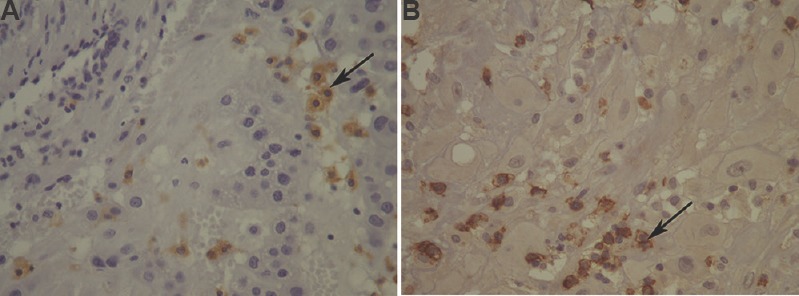
(A) Macrophages in the decidual stroma (arrow; CD68, ×400) (B) natural killer (NK) cells in the decidual stroma (arrow; CD56, ×400).
Table III.
Number of natural killer (NK) cells and macrophages in the decidual stroma in relation to the trophoblastic invasion of decidual spiral arteries
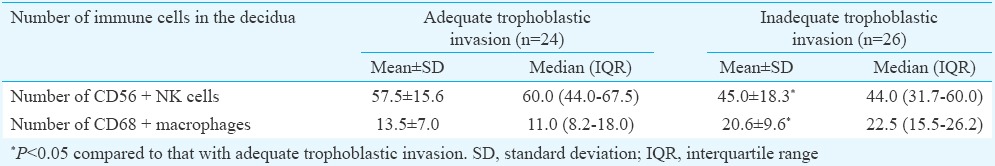
Using histopathological sections after immunohistochemical visualization of trophoblastic cells with CK7 antibody, a comparative review was done of the adequate trophoblastic invasion and transformed spiral artery from the control group (Fig. 3A) and the inadequate trophoblastic invasion and untransformed spiral artery of the decidua in pre-eclampsia (Fig. 3B).
Fig. 3.
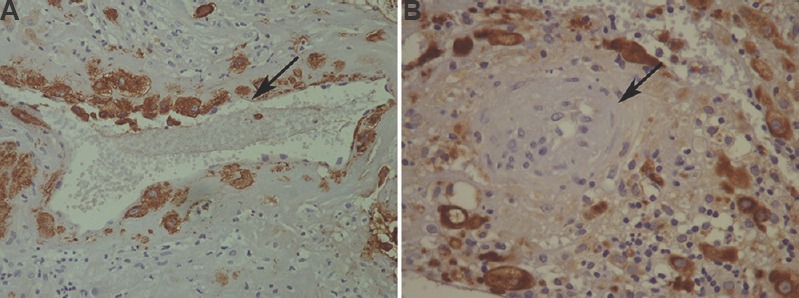
Distribution of trophoblastic cells in a decidua: (A) transformed spiral artery with presence of trophoblastic cells in more than a half of the circumference of the artery (arrow; CK7, ×400) (B) untransformed spiral artery, trophoblastic cells are present only in the decidual stroma (arrow; CK7, ×400).
Discussion
In our study group pre-eclampsia with IUGR was seen in 63.3 per cent women, higher than that reported in literature, around 30 per cent of cases of preeclampsia14. This could be due to the composition of our study group in which more than two-thirds were patients with severe pre-eclampsia often associated with foetal growth restriction and a more lenient criterion for the diagnosis of IUGR. There was a difference in parity and gestational age between the study and control groups, as the control group consisted of healthy women with uncomplicated pregnancies, without premature rupture of membranes and chorioamnionitis, delivered by caesarean section which was mostly indicated by previous caesarean section, hence greater parity and term delivery.
As the composition of immune cells in the decidua is complex and as there is a great variety of immunocompetent decidual cells, changes in their population could be an important factor in aetiopathogenesis of various disorders in pregnancy, particularly those dependent on implantation. Although the distribution of immune cells in the decidua has been extensively explored, their role has not been fully understood, and the data on the presence of any immunocompetent cell are highly divergent15. The number of decidual NK cells in pre-eclampsia may be without significant change relative to control16 and significantly lower17,18,19,20 or even significantly higher21,22. In our study, there was a significantly higher number of decidual macrophages in pre-eclampsia compared to uncomplicated pregnancy, as shown earlier in several studies23,24,25,26, while other groups reported no difference or even the lower number of decidual macrophages in pre-eclampsia18,19,27. There are differences in the way of decidual tissue sampling, the method of quantifying immunocompetent cells and the difference in the gestational age of the test and control groups.
In our study, the number of CD56+ NK cells in the decidua was significantly lower in pre-eclampsia, while the number of macrophages in this group was significantly higher. We also examined the ratio of the number of these immune cells in the decidua and distribution of trophoblastic cells within the arterial blood vessels of the decidua after their immunohistochemical visualization using anti-CK7 antibody to CK. The number of CD56+ NK cells in the decidua was significantly lower in the group with poor trophoblast invasion, while the number of macrophages was higher in this group. These results were consistent with the results obtained in the study group with pre-eclampsia compared to the control group. Activated macrophages induce apoptosis of EVT in vitro28 which results in increased death of trophoblastic cells that could be central for poor placentation in pre-eclampsia. There was no significant difference in the distribution of these immune cells in the decidua in relation to the severity of pre-eclampsia, while the number of NK cells and macrophages in the decidua in pre-eclampsia with IUGR was higher than in the subgroup without IUGR. This could imply the role of decidual immune cells in the aetiopathogenesis of pre-eclampsia, but with no direct impact on the severity of the clinical manifestation, which is due to generalized systemic endothelial reaction and multifactorial aetiology. It seems that the clinical manifestations cannot be easily connected with the number of these immune cells in the decidua. Although altered placentation is a key feature of pre-eclampsia, it is probably not sufficient to explain all the symptoms associated with pre-eclampsia. Therefore, some other pre-existing maternal factors must be present to result in a pre-eclamptic syndrome. A lower number of decidual NK cells in pre-eclampsia compared to uncomplicated pregnancy and higher in pre-eclampsia with IUGR compared to pre-eclampsia without IUGR may point to different aetiopathogenesis of pre-eclampsia with and without IUGR. Stallmach et al21 also found the increased number of NK cells in the decidua in the case of pre-eclampsia with IUGR. Changes in placental bed in terms decidual vasculopathy are rather the characteristics of the pre-eclampsia than the growth restriction of the fetus29. Placentas in the late-onset of pre-eclampsia are similar to the placentas with normal pregnancy and with newborns who are of normal birth weight20. It is primarily IUGR, but not alone pre-eclampsia that is associated with changes in placental morphology including changes in villous and foetal vascularization30. The assumption that pre-eclampsia with and without IUGR may have different aetiopathogenesis has been supported by the results of other studies29,30.
There were some limitations to this study. First, there was a difference in gestational age between the study and control groups, but for ethical reasons, could not be avoided. Second, the insufficient implantation underlying pre-eclampsia takes place in the early pregnancy, while the clinical manifestations of the disease appear only late in pregnancy. Since the period between implantation and tissue sample collection for analysis was long, whether these changes observed in the composition of immune cells reflected the mechanisms involved in the pathogenesis of pre-eclampsia or it was the consequence of a disease, was not known. Third, not only the number of immune cells, but also their phenotype and cytokine profiles are important which have not been done.
Alterations in the number of NK cells and macrophages in pre-eclampsia indicate the important role of immune cells in the decidua in the pathogenesis of inadequate placentation, but whether it is their absolute number, percentage, presence at certain gestational ages, their immune phenotype, functional activity, receptors or gene expression are subject of extensive multidisciplinary research. This might lead to the development of diagnostic screening tests for the early detection of the disorder, adequate access and monitoring, and also therapeutic options for the improvement of maternal and perinatal outcome.
Footnotes
Conflicts of Interest: None.
References
- 1.Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1998;19:241–52. doi: 10.1016/s0143-4004(98)90055-8. [DOI] [PubMed] [Google Scholar]
- 2.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–58. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–54. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54:281–94. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 5.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–74. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 7.Kopcow HD, Karumanchi SA. Angiogenic factors and natural killer (NK) cells in the pathogenesis of preeclampsia. J Reprod Immunol. 2007;76:23–9. doi: 10.1016/j.jri.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak-Kim J, Gilman-Sachs A. Clinical implication of natural killer cells and reproduction. Am J Reprod Immunol. 2008;59:388–400. doi: 10.1111/j.1600-0897.2008.00596.x. [DOI] [PubMed] [Google Scholar]
- 9.Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18:458–71. doi: 10.1093/humupd/dms015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–82. doi: 10.1111/j.1600-0897.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 11.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26:877–97. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 12.Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–22. [PubMed] [Google Scholar]
- 13.Roberts JM. Pregnancy related hypertension. In: Creasy RK, Resnik R, Iams JD, editors. Maternal-fetal medicine. 5th ed. USA: Saunders, Elsevien Inc; 2004. pp. 859–97. [Google Scholar]
- 14.Srinivas SK, Edlow AG, Neff PM, Sammel MD, Andrela CM, Elovitz MA. Rethinking IUGR in preeclampsia: dependent or independent of maternal hypertension? J Perinatol. 2009;29:680–4. doi: 10.1038/jp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh CC, Chao KC, Huang SJ. Innate immunity, decidual cells, and preeclampsia. Reprod Sci. 2013;20:339–53. doi: 10.1177/1933719112450330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Rodríguez EN, Nava-Salazar S, Mendoza-Rodríguez CA, Moran C, Romero-Arauz JF, Ortega E, et al. Persistence of decidual NK cells and KIR genotypes in healthy pregnant and preeclamptic women: a case-control study in the third trimester of gestation. Reprod Biol Endocrinol. 2011;9:8. doi: 10.1186/1477-7827-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockwood CJ, Huang SJ, Chen CP, Huang Y, Xu J, Faramarzi S, et al. Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol. 2013;183:841–56. doi: 10.1016/j.ajpath.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieger L, Segerer S, Bernar T, Kapp M, Majic M, Morr AK, et al. Specific subsets of immune cells in human decidua differ between normal pregnancy and preeclampsia - A prospective observational study. Reprod Biol Endocrinol. 2009;7:132. doi: 10.1186/1477-7827-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams PJ, Bulmer JN, Searle RF, Innes BA, Robson SC. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction. 2009;138:177–84. doi: 10.1530/REP-09-0007. [DOI] [PubMed] [Google Scholar]
- 20.Eide IP, Rolfseng T, Isaksen CV, Mecsei R, Roald B, Lydersen S, et al. Serious foetal growth restriction is associated with reduced proportions of natural killer cells in decidua basalis. Virchows Arch. 2006;448:269–76. doi: 10.1007/s00428-005-0107-z. [DOI] [PubMed] [Google Scholar]
- 21.Stallmach T, Hebisch G, Orban P, Lü X. Aberrant positioning of trophoblast and lymphocytes in the feto-maternal interface with pre-eclampsia. Virchows Arch. 1999;434:207–11. doi: 10.1007/s004280050329. [DOI] [PubMed] [Google Scholar]
- 22.Akhlaq M, Nagi AH, Yousaf AW. Placental morphology in pre-eclampsia and eclampsia and the likely role of NK cells. Indian J Pathol Microbiol. 2012;55:17–21. doi: 10.4103/0377-4929.94848. [DOI] [PubMed] [Google Scholar]
- 23.Schonkeren D, van der Hoorn ML, Khedoe P, Swings G, von Beelen E, Claas F, et al. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am J Pathol. 2011;178:709–17. doi: 10.1016/j.ajpath.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schröder W, et al. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta. 1999;20:229–33. doi: 10.1053/plac.1998.0373. [DOI] [PubMed] [Google Scholar]
- 25.Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1 beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–52. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilczynski JR, Tchórzewski H, Banasik M, Glowacka E, Wieczorek A, Lewkowicz P, et al. Lymphocyte subset distribution and cytokine secretion in third trimester decidua in normal pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2003;109:8–15. doi: 10.1016/s0301-2115(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Romero R, Cushenberry E, Kim YM, Erez O, Nien JK, et al. Distribution of CD14+ and CD68+ macrophages in the placental bed and basal plate of women with preeclampsia and preterm labor. Placenta. 2007;28:571–6. doi: 10.1016/j.placenta.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–52. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- 29.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61:1254–60. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 30.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113:580–9. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]


