Abstract
Background & objectives:
Japanese encephalitis (JE) caused by mosquito-borne Flavivirus is one of the leading causes of viral encephalitis in Asia. Control strategies include vector control and human vaccination. Due to lack of immunization programmes in endemic regions, there are still high mortality and morbidity. A live-attenuated SA 14-14-2 JE vaccine (LAJEV) has been licensed and used in Asian countries, including India. We report the assessment of immunogenicity and safety of the vaccine in adults during the first mass adult vaccination campaign carried out in Assam, India.
Methods:
One thousand and seventy five adults (aged ≥15 yr) who received LAJEV were monitored for adverse events following immunization for one year. The safety assessment of vaccinated population was evaluated till 28 days and at 6 and 12 months. Blood samples collected from the enrolled participants were tested by plaque reduction neutralization test (PRNT50) to assess the neutralizing antibody titres (NATs) before vaccination and 28 days, six and 12 months post-vaccination (PV).
Results:
Among the 1075 vaccinated individuals, four reported minor adverse effects from 30 min to 28 days PV. Based on the pre-vaccination NAT, the study participants were categorized as seronegative, moderately seropositive and strongly seropositive. Nearly 85.5 per cent of JE seronegative participants seroconverted by 28 days PV. The geometric mean titre (GMT) in all the three groups increased by 28 days and decreased by six and 12 months PV. Nearly 60 per cent of the moderately positive individuals exhibited four-fold rise in GMT, 28 days PV. Almost 95.5 per cent of the participants in the study population remained seroprotected at the end of 12 months PV.
Interpretation & conclusions:
This study on immunogenicity and safety of LAJEV in adults showed that a single dose of the live-attenuated vaccine was safe and induced protective immunity to both JE seronegative and naturally seropositive adults. Further study is required to find out long term protective efficacy of this vaccine.
Keywords: Immunogenicity, live-attenuated Japanese encephalitis vaccine, safety, seroprotection
Japanese encephalitis (JE) is a mosquito-borne disease which has a complex interaction between human beings, principal hosts such as pigs and birds and mosquito vectors (Culex sp.)1. It has been estimated that approximately 50,000 cases of JE occur annually, with 25-35 per cent case fatality rates and more than 30 per cent of survivors suffer from severe long-term disabilities2. JE has recently spread widely in South-East Asia as well as into new geographical areas including Australia and the UK3. Since the first report of JE in 1955, frequent outbreaks have been reported from northern, northeastern and southern parts of India4,5.
The disease has been endemic in Assam, a northeastern State of India since 19766. Initially, young children of rural areas were known to be vulnerable to the disease. However, following mass JE vaccination campaigns among children, adult JE cases were found to outnumber paediatric cases in different States of India, including Assam6. Considering the situation, the Government of Assam introduced a mass vaccination drive in a campaign mode among adults (≥15 yr) with the live-attenuated SA 14-14-2 JE vaccine (LAJEV) in Sivasagar, a JE-endemic district of Assam, during October-November 20117. The mass vaccination campaign in adults was an initiative taken by the Health Department of Assam, Government of India, on a pilot basis. The protective effect and safety of a single dose of LAJEV in children have shown promising results8,9. It has also been used in adults in some JE-endemic districts of Nepal10. However, the question remained whether LAJEV could be used among adults as safely as it had been used for children in India11. Hence, the present study was undertaken to estimate the immunogenicity and safety of a single dose of LAJEV in adult population in Assam, India.
Material & Methods
The Government of Assam undertook the first adult JE mass vaccination campaign in Sivasagar, India, from October 9 to November 28, 2011, using lyophilized LAJEV (CD-JEVAX™, Chengdu Institute of Biological Products, Chengdu, PR China). Our study was a nested study in line with the government's JE vaccination campaign. This observational study was conducted to estimate the immunogenicity elicited by LAJEV in adults (≥15 yr) over a period of 12 months in the study area. The safety of the vaccine was evaluated as a second objective. Adequate care was taken in selection of the area for immunogenicity and safety studies. Demow, a moderately JE-affected primary health centre (PHC), was selected avoiding highly JE-endemic or very low JE-reporting areas. The endemicity was based on case load per year per PHC and thus categorized as high (>10 cases per yr), moderate (1-10 cases per yr) and low (<1 case per yr). Two villages, Khongia, inhabited mostly by tea tribes (Adivasi community), and an adjoining village, Hiloidhari, inhabited by ethnic Assamese population of the PHC, were included in the study.
Sample size: Administration of the JE vaccine was solely undertaken by the Government of Assam. However, to estimate the immunogenicity and safety of the vaccine, sample size was calculated based on the conservative estimates. Assuming a natural seropositivity of about 90 per cent with an absolute precision of 2 per cent and the confidence level of 95 per cent, the sample size was calculated to be 864. We took into consideration 25 per cent loss due to various causes. To compensate these losses, the sample size was increased by 25 per cent. Thus, the total sample required was 864+216=1080, which was rounded up to 1100. These participants were selected from the two contiguous villages selected on the basis of practical considerations including ease of approach and population receptiveness for the one year follow up. Informed written consent was obtained from all the participants before enrolment in the study. Complete participant details were recorded in a pre-designed proforma. The study was approved by the Institutional Ethics Committee of the ICMR- Regional Medical Research Centre, Dibrugarh, Assam.
Study participants: Healthy adults aged ≥15 yr suitable for vaccination as per the government's programme and residing in the study locations were eligible for the study. The exclusion criteria included: any vaccination in the four weeks prior to the study; history of seizures, allergic disease or reactions likely to be exacerbated by any component of the vaccine; immunodeficiency, HIV; any major congenital abnormality requiring surgery or chronic treatment and a history of documented suspected encephalitis or meningitis. All the adults at the study sites were identified through a door-to-door survey. The entire adult population of 1293 participants of the two villages voluntarily agreed to participate in the study. However, among them, 1075 participants turned up for vaccination at the government vaccination booths and were subsequently followed up after vaccination.
Immunogenicity assessment: Blood samples (4 ml) for immunogenicity were collected before vaccination (day 0) and post-vaccination (PV) on 28 days, six and 12 months. Serum samples were separated, appropriately labelled and stored at -70°C until further processing. Serum samples were tested for JE virus neutralizing antibody titre (NAT) by plaque reduction neutralization test (PRNT)12. BHK-21 cell line and an Indian JEV strain (GP 057434), both received from ICMR-National Institute of Virology, Pune, India were used for the assay. Initially, the serum samples were heat inactivated for 30 min at 56°C. The 4-fold diluted serum samples (1:10, 1:40, 1:160, 1:640) were mixed with 100 plaque-forming units of JEV strain, and infectivity was determined in pre-seeded BHK 21 cell monolayer. The serum dilution that showed a 50 per cent neutralizing dose was considered the neutralizing end point12.
Safety assessment: Following vaccination, the participants were observed in the vaccination facility for local or general reactions for 30 min. The participants were visited daily from one to seven days PV to monitor any adverse event (AE). Further, AEs were recorded at 14, 21, 28 days and at months six and 12 (Figure). Local reactions monitored included pain, erythema and swelling at the injection site.
Figure.
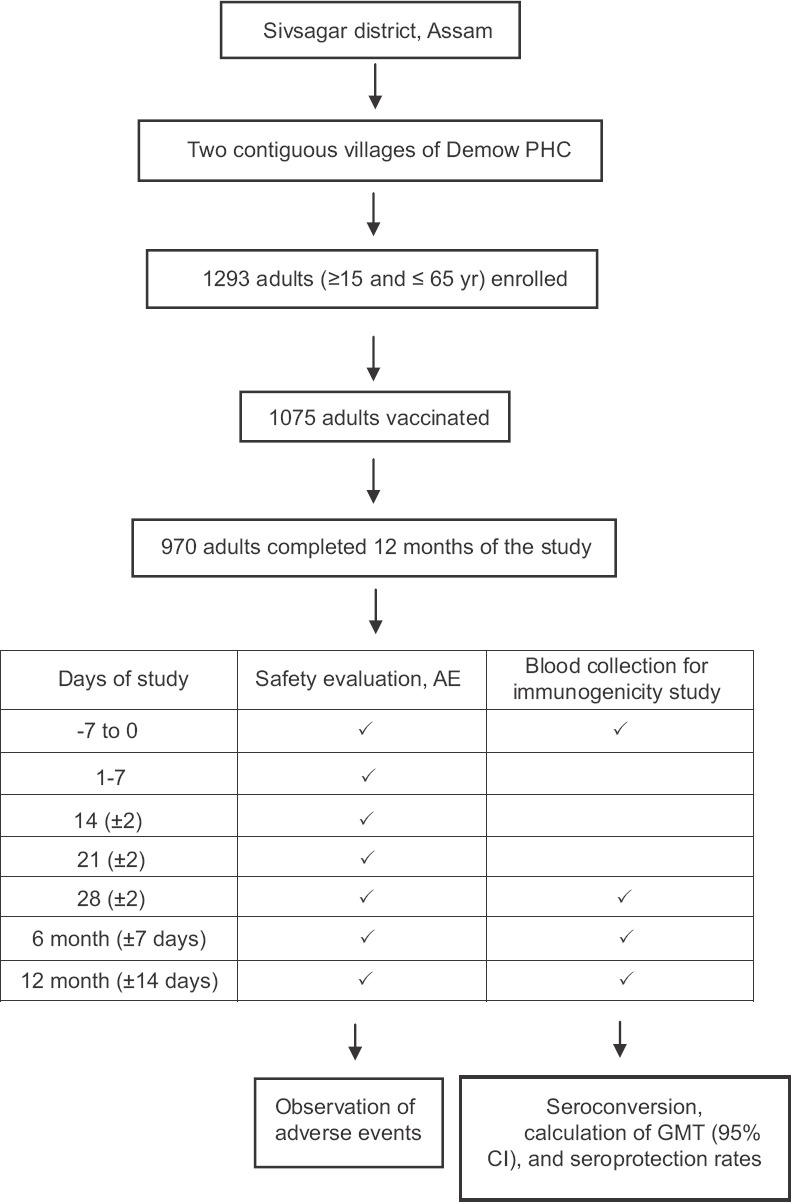
Study profile and disposition of participant; PHC, primary health centre; AE, adverse events; GMT, geometric mean titre; CI, confidence interval.
Statistical analysis: Information recorded in the proforma was entered in Epi Info, version 7 (CDC, USA). A cut-off value to determine seropositivity and seroprotectivity of NAT was defined as PRNT50 ≥1013. Seropositive participants were defined as those having a reciprocal antibody titre above or equal to the cut-off value and seronegative participants were those falling below the cut-off value. PRNT50 NAT≥10 in seronegative individuals or a ≥4-fold rise in titre of seropositive individuals at baseline was defined as seroconverted14. The fold increase was calculated as the individuals’ pre-vaccination (baseline) immunogenicity value by PV value15. Subsequently, geometric mean titre (GMT) was calculated as the antilog of the mean of individual log-transformed titres.
Results
During October 2011, of the 1293 adults enrolled in the study, 1075 turned up for vaccination. The ratio of male to female was 1.04, and the median age was 33 yr (744 adults of age 15-35 yr, 411 of 36-55 yr and 138 of >56 yr) at enrolment. Among the vaccinated individuals, 79 were lost to follow up during the 28 days PV, 92 during six months PV and 91 during 12 months PV. In total, 105 samples were missed in at least one of the blood collection drives. Therefore, of the 1075 individuals, only 970 participants from whom all four blood samples could be collected, were considered as the final study participants for the immunogenicity study.
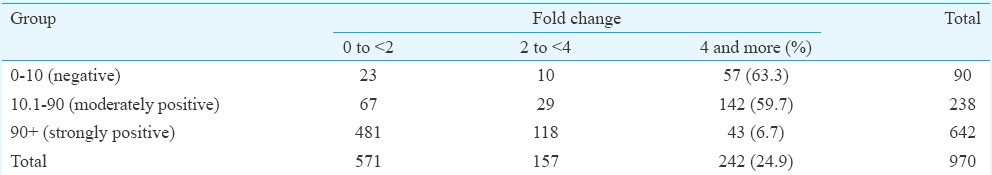
Immunogenicity results: The participants were categorized into three groups based on the pre-vaccination PRNT50 titre, namely, seronegative (titre ≤10), moderately seropositive (titre 10.1-90) and strongly seropositive (titre ≥90.1) individuals. Seronegative individuals comprised 9.27 per cent (90/970) of the study population as compared to 24.53 per cent (238/970) of moderately seropositive and 66.18 per cent (642/970) of strongly seropositives. Seroprotection in the seronegative group was observed in 85.5 per cent (77/90) of the participants at 28 days PV (Table I). The GMT before vaccination ranged from 6.22 to 373.82 (Table II). All participants at 28 day follow up had an increase in GMT. However, the GMTs were found to show a downwards trend by six months PV. The GMTs at 12 months PV were found to further decline in all the three groups. The titres were however, consistently maintained above the protective threshold (≥10) till the end of the study period. Fold changes in GMT on day 28 PV were also monitored (Table III). It was observed that 142 of 238 (60%) moderately positive participants and 43 of the 642 (6.7%) strongly positive participants exhibited a 4-fold rise in GMT after 28 days PV. Nearly 95.5 per cent [95% confidence interval (CI): 94.2-96.8] maintained the protective antibody titre up to 12 months PV.
Table I.
Frequency distribution of antibody response
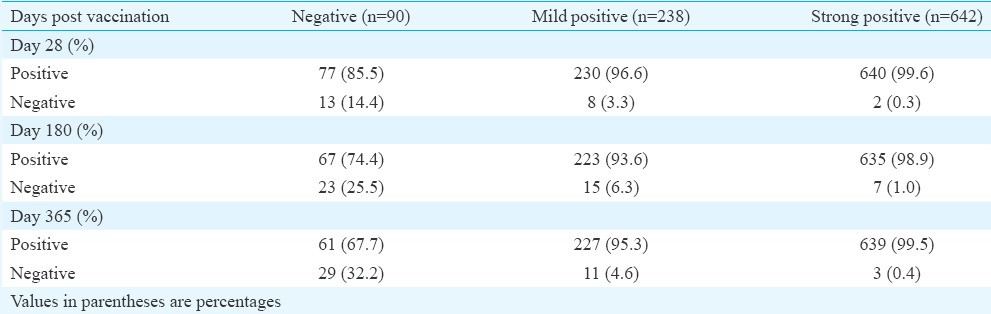
Table II.
Geometric mean titre (GMT) of three groups (seronegative, moderately seropositive and strongly seropositive) during pre- and post-vaccination at different study intervals
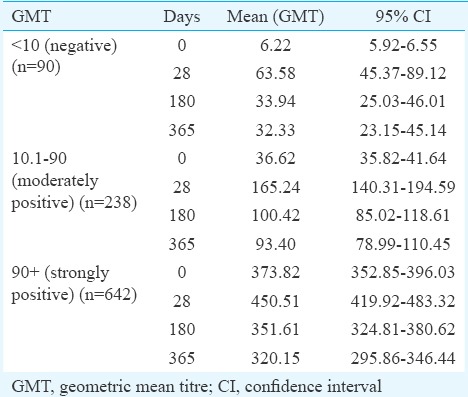
Table III.
Fold changes and 4-fold rise by 28 days post-vaccination
Safety results: Among the vaccinated participants, only four individuals (3 female, 1 male) showed minor symptoms such as erythema, rash, irritability, nasopharyngitis and fever within 28 days of observation period. Mild severity was observed after administration of the vaccine in the individuals reporting AE. One participant showed grade 3 (>10 cm diameter) erythema, rash, irritability and nasopharyngitis while another participant showed dizziness within 30 min of vaccination. One participant developed grade 2 fever (38.5-38.9°C) on 0 and 1 day PV while another participant reported grade 2 erythema (5.1-10 cm diameter), rash and irritability on day 28 of vaccination. There were no reports of serious AE or drug withdrawal during the study period. However, seven deaths were recorded at one year PV period (Table IV).
Table IV.
Details of deaths occurred during the study period
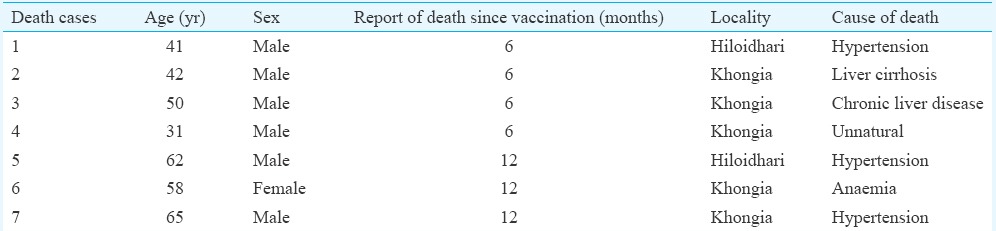
Discussion
Vaccination plays an important role in the control of JE. However, it has been reported that half of the 24 endemic countries do not have routine JE immunization programmes16. Lack of vaccination programmes has led to high mortality and morbidity in endemic areas. Implementation of JE vaccination in routine immunization programmes in India and in other Asian countries has largely contributed in reducing the disease burden in children, although in India, this situation is further complicated by the increasing number of cases in adult age group in different states17,18.
This study established the hypothesis that a single dose of the LAJEV in adults of Assam, India, provided considerable safety and immunogenicity. The most accepted test to measure the functional antibody to neutralize a virus is PRNT50 which was used in this study as recommended by the World Health Organization PRNT50 titre of ≥10 as a threshold of protection from JE in humans13. The vaccine elicited high immunogenicity showing seroprotection rates of 85.5 per cent after 28 days of a single dose. The findings showed better immunogenicity than a previous report of 79.63 per cent seroprotection rate 28 days PV in adults (≥18 to ≤50 yr) in Indian population19. However, seroprotection rate at 28 days PV in 10-month old children in Philippines was 92.1 per cent, and in 10-12 month old children in Bangladesh, the rates ranged from 80.2 to 86.3 per cent which was consistent with our study20,21. Four-fold rise was observed for 60 per cent moderately positive individuals after 28 days PV. As expected, strongly seropositive participants showed the least antibody rise. A considerable subset of the seropositive individuals showed a four fold rise in titre. This indicated that the vaccination induced a booster effect on the pre-existing response without any negative interference.
A total of 95.5 per cent of the individuals maintained the protective antibody titre up to 12 months PV. This finding was similar to earlier studies conducted in Korea and Nepal where children and adolescents showed 93.9 and 98.5 per cent, respectively, protective antibody 12 months PV9,22. The immunogenicity dropped from 63.58 per cent at 28 days PV to 33.94 per cent at six months PV and 32.33 per cent at 12 months PV in seronegative individuals. Similar trend was also observed in GMTs of moderately and strongly positive individuals. In a clinical trial study in Thailand, similar immunogenicity drop was observed23. Thus, these results may suggest that the immune response reaches a plateau phase.
In this study, the vaccine was well tolerated and found safe. Systemic reactions for all cases were mild and resolved concurrently. Events as rare as 3 in 10,000 were detected in a short-term safety study conducted in China in children24. However, in children in the age group of nine months to six years, LAJEV had demonstrated local and systemic AEs14,25. Following vaccination, no reports of meningitis or encephalitis were observed in our study. Although all districts, including Sivasagar district in upper Assam, are endemic to JE, no JE cases were reported during the study. During the study period, seven deaths were recorded. The causal relationship was improbable due to the following reasons: the first death was recorded six months after the vaccination campaign; six cases suffered from chronic liver disease, hypertension or anaemia; one died of unnatural death. These cases were apparently healthy and did not complain of any ailment at the time of enrolment in the study. Previous studies have reported two deaths and encephalitis case following the administration of LAJEV but were non-conclusive and judged as unrelated to the vaccine26,27. Therefore, vigilant surveillance and complete investigations of any event following vaccination should be emphasized.
Our study had some limitations. The possibility of selection bias due to attrition cannot be neglected. Moreover, as people who voluntarily provided written informed consent were included in the study, the possibility of selection bias and lack of generalization to all tea workers need to be recognized. Though severe AEs were not found during the study, prolonged monitoring of vaccines would be required. The methodological differences of titre calculations along with the age differences in studies conducted till now would possibly reflect the antibody response and cannot be directly compared with our study28. The immune status and genetic aspect of the 25 per cent of the seronegative individuals who did not seroconvert require further study. Future study is essential to address the long-term protection and vaccine effectiveness of a single dose of this vaccine.
In conclusion, our findings provided evidence that a single dose of LAJEV was safe and elicited protective immunity where 95.5 per cent of the adults in the study population remained seroprotected at the end of 12 months PV. Thus, it can be expected that immunization strategy will thereby increase the herd immunity and reduce the disease burden in adults.
Acknowledgment
The authors acknowledge the Indian Council of Medical Research, New Delhi, India, for providing financial grant for the study. The authors thank the research staff involved in the study.
Footnotes
Conflicts of Interest: None.
References
- 1.Buescher EL, Scherer WF. Ecologic studies of Japanese encephalitis virus in Japan. IX. Epidemiologic correlations and conclusions. Am J Trop Med Hyg. 1959;8:719–22. doi: 10.4269/ajtmh.1959.8.719. [DOI] [PubMed] [Google Scholar]
- 2.Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol. 2002;267:171–94. doi: 10.1007/978-3-642-59403-8_9. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(12 Suppl):S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 4.Reuben R, Gajanana A. Japanese encephalitis in India. Indian J Pediatr. 1997;64:243–51. doi: 10.1007/BF02752458. [DOI] [PubMed] [Google Scholar]
- 5.Kabilan L, Rajendran R, Arunachalam N, Ramesh S, Srinivasan S, Samuel PP, et al. Japanese encephalitis in India: an overview. Indian J Pediatr. 2004;71:609–15. doi: 10.1007/BF02724120. [DOI] [PubMed] [Google Scholar]
- 6.Borah J, Dutta P, Khan SA, Mahanta J. A comparison of clinical features of Japanese encephalitis virus infection in the adult and pediatric age group with acute encephalitis syndrome. J Clin Virol. 2011;52:45–9. doi: 10.1016/j.jcv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Vashishtha VM. Japanese encephalitis vaccines. In: Vashishtha VM, Choudhury P, Bansal CP, Yewale VN, Agarwal R, editors. IAP Guidebook on Immunization 2013-2014. Gwalior: National Publication House, Indian Academy of Pediatrics; 2014. pp. 303–16. [Google Scholar]
- 8.Ma X, Yu YX, Wang SG. Observations on safety and serological efficacy from a large-scale field trial of Japanese encephalitis vaccine. Chin J Biol. 1993;6:188–91. [Google Scholar]
- 9.Tandan JB, Ohrr H, Sohn YM, Yoksan S, Ji M, Nam CM, et al. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: a case-control study in Nepalese children 5 years after immunization. Vaccine. 2007;25:5041–5. doi: 10.1016/j.vaccine.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 10.Upreti SR, Janusz KB, Schluter WW, Bichha RP, Shakya G, Biggerstaff BJ, et al. Estimation of the impact of a Japanese encephalitis immunization program with live, attenuated SA 14-14-2 vaccine in Nepal. Am J Trop Med Hyg. 2013;88:464–8. doi: 10.4269/ajtmh.12-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Observed rate of vaccine reactions. Japanese encephalitis vaccine. Switzerland: Global Vaccine Safety Essential Medicines & Health Products; 2014. [Google Scholar]
- 12.Cohen BJ, Audet S, Andrews N, Beeler J WHO Working Group on Measles Plaque Reduction Neutralization Test. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26:59–66. doi: 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 13.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2-3 September, 2004. Vaccine. 2005;23:5205–11. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Feroldi E, Pancharoen C, Kosalaraksa P, Chokephaibulkit K, Boaz M, Meric C, et al. Primary immunization of infants and toddlers in Thailand with Japanese encephalitis chimeric virus vaccine in comparison with SA14-14-2: a randomized study of immunogenicity and safety. Pediatr Infect Dis J. 2014;33:643–9. doi: 10.1097/INF.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 15.Nauta J. Statistics in clinical vaccine trials. 1st ed. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg; 2011. [Google Scholar]
- 16.Background Paper on Japanese Encephalitis Vaccines. SAGE Working Group on Japanese encephalitis vaccines, October 1. Geneva: WHO; 2014. [accessed on August 11, 2015]. Available from: http://www.who.int/immunization/sage/meetings/2014/october/1_JE_Vaccine_Background_Paper.pdf . [Google Scholar]
- 17.Kumari R, Joshi PL. A review of Japanese encephalitis in Uttar Pradesh, India. WHO South East Asia J Public Health. 2012;1:374–95. doi: 10.4103/2224-3151.207040. [DOI] [PubMed] [Google Scholar]
- 18.Taraphdar D, Sarkar A, Mukhopadhyay BB, Chakraborty D, Khatun T, Chatterjee S. Increasing trend of Japanese encephalitis cases in West Bengal, India - A threat to paediatric population. Asian Pac J Trop Dis. 2012;2:358–61. [Google Scholar]
- 19.Singh A, Mitra M, Sampath G, Venugopal P, Rao JV, Krishnamurthy B, et al. A Japanese encephalitis vaccine from India induces durable and cross-protective immunity against temporally and spatially wide-ranging global field strains. J Infect Dis. 2015;212:715–25. doi: 10.1093/infdis/jiv023. [DOI] [PubMed] [Google Scholar]
- 20.Victor J, Gatchalian S, Yao Y, Zhou B, Zhang L, Yoksan S, et al. Comparison of the immunogenicity and safety of measles vaccine administered alone or with live, attenuated Japanese encephalitis SA 14-14-2 vaccine in Philippine infants. Vaccine. 2014;32:306–8. doi: 10.1016/j.vaccine.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Zaman K, Naser AM, Power M, Yaich M, Zhang L, Ginsburg AS, et al. Lot-to-lot consistency of live attenuated SA 14-14-2 Japanese encephalitis vaccine manufactured in a good manufacturing practice facility and non-inferiority with respect to an earlier product. Vaccine. 2014;32:6061–6. doi: 10.1016/j.vaccine.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Kwon HJ, Lee SY, Kim KH, Kim DS, Cha SH, Jo DS, et al. The immunogenicity and safety of the live-attenuated SA 14-14-2 Japanese encephalitis vaccine given with a two-dose primary schedule in children. J Korean Med Sci. 2015;30:612–6. doi: 10.3346/jkms.2015.30.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. [accessed on September 16, 2015]. Available from: http://www.clinicaltrials.gov/ct2/show/results/NCT01092507 .
- 24.Liu ZL, Hennessy S, Strom BL, Tsai TF, Wan CM, Tang SC, et al. Short-term safety of live attenuated Japanese encephalitis vaccine (SA14-14-2): results of a randomized trial with 26,239 subjects. J Infect Dis. 1997;176:1366–9. doi: 10.1086/517323. [DOI] [PubMed] [Google Scholar]
- 25.Kim DS, Houillon G. A Randomized Study of the Immunogenicity and Safety of Japanese Encephalitis Chimeric Virus Vaccine (JE-CV) in Comparison with SA 14-14-2 Vaccine in Children in South Korea. 8th World Congress of the World Society for Pediatric Infectious Diseases (WSPID) – Cape Town, South Africa, 19-22 November. 2013 [Google Scholar]
- 26.Jia N, Zhao QM, Guo XF, Cheng JX, Wu C, Zuo SQ, et al. Encephalitis temporally associated with live attenuated Japanese encephalitis vaccine: four case reports. BMC Infect Dis. 2011;11:344. doi: 10.1186/1471-2334-11-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Lin H, Zhu Q, Wu C, Zhao Z, Zheng H. Safety of Japanese encephalitis live attenuated vaccination in post-marketing surveillance in Guangdong, China, 2005-2012. Vaccine. 2014;32:1768–73. doi: 10.1016/j.vaccine.2013.11.107. [DOI] [PubMed] [Google Scholar]
- 28.Xin YY, Ming ZG, Peng GY, Jian A, Min LH. Safety of a live-attenuated Japanese encephalitis virus vaccine (SA14-14-2) for children. Am J Trop Med Hyg. 1988;39:214–7. doi: 10.4269/ajtmh.1988.39.214. [DOI] [PubMed] [Google Scholar]


