Abstract
Background & objectives:
Scrub typhus is a re-emerging mite-borne rickettsiosis, which continues to be underdiagnosed, with lethal consequences. The present study was conducted to determine the seasonality, clinical presentation and predictors of mortality in patients with scrub typhus at a tertiary care teaching hospital in northern India.
Methods:
Scrub typhus was suspected in patients attending the hospital as per the standard case definition and serological evidence was obtained by performing an IgM ELISA.
Results:
A total of 284 patients with scrub typhus from urban and rural areas were seen, predominantly from July to November. The most common clinical presentation was a bilateral community-acquired pneumonia (CAP), which resembled pneumonia due to atypical pathogens and often progressed to acute respiratory distress syndrome (ARDS). An acute undifferentiated febrile illness (AUFI) or a febrile illness associated with altered sensorium, aseptic meningitis, shock, abdominal pain, gastrointestinal bleeding or jaundice was also seen. Eschars were seen in 17 per cent of patients, and thrombocytopenia, transaminitis and azotaemia were frequent. There were 24 deaths (8.5%) caused predominantly by ARDS and multi-organ dysfunction. The mortality in patients with ARDS was high (37%). ARDS [odds ratio (OR)=38.29, 95% confidence interval (CI): 9.93, 147.71] and acute kidney injury (OR=8.30, 95% CI: 2.21, 31.21) were the major predictors of death.
Interpretation & conclusions:
The present findings indicate that scrub typhus may be considered a cause of CAP, ARDS, AUFI or a febrile illness with multisystem involvement, in Uttarakhand and Uttar Pradesh, especially from July to November. Empiric therapy of CAP may include doxycycline or azithromycin to ensure coverage of underlying unsuspected scrub typhus.
Keywords: India, mortality, outcomes, pneumonia, scrub typhus, seasonality
Scrub typhus is an acute febrile illness with frequent multisystem involvement caused by Orientia tsutsugamushi and transmitted by bites of infected larvae (chiggers) of trombiculid mites. The target cells of O. tsutsugamushi are endothelial cells, monocytes and other cell types and the disease is associated with vasculitis and endothelial dysfunction1. Scrub typhus has been termed as the ‘single most prevalent, under-recognized, neglected and severe, but easily treatable disease in the world’2. With delays in diagnosis and treatment, the mortality can exceed 30 per cent1. In India, initial reports of scrub typhus appeared in the 1930s3, and a large number of cases were identified in the Second World War4. In later years, scrub typhus has been reported with increasing frequency from diverse ecologies – initially from southern India5 and later from the Himalayan regions6, the plains of northern India7, coastal areas8, desert regions9 and even residential localities in metropolitan cities10.
Scrub typhus was first reported in the Kumaon hills (now Uttarakhand) in 193811, while serologically confirmed cases were reported from Uttar Pradesh in 194512. In Uttarakhand, a few cases of scrub typhus were reported in 199213 and 200914, while reports from Uttar Pradesh have been uncommon. Since 2012, when the National Centre for Disease Control (NCDC) confirmed a multi-State outbreak of scrub typhus15, an increased number of patients have been seen in rural as well as urban areas of Uttarakhand and adjoining Uttar Pradesh with diverse clinical manifestations and with severe morbidity and significant mortality.
The present study was conducted to determine the seasonality, clinical features, outcomes and predictors of mortality in patients with scrub typhus admitted in a tertiary care hospital in Uttarakhand.
Material & Methods
This study was conducted at the Himalayan Hospital, a 750-beded tertiary care teaching hospital located at Jolly Grant, 25 km from Dehradun, Uttarakhand, India, from August 2012 to December 2013. This hospital caters to patients from the seven districts of the Garhwal division, some districts of the Kumaon division and the adjoining districts of Uttar Pradesh and Himachal Pradesh.
Case definitions and data sources: Cases of scrub typhus were suspected on the basis of presence of one or more of the following features - (i) acute-onset fever with or without headache, cough with or without shortness of breath, abdominal pain, conjunctival infection, lymphadenopathy or rash; (ii) presence of a primary punched out ulcer or eschar; (iii) acute febrile illness, in which other causes had been excluded and in which defervescence occurred within 48 h of administration of tetracyclines16.
Data on cases of scrub typhus were collected prospectively among consecutive patients presenting to Himalayan Hospital during the study period. Serological evidence of scrub typhus was obtained by demonstration of IgM antibodies to 56 kDa antigen of O. tsutsugamushi, using a commercial IgM ELISA (Scrub Detect™, InBios International Inc., Seattle, Washington, USA). The cut-off used for the IgM ELISA was an optical density of >0.5 as used in other studies17.
Information on putative risk factors for exposure to mite larvae was elicited, and any relevant investigations and therapy before admission were noted. Each patient was examined in detail for the presence of rash, eschar and lymphadenopathy. Patients underwent a complete haemogram, urinalysis, assessment of renal and hepatic function, chest X-ray and evaluation for other causes of fever (malaria, dengue and typhoid), as considered appropriate. Patients presenting with neurological symptoms underwent cerebrospinal fluid (CSF) examination and imaging.
Systemic involvement in scrub typhus was defined as follows: (i) pneumonia: radiologic evidence of opacities; (ii) acute respiratory distress syndrome (ARDS): presence of bilateral infiltrates with severe hypoxaemia with normal cardiac function; (iii) acute hepatitis: total bilirubin >2.5 mg/dl and/or elevations of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) 2.5 times above the upper limit for normal readings (i.e., >100 IU/l); (iv) acute renal failure: serum creatinine >2.0 mg/dl; (v) shock: systolic BP <90 mm Hg; (vi) acute confusional state: altered sensorium in a patient with scrub typhus with/without CSF changes; (vii) multi-organ dysfunction: progressive abnormalities in two or more systems.
The study protocol was approved by the Institutional Review Committee of the Himalayan Institute of Medical Sciences.
Statistical analysis: Association between categorical variables was assessed using Chi-squared test. Student's t test (unpaired) and Mann–Whitney U-test were used to compare means and medians, respectively. The multivariate logistic regression analyses of outcomes of death due to scrub typhus were performed, and variables were included in the final model if the univariate analysis showed P<0.25 or if the variable was of known clinical importance. Statistical analysis was done using STATA 11 (Stata Corp, College Station, Texas, USA).
Results
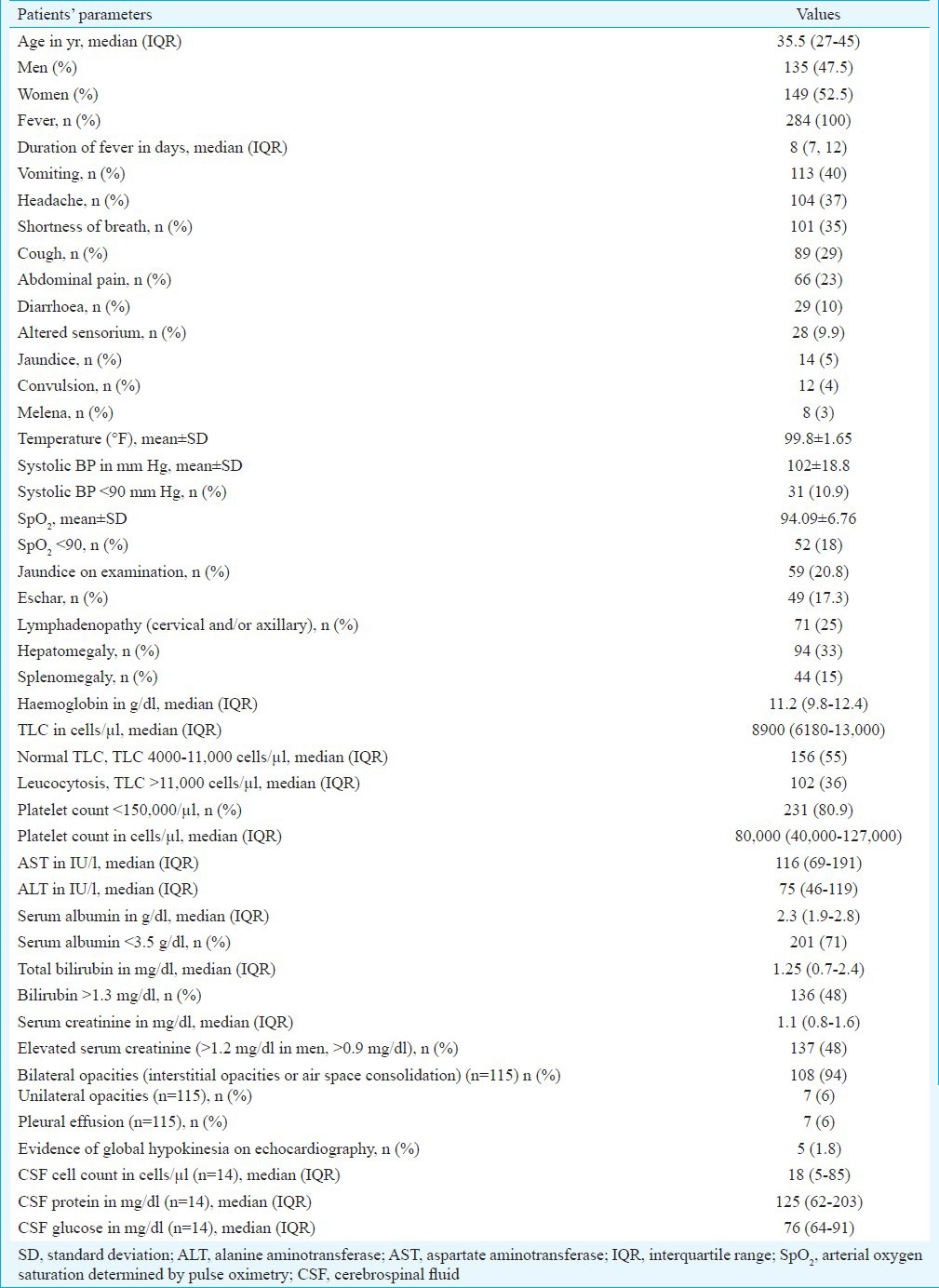
A total of 284 adults were diagnosed with scrub typhus during the study period (143 cases in 2012 and 141 cases in 2013). During this period, a total of 2524 cases of acute febrile illnesses were seen. The predominant causes were dengue (1278 cases, 50.6%), typhoid fever (575 cases, 22.8%), malaria (380 cases, 15.1%), scrub typhus (284 cases, 11.2%) and leptospirosis (7 cases, 0.3%). The distribution with regard to States, districts and rural-urban residence of the patients with scrub typhus is shown in Table I. There were cases from both urban and rural areas, and in Haridwar district, the cases from urban areas outnumbered those from the rural area. The presentation according to the month of the year is shown in Fig. 1. Scrub typhus in the region had a consistent seasonal pattern with a few cases in the months between January and June and a predilection for occurrence in the months from July to November. Scrub typhus occurred in both men and women with no predilection for gender (Table II).
Table I.
Distribution of cases of scrub typhus: according to State, district and urban-rural residence
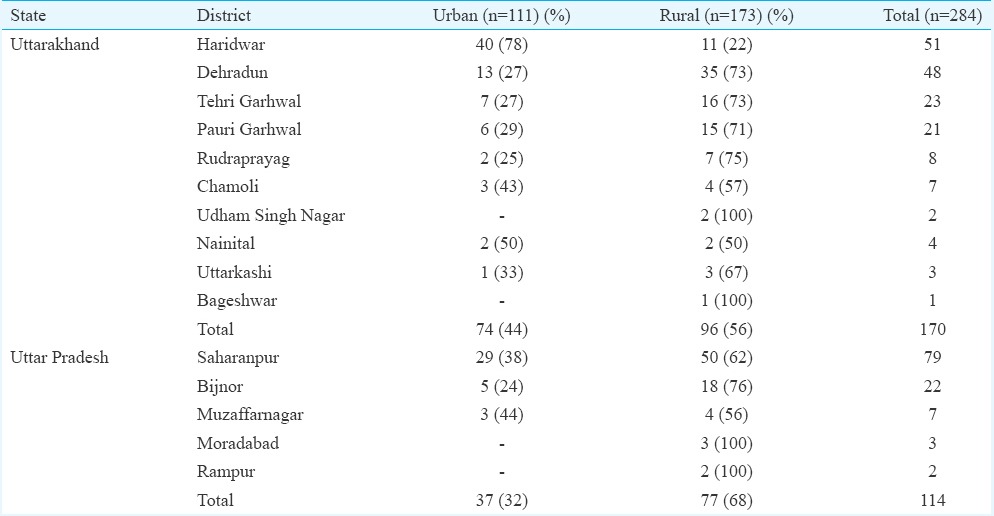
Fig. 1.
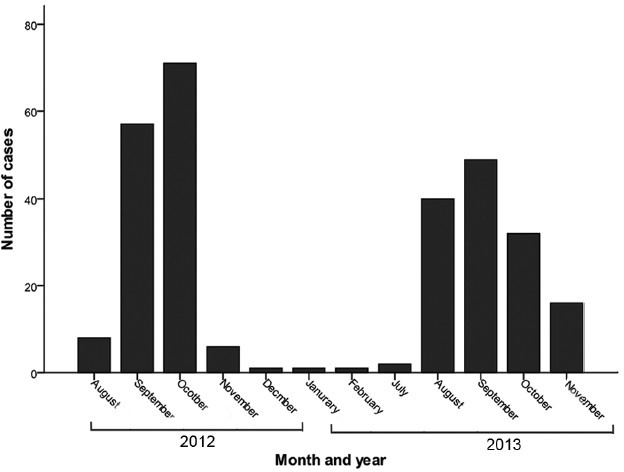
Scrub cases by month and year from August 2012 to November 2013.
Table II.
Demographic characteristics, symptoms, signs and laboratory features in patients with scrub typhus (n=284)
Risk factors for exposure to mites and previous diagnosis/treatment for scrub typhus: A majority of patients reported the presence of bushes around the houses (71%) and rodents at home (61%). Nearly half of the patients resorted to open air defecation (49%) or travelled to the forest for the collection of fodder (48%). None of the patients recalled a bite in the period before onset of illness. Details of earlier investigations were available in 172 patients, of whom only one patient (in 2013) had been subjected to any diagnostic test for scrub typhus. Patients presenting with pneumonia had received earlier treatment, usually only with a beta-lactam antibiotic or a respiratory fluoroquinolone (like levofloxacin).
Clinical and laboratory features: The clinical and laboratory features of the patients are given in Table II. A non-productive cough appearing a few days after the onset of fever and shortness of breath, which was often rapidly progressive, was the most common systemic symptom. Abdominal pain arousing the suspicion of an acute abdomen, symptoms of altered sensorium and convulsions were also noted in some patients. A few patients presented with jaundice and symptoms of upper gastrointestinal (GI) bleeding. The clinical course of patients was sometimes unexpected, with the onset or rapid progression of hypoxaemia despite early therapy and the appearance of cardiac arrhythmias.
On clinical examination, the most frequent abnormality was found to be hepatomegaly, followed by cervical and/or axillary adenopathy (Table II), conjunctival icterus and hypoxaemia on pulse oximetry. A striking and consistent finding in patients with pneumonia was the relative paucity of physical signs despite the often extensive involvement of both lungs. Eschar was seen in 49 (17%) patients in the neck, axilla, abdomen, inguinal and pubic regions (Fig. 2). When the presence of eschars was compared across clinical presentations of scrub typhus, eschars were particularly associated with the presence of ARDS (P=0.008), wherein these were documented in nearly one-third of cases.
Fig. 2.
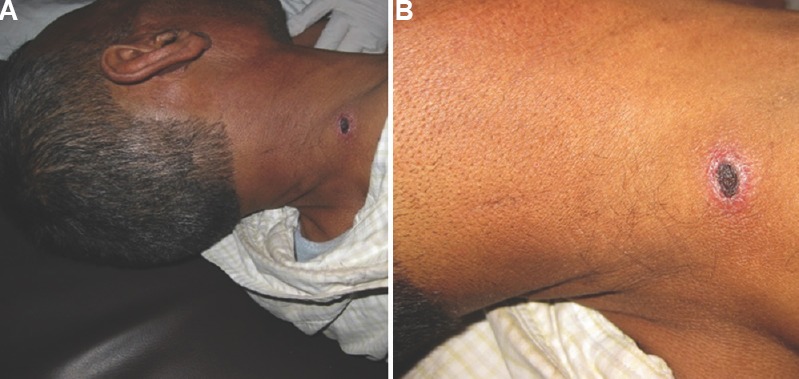
Eschars in patients with scrub typhus. (A) An eschar on the neck of a patient with scrub typhus. (B) A close up of the eschar shows the typical cigarette burn appearance of an eschar.
On laboratory evaluation, the leucocyte count was normal in more than half of cases and elevated in one-third of patients (Table II). The most common laboratory abnormalities were elevation of transaminases, thrombocytopenia, hypoalbuminaemia, while elevated total bilirubin level and serum creatinine were seen in nearly half of patients (Table II). Due to lack of a productive cough, sputum examinations could not be done in patients. The X-rays revealed bilateral interstitial patterns (with reticulonodular and ground-glass opacities) initially, extensive air-space consolidation in patients with ARDS (Fig. 3) and occasional pleural effusion. CSF examination was done in 14 patients. The pattern was similar to aseptic meningitis, with a predominant mononuclear pleocytosis (median count of 85 cells/μl) and raised CSF protein (median - 125 mg/dl), with normal CSF glucose [median (interquartile range [IQR]) of 76 mg/dl (64, 91)] (Table II).
Fig. 3.
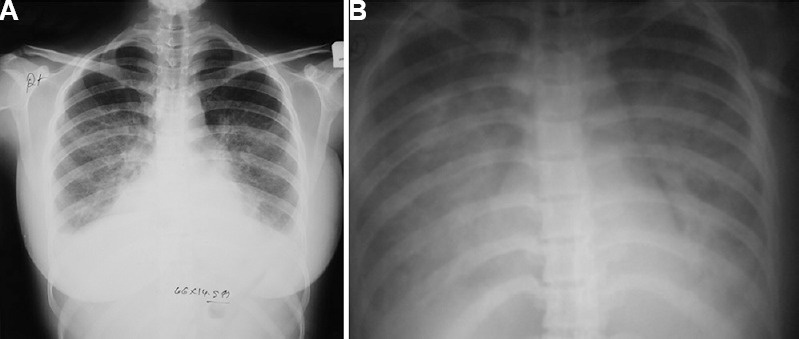
(A) A patient with scrub typhus presenting with fever and shortness of breath. X-ray shows an interstitial pattern with ground glass opacities in bilateral lower lobes. (B) An X-ray of a patient with scrub typhus who progressed rapidly to acute respiratory distress syndrome. X-ray showing extensive air space consolidation.
Table III shows the clinical presentations of scrub typhus patients. It was most often presented as community-acquired pneumonia (CAP) (in 115 cases), which was bilateral and frequently complicated by ARDS. ARDS was seen in 46 cases (16.2%) overall and in 40 per cent of the cases of pneumonia. Patients with scrub typhus and pneumonia also had significant thrombocytopenia, with a median (IQR) platelet count of 60,000/μl (35000-105000).
Table III.
Clinical presentation and causes of death seen in patients with scrub typhus (n=284)
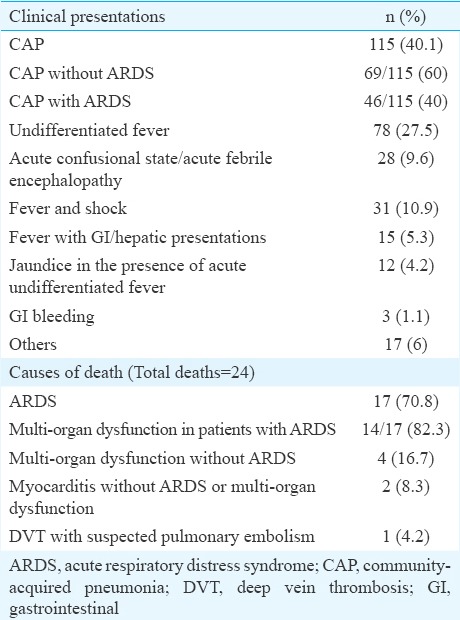
The other clinical presentations of scrub typhus were an acute undifferentiated febrile illness (AUFI), acute febrile encephalopathy or fever followed by shock, jaundice and GI bleeding (Table III). The median (IQR) durations of fever in the different presentations were similar.
Treatment, outcomes and predictors of mortality: Patients received oral doxycycline and/or intravenous (iv) azithromycin18, along with beta-lactam antibiotics (in the case of pneumonia), along with supportive care which included oxygen, mechanical ventilation, haemodialysis and blood products. Steroids were given to patients with shock, requiring vasopressors, and also often to those with early ARDS. Treatment was successful in 242 (85.2%) patients. Forty two patients (14.8%) had unfavourable outcomes after admission and despite receiving therapy for scrub typhus. This included deaths in 24 patients (8.5%), while 18 patients (6.3%) who were also seriously ill left against medical advice (LAMA). The major causes of death were ARDS and multi-organ dysfunction (Table III). Of the 46 patients with ARDS, 19 (41%) recovered successfully while the unfavourable outcomes of death and LAMA were seen in 17 (37%) and 10 (22%), respectively. Two patients died of myocarditis without ARDS or multi-organ dysfunction (Table III).
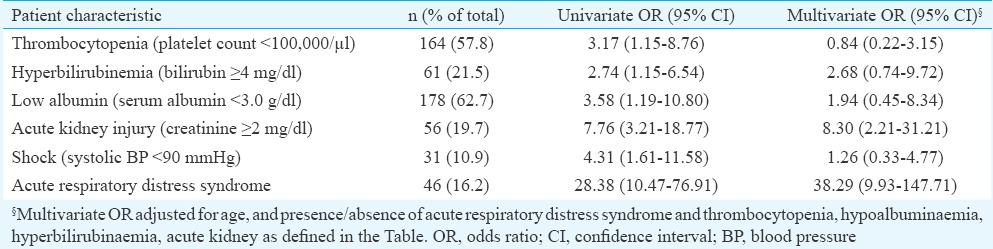
Deaths usually occurred in the second week of illness, and the duration of fever on admission in those who died was not significantly different than in those who survived (Table IV). In-hospital mortality was predominantly early: the median duration of stay in those who died was two days, compared to a median of six days in those who survived (P<0.001) (Table IV). While age, gender and residence were not associated with the risk of death, an increase in total leucocyte count and serum creatinine and a decrease in platelet count, serum albumin and oxygen saturation were significantly associated with death (Table IV). On univariate analysis, the indicators of severe sepsis (thrombocytopenia, azotaemia, hyperbilirubinaemia, ARDS, shock) as well as hypoalbuminaemia were all associated with increased odds of death (Table V). However, on multivariate logistic regression analysis, ARDS [odds ratio (OR)=38.29, 95% confidence interval (CI): 9.93, 147.71] and acute kidney injury (OR=8.30, 95% CI: 2.21, 31.21) remained strong risk factors for mortality, while the associations of thrombocytopenia, hyperbilirubinaemia, low albumin and shock were no longer significant (Table V).
Table IV.
Comparison of demographic, clinical and laboratory characteristics of those who died versus those with a successful outcome
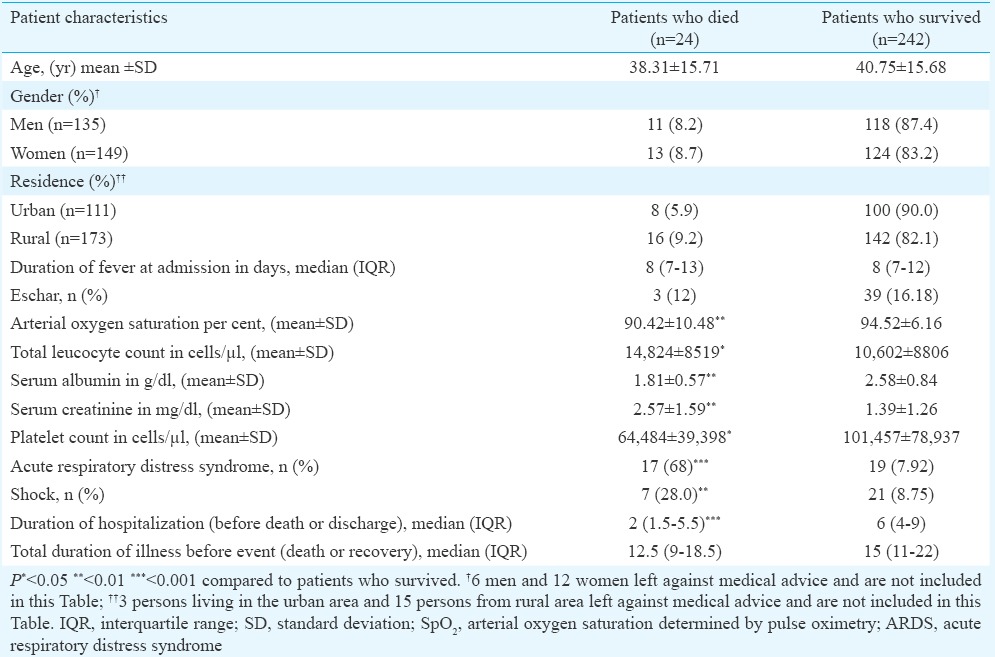
Table V.
Univariate and multivariate odds ratios of selected patient characteristics for the outcome of death
Discussion
Our study included 284 patients with scrub typhus from northern India, predominantly from Uttarakhand and from a few adjoining districts of Uttar Pradesh. Scrub typhus has been described as an occupational disease of rural residents19, but a substantial proportion of our patients were from urban areas of both States. A seasonal pattern with occurrence in July-November was evident, and the maximum cases were seen in September and October. This seasonal pattern was consistent with reports from north India6, western India8 and north-eastern India20 but was different from the pattern observed in south India, where the disease has been reported during the cooler months, from October to February5.
The most common clinical presentation was CAP, with a non-productive cough, bilateral infiltrates, and the paucity of physical signs, resembling pneumonia caused by atypical organisms such as Legionella spp., Chlamydia pneumoniae and Mycoplasma pneumoniae21, which often progressed to ARDS. A similar predominance of pneumonia as the most common presentation and ARDS as the major complication has been noted in other studies9,17.
The epidemiologic and clinical features that suggest a diagnosis of scrub typhus in a patient with febrile illness are a history of environmental exposure, the presence of eschar, maculopapular rash and adenopathy22. In our study, none of these features occurred consistently in majority of patients. Eschar was seen only in 17 per cent of patients. The low frequency of eschar (10-19%) has been reported earlier6,8,20, with some reports failing to find eschars in any patients10,23. The laboratory abnormalities comprised normal or elevated leucocyte counts, thrombocytopenia, an AST-predominant transaminitis, hyperbilirubinaemia, hypoalbuminaemia and azotaemia. The CSF abnormalities were suggestive of aseptic meningitis. Elevated transaminases (especially AST) and thrombocytopenia have been consistently reported in other studies from India8,9,17, but these are also common in other acute febrile illnesses such as dengue fever24,25. The finding of thrombocytopenia in patients with scrub typhus and pneumonia may be diagnostically helpful as only a few causes of CAP are associated with a low platelet count26.
The deaths in patients resulted largely from ARDS, associated in most cases with multi-organ dysfunction. ARDS has been documented as occurring in 9.5-60 per cent cases from different parts of India6,8,17. However, in a study from Sikkim, ARDS was not reported at all27. ARDS was associated with a high mortality rate (37%) in our study, similar to the 33 per cent reported in another recent study from Rajasthan9. This may be related to delayed presentation of patients, low awareness of scrub typhus among healthcare providers in the region, and previous ineffective empiric therapy for CAP, which consisted of only beta-lactams such as ceftriaxone or a respiratory fluoroquinolone, which do not provide coverage for scrub typhus. The treatment of patients presenting with CAP with a combination of a beta-lactam agent and macrolide (azithromycin) or doxycycline as suggested in the therapeutic recommendations for CAP to ensure coverage of atypical pathogens28, would have ensured coverage for underlying scrub typhus. This could have prevented the progression to ARDS and a fatal outcome.
The strengths of our study was large number of patients drawn from urban and rural areas. The study had certain limitations. It was conducted at a tertiary care centre, and the clinical spectrum and profile might not be representative of scrub typhus at other levels of care. Further, ELISA targeting specific IgM antibodies against a 56 kDa recombinant antigen of O. tsutsugamushi was used to obtain serological evidence of scrub typhus since isolation facilities, indirect immunofluorescence and polymerase chain reaction (PCR)-based assays were not available.
In conclusion, our results showed cases of scrub typhus as an important cause of acute febrile illness presenting as an undifferentiated fever, bilateral pneumonia resembling atypical pneumonia or fever with diverse sepsis syndromes in areas of Uttarakhand and Uttar Pradesh, particularly from July to November. In resource-limited settings and pending laboratory confirmation, clinicians may implement a ‘suspect and treat’ strategy and initiate prompt treatment with doxycycline or azithromycin, to prevent serious morbidity and fatality in this potentially treatable and curable illness. CAP was the most common presentation of this re-emerging, under-recognized and widely prevalent disease. Empiric therapy of patients with CAP may, therefore, include macrolides (azithromycin) or doxycycline in addition to a beta-lactam agent, to ensure coverage against atypical pathogens, including O. tsutsugamushi, since scrub typhus may be an underlying and unsuspected cause.
Acknowledgment
Authors acknowledge the help provided by Dr Manoj Chandday, the staff of the Medical Record Section and the Serology Laboratory in the compilation of data. Authors thank Dr J.P. Sharma, Medical Superintendent Himalayan Hospital, for facilitating data retrieval and Drs Biswaroop Chatterjee and Madhavi Bhargava for critical comments. This work was supported by funding from HIHT University and Jamsetji Tata Trust, Mumbai India.
Footnotes
Conflicts of Interest: None.
References
- 1.Watt G. Scrub typhus. In: Warrell DA, Cox TM, Firth JD, editors. Oxford textbook of medicine. 5th ed. Oxford: Oxford University Press; 2010. pp. 7–6. 40. [Google Scholar]
- 2.Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–7. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy BC. Typhus fever; with special reference to its incidence in India. J Indian Med Assoc 1945. 1946;15:135–46. [PubMed] [Google Scholar]
- 4.Sayen JJ, Pond HS, Forrester JS, Wood FC. Scrub typhus in Assam and Burma; a clinical study of 616 cases. Medicine (Baltimore) 1946;25:155–214. doi: 10.1097/00005792-194605000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, et al. Outbreak of scrub typhus in Southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359–64. doi: 10.1111/j.1749-6632.2003.tb07391.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahajan SK, Rolain JM, Kashyap R, Bakshi D, Sharma V, Prasher BS, et al. Scrub typhus in Himalayas. Emerg Infect Dis. 2006;12:1590–2. doi: 10.3201/eid1210.051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhry D, Garg A, Singh I, Tandon C, Saini R. Rickettsial diseases in Haryana: not an uncommon entity. J Assoc Physicians India. 2009;57:334–7. [PubMed] [Google Scholar]
- 8.Narvencar KP, Rodrigues S, Nevrekar RP, Dias L, Dias A, Vaz M, et al. Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. 2012;136:1020–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma R, Krishna VP, Manjunath, Singh H, Shrivastava S, Singh V, et al. Analysis of two outbreaks of scrub typhus in Rajasthan: a clinico-epidemiological study. J Assoc Physicians India. 2014;62:24–9. [PubMed] [Google Scholar]
- 10.Mittal V, Gupta N, Bhattacharya D, Kumar K, Ichhpujani RL, Singh S, et al. Serological evidence of rickettsial infections in Delhi. Indian J Med Res. 2012;135:538–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Blewitt B. Fevers of the typhus group in the Bhimtal area of Kumaon hills. J Royal Armed Corps. 1938;70:241–5. [Google Scholar]
- 12.Padbidri VS, Gupta NP. Rickettsiosis in India: a review. J Indian Med Assoc. 1978;71:104–7. [PubMed] [Google Scholar]
- 13.Singh P. Scrub typhus, a case report: Military and regional significance. MJAFI. 2004;60:89–90. doi: 10.1016/S0377-1237(04)80174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad S, Srivastava S, Verma SK, Puri P, Shirazi N. Scrub typhus in Uttarakhand, India: a common rickettsial disease in an uncommon geographical region. Trop Doct. 2010;40:188–90. doi: 10.1258/td.2010.090447. [DOI] [PubMed] [Google Scholar]
- 15.Mittal V, Bhattacharya D, Chhabra M. Multi-state outbreak of scrub typhus. NCDC Newsl. 2013;2:3–4. [Google Scholar]
- 16.World Health Organization. WHO recommended surveillance standards. 2nd ed. Geneva: World Health Organization, Department of Communicable Disease Surveillance and Response; 1999. [Google Scholar]
- 17.Varghese GM, Trowbridge P, Janardhanan J, Thomas K, Peter JV, Mathews P, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39–43. doi: 10.1016/j.ijid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR Guidelines for diagnosis and management of Rickettsial diseases in India. Indian J Med Res. 2015;141:417–22. doi: 10.4103/0971-5916.159279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organisation. Frequently asked questions: Scrub Typhus. [accessed on November 8, 2015]. Available from: http://www.searo.who.int/entity/emerging_diseases/CDS_faq_Scrub_Typhus.pdf .
- 20.Sharma PK, Ramakrishnan R, Hutin YJ, Barui AK, Manickam P, Kakkar M, et al. Scrub typhus in Darjeeling, India: opportunities for simple, practical prevention measures. Trans R Soc Trop Med Hyg. 2009;103:1153–8. doi: 10.1016/j.trstmh.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. 2006;12(Suppl 3):12–24. doi: 10.1111/j.1469-0691.2006.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim I, Walker DH. Scrub typhus. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: principles, pathogens, and practice. 3rd ed. Philadelphia, USA: Saunders, Elsevier; 2011. pp. 334–8. [Google Scholar]
- 23.Bhargava A, Karadwal S, Mittal G, Kaushik R, Sharma A. Scrub typhus at a district hospital in Tehri Garhwal, Uttarakhand, India: report of a rapid response investigation. J Commun Disord. 2014;46:40–3. [Google Scholar]
- 24.Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, Thomas EM, et al. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors - An experience from a tertiary care hospital in South India. Trop Doct. 2010;40:230–4. doi: 10.1258/td.2010.100132. [DOI] [PubMed] [Google Scholar]
- 25.Kittitrakul C, Silachamroon U, Phumratanaprapin W, Krudsood S, Wilairatana P, Treeprasertsuk S. Liver function tests abnormality and clinical severity of dengue infection in adult patients. J Med Assoc Thai. 2015;98(Suppl 1):S1–8. [PubMed] [Google Scholar]
- 26.Cunha BA, Hage JE. Community-acquired pneumonia: diagnostic vs. prognostic significance of the platelet count. Chest. 2011;139:1255–6. doi: 10.1378/chest.10-3146. [DOI] [PubMed] [Google Scholar]
- 27.Gurung S, Pradhan J, Bhutia PY. Outbreak of scrub typhus in the North East Himalayan region-Sikkim: an emerging threat. Indian J Med Microbiol. 2013;31:72–4. doi: 10.4103/0255-0857.108729. [DOI] [PubMed] [Google Scholar]
- 28.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]


