Abstract
Background
B-cell depleting drugs show promise for treating multiple sclerosis.
Objective
We sought predictors of optimal response to rituximab, a B-cell depleting antibody, to help guide therapy selection.
Methods
We performed a post hoc study of 30 relapsing multiple sclerosis patients with breakthrough disease while on beta-interferon or glatiramer acetate who were treated with add-on rituximab. Standardized neurologic examinations, brain magnetic resonance imaging, and cerebrospinal fluid were obtained before and after rituximab. Tissue biomarkers were measured. Optimal responders were defined as having no evidence of disease activity.
Results
At baseline, optimal responders with no evidence of disease activity had higher IgG indices (P = 0.041), and higher CXCL13 indices ((cerebrospinal fluid CXCL13/serum CXCL13)/albumin index; P = 0.024), more contrast enhancing lesions (P = 0.002), better 25 foot timed walk (P = 0.001), and Expanded Disability Status Scale (P = 0.002). Rituximab treatment led to reduced cerebrospinal fluid biomarkers of tissue destruction: myelin basic protein (P = 0.046), neurofilament light chain (P < 0.001), and of inflammation (CXCL13 index; P = 0.042).
Conclusions
Multiple sclerosis patients with optimal response to rituximab had higher cerebrospinal fluid IgG and CXCL13 indices, more gadolinium-enhancing lesions, and less disability at baseline. Rituximab treatment led to decreased markers of inflammation and tissue damage. If validated, these results will help identify multiple sclerosis patients who will respond optimally to B-cell depletion.
Keywords: Rituximab, outcome measurement, treatment response, neurofilament heavy, MBP, BAFF
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system affecting 2.3 million people worldwide.1 Given the growing number of disease modifying treatments (DMTs) with diverse mechanisms of action for relapsing forms of MS, the treatment goal of achieving ‘no evidence of disease activity’ (NEDA) has emerged. Unfortunately, the choice of DMT for an individual remains largely heuristic. Biomarkers predictive of response to a particular therapy would improve care by minimizing further relapses and limiting associated disability and costs.
Clinical disease characteristics, demographics, magnetic resonance imaging (MRI) parameters, and biomarkers in serum and cerebrospinal fluid (CSF) have been advanced as predictors of prognosis and response to treatment. Studies suggest that DMTs are more effective when started earlier in the disease course, in younger patients, and in those with more enhancing lesions, presumably when MS is most inflammatory.2 Individual biomarkers have not yet been shown to predict response to specific DMTs, except for the negative relationship with neutralizing antibodies to interferons and natalizumab.3 Biomarkers of tissue damage have been shown to correlate with (a) disability (neurofilament light; NFL),4 (neurofilament heavy; NFH);5 (b) contrast-enhancing lesions (CELs);6 myelin basic protein (MBP);7 and (c) CSF inflammation (CXCL13),8 and osteopontin.9–13 These markers have been hypothesized to provide information on responses to treatment,14 and have sometimes been used as endpoints in clinical trials.15
We utilized samples and data collected during a prospective, MRI-blinded phase II trial of rituximab as an add-on treatment for relapsing MS16 to evaluate predictors of treatment response. Rituximab, a B lymphocyte depleting monoclonal antibody targeting CD20, decreased numbers of CELs and relapses in early phase trials, including as an add-on therapy to platform DMTs.16–18 CSF B and T lymphocytes and levels of serum and CSF CXCL1319 were also reduced. In our add-on trial in suboptimal responders to beta-interferon or glatiramer acetate platform therapies, 30 patients were treated with 4-weekly intravenous rituximab doses of 375 mg/m2. A subset of 24 patients had CSF and serum obtained prior to and approximately 24 weeks after treatment. Using NEDA criteria, we identified all ‘optimal responders’ and sought baseline laboratory, imaging and clinical biomarkers associated with NEDA after rituximab treatment.
Methods
Standard protocol approvals, setting and patient selection
The phase II trial of rituximab as add-on therapy from which the current study derived was reported previously.16 The trial started at a time when rituximab was only used for non-Hodgkin’s lymphoma and thus the dose used was 4-weekly infusions of 375 mg/m2, the standard oncological dosing regimen at that time. Oral acetaminophen and diphenhydramine, but no corticosteroids, were given as pre-treatment before each infusion. The Washington University in Saint Louis Human Research Protection Office approved this study. Informed consent was obtained from each patient at enrollment. Twenty-four of the 30 subjects had CSF and serum collected one week prior to the initial rituximab infusion and again approximately 24 weeks after the first infusion. Baseline MRI prior to initial infusion, performed within one week of pre-treatment CSF collection and all post-treatment MRIs were evaluated. MRI and clinical safety assessments were also performed at week 52 when most subjects had detectable circulating B cells.
Determination of NEDA in response to treatment
Optimal responders with NEDA were identified prior to assessment of any putative biomarkers. NEDA was defined as having no new or enhancing lesions and either stable or improved clinically with no relapses and no disability worsening as measured using the Expanded Disability Status Scale (EDSS) during the 6 months of B-cell depletion following rituximab treatment. Non-NEDA subjects had relapsed or had clinical deterioration, or had a new or active lesion on any of three brain MRIs performed at weeks 12, 16, and 20 weeks after B-cell depletion.
Sample processing
CSF was immediately placed on ice at the time of lumbar puncture. CSF and serum samples were each centrifuged at 1250g for 15 minutes at 4℃, aliquoted, and stored at –80℃. CSF cell count, protein, glucose, and IgG index were performed by the Barnes-Jewish Hospital clinical laboratory.19
Enzyme-linked immunosorbent assay
CSF levels of MBP, NFH, and NFL were determined per manufacturer’s instructions by human MBP, human phosphorylated NFH, and human NFL enzyme-linked immunosorbent assay (ELISA) kits (Beckman Coulter, Brea, CA, USA; and BioVendor, Modrice, Czech Republic; Uman Diagnostics, Umea, Sweden, respectively). CXCL13, CCL19, and, B-cell activating factor (BAFF) in serum and CSF were measured using human Quantikine ELISA kits (all from R&D Systems, Minneapolis, MN, USA). Antibodies to recombinant myelin oligodendrocyte glycoprotein (rMOG) were measured using a homemade ELISA as described.20 Index values for CXCL13 and BAFF were calculated similarly to IgG index using the following equation:
Index = (CSF level/serum level)÷(CSF albumin/serum albumin)
Statistical analysis
For comparisons between patient groups, Fisher’s exact test and the Wilcoxon test were used. For correlation analyses, the Spearman correlation coefficient (rs) by rank was used. To evaluate the validity of a test, receiver operating characteristic analysis was performed. A P value of less than 0.05 was considered significant. As these were exploratory correlations, no corrections for multiple testing were performed. Tests were done using PASW Statistics Gradpack 22 (SPSS, Armonk, NY, USA) and GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient demographics and response
Thirty subjects were enrolled into the original study, 24 with both CSF and serum available for biomarker analyses.16, 21 Among the 30, we identified nine responders who met the criteria for NEDA (six with CSF and serum samples available) and 21 subjects were classified as non-NEDA (18 with CSF and serum) (Table 1). Lack of achieving NEDA was due to an enhancing lesion on any of the three post-rituximab brain MRIs in 11 subjects, clinical relapse plus a gadolinium-enhaning lesion on a post-rituximab brain MRI in three subjects, or clinical relapse, gadolinium-enhancing lesion, plus decline on 25 foot timed walk (25FTW) in one, or clinical relapse plus deterioration on the 25FTW in two, or clinical relapse alone in one, or decline in EDSS in one subject, or deterioration by more than 20% on nine-hole peg test in two. Thus, 21 subjects did not fully achieve NEDA. Notably, 83% (25/30) of subjects in the study met the original predefined positive response of 50% or greater reduction in enhancing lesion numbers after treatment.
Table 1.
Baseline demographic and disease characteristics.
| Non-NEDA |
NEDA |
All subjects |
||||
|---|---|---|---|---|---|---|
| CSF available | All | CSF available | All | CSF available | All | |
| Number | 18 | 21 | 6 | 9 | 24 | 30 |
| Age, years (mean ± SD) | 41.9 ± 6.7 | 42.2 ± 6.2 | 38.0 ± 11.4 | 39.7 ± 10.2 | 40.9 ± 7.8 | 41.5 ± 7.5 |
| Gender (% female) | 55.6 | 61.9 | 100 | 100 | 66.7 | 73.3 |
| Race (% Caucasian, % black) | 88.9, 11.1 | 90.5, 9.5 | 100, 0 | 100, 0 | 91.7, 8.3 | 93.3, 6.7 |
| Duration of disease, years (mean ± SD) | 10.9 ± 7.9 | 10.6 ± 7.6 | 9.2 ± 8.3 | 8.4 ± 7.2 | 10.5 ± 7.8 | 10.0 ± 7.4 |
| MS type (% RRMS, % SPMS) | 72.2, 27.8 | 76.2, 23.8 | 100, 0 | 100, 0 | 79.2, 20.8 | 83.3, 16.7 |
| Baseline number of enhancing lesions (mean ± SD) | 1.4 ± 1.7 | 1.3 ± 1.6 | 4.8 ± 3.3 | 6.0 ± 5.0 | 2.29 ± 2.6 | 2.7 ± 3.6 |
| EDSS, median (minimum, maximum) | 6.0 (3.0, 6.5) | 6.0 (3.0, 6.5) | 3.5 (3.0, 6.0) | 3.0 (2.0, 6.0) | 5.8 (3.0, 6.5) | 4.0 (2.0, 6.5) |
NEDA: no evidence of disease activity; CSF: cerebrospinal fluid; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; RRMS: relapsing–remitting multiple sclerosis; PPMS: primary progressive multiple sclerosis; SD: standard deviation.
Demographic, clinical, and imaging predictors of optimal treatment response
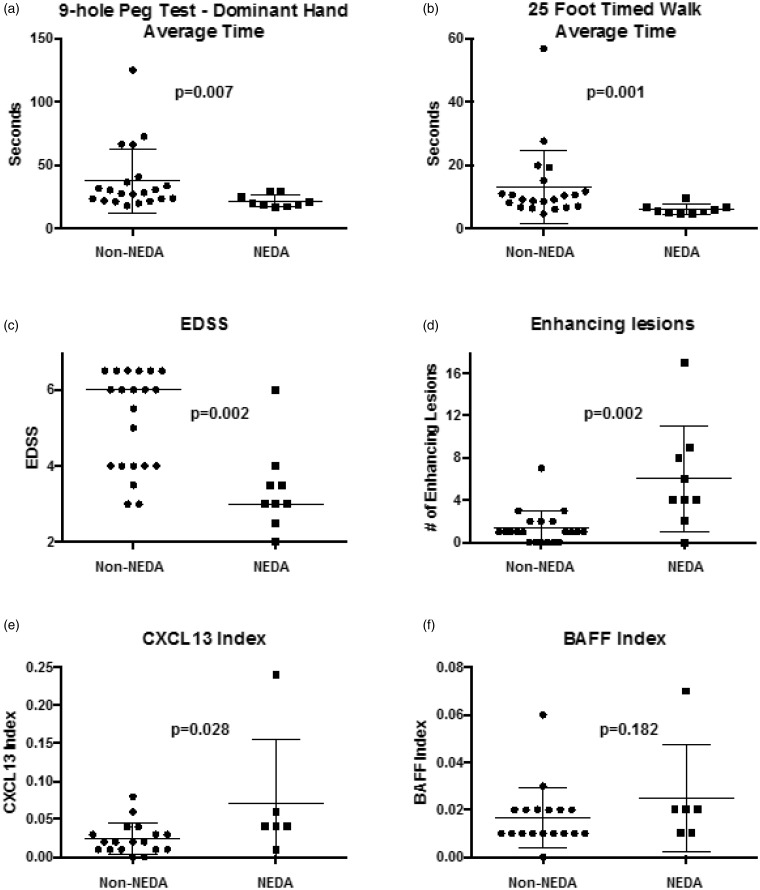
None of the eight male subjects met the criteria for NEDA, whereas all nine of those achieving NEDA were women (P = 0.067) (Table 1). Race, age, prior history of progression and disease duration did not help identify optimal responders (P = 0.999, 0.820, 0.286, and 0.329, respectively). Compared to the non-NEDA subjects, at baseline those with NEDA had faster nine-hole peg test times with the dominant hand (median 19.6 seconds for NEDA responders versus 28.3 seconds for non-NEDA responders, P = 0.007; Figure 1(a)), non-dominant hand (median 19.9 seconds versus 31.3 seconds, P = 0.001), 25FTW times (median 5.5 seconds in optimal responders versus 9.2 in non-NEDA subjects, P = 0.001; Figure 1(b)) and lower EDSS (median of 3.0 in the optimal responders versus 6.0 in the non-NEDA group, P = 0.002; Figure 1(c)). Optimal responders had a higher baseline number of CELs than the non-NEDA group (median of four CELs in NEDA versus one for non-NEDA, P = 0.002; Figure 1(d)). Seven of eight patients (88%) with four or more CELs on the baseline MRI immediately prior to rituximab treatment were ideal responders. Neither the number nor the volume of fluid-attenuated inversion recovery lesions or T1 hypointensities were different between responder groups.
Figure 1.
At baseline, optimal responders were better clinically, with faster (a) nine-hole peg test times (dominant hand, P = 0.007) and (b) 25 foot timed walk times (P = 0.001). Optimal responders also had (c) lower Expanded Disability Status Scale (EDSS, P = 0.002) and (d) a higher number of contrast-enhancing lesions (P = 0.002). (e) Intrathecally synthesized CXCL13 calculated by the ‘CXCL13 index’ predicted optimal response to rituximab (P = 0.028). (f) The BAFF index did not predict optimal responders. NEDA: no evidence of disease activity.
Biomarker predictors of ideal treatment response
CSF and serum samples were evaluated for potential biomarkers of tissue destruction, including MBP, NFH, and NFL, and for B-cell biomarkers including CXCL13, a B-cell chemoattractant that is critical for the development of lymphoid follicles, CCL19 which attracts activated B cells and other cell types, and BAFF, a B-cell activator. MBP, NFH, and NFL levels were mostly undetectable in serum.
At baseline, the CSF median IgG index was significantly higher in the NEDA patients (1.17 versus 0.67, P = 0.041). Five of the nine CSF parameters were larger in magnitude in the NEDA patients, although not significantly (MBP 1.99 ng/ml for optimal responders versus 0.79 ng/ml in the non-NEDA group, NFL 3064.0 pg/ml versus 876.2 pg/ml, CXCL13 40.5 pg/ml versus 9.9 pg/ml, BAFF 121.7 pg/ml versus 102.2 pg/ml, CCL19 528.3 pg/ml versus 305.9 pg/ml). No differences were noted for CSF NFH (157.8 pg/ml in NEDA responders versus 185.1 pg/ml in non-NEDA), CSF antibodies to rMOG (0.26 versus 0.31), and albumin index (5.2 versus 6.9). Serum CXCL13 and BAFF levels were not significantly higher in optimal responders than non-NEDA subjects (129.9 pg/ml versus 69.8 pg/ml and 1163.9 pg/ml versus 1100.1 pg/ml, respectively).
Optimal responders had higher baseline CEL numbers. Because CXCL13 CSF levels may be increased due to the transfer of serum CXCL13 across the damaged blood brain barrier, a ‘CXCL13 index’ was created to estimate CXCL13 derived by intrathecal production. The CXCL13 index in the optimal responders was greater (mean 0.071) than in the non-NEDA group (0.024, P = 0.028; Figure 1(e)). A BAFF index created similarly was not significantly different between groups (0.025 for NEDA group versus 0.018; Figure 1(f)).
To examine how well these tests could predict patients who achieve NEDA, a receiver operating characteristic analysis was conducted. The area under the curve for the CXCL13 index was 0.806, indicating that this test alone could predict an optimal response to rituximab 81% of the time. Clinical measures had equally strong predictive power, with the nine-hole peg test (non-dominant hand) predicting an optimal response 88% of the time, 25FTW in 88%, CELs in 86%, and EDSS in 86%.
The NEDA group was all female relapsing–remitting MS patients. To examine the effect of gender and MS subtype disparities between the two groups, the NEDA group was compared with the subset of non-NEDA consisting of only female relapsing–remitting MS patients. This reduced the sample size to 19 and lowered the power to detect differences. Two baseline predictors were significant, higher CSF NFL and CEL number, with a strong trend towards the CSF CXCL13 index being predictive as well.
Effect of treatment with rituximab on biomarkers
The effect of rituximab treatment on potential biomarkers was examined to determine if these biomarkers could act as surrogates of treatment response. We also considered that normalization of the biomarkers of tissue destruction and inflammation might complement the definition of NEDA.
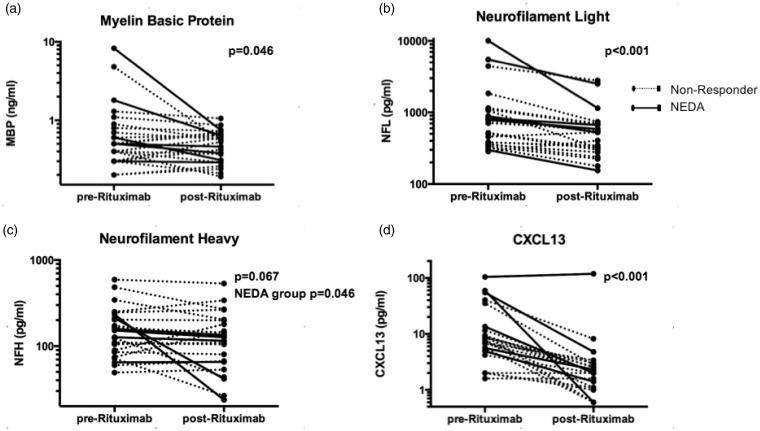
Several putative biomarkers of tissue destruction decreased following rituximab treatment; CSF MBP (1.09 ng/ml at baseline versus 0.51 ng/ml after treatment, P = 0.046; Figure 2(a)) and NFL (1423.1 pg/ml versus 656.0 pg/ml, P < 0.001; Figure 2(b)). These differences were proportionally larger in the NEDA cohort. In the NEDA cohort CSF MBP decreased from 1.99 ng/ml at baseline to 0.47 ng/ml after treatment (P = 0.028) and NFL decreased from 3064.0 pg/ml to 931.3 pg/ml (P = 0.028). In the non-NEDA group, CSF MBP decreased non-significantly from 0.79 ng/ml at baseline to 0.53 ng/ml after treatment (P = 0.320), and NFL decreased from 876.2 pg/ml to 564.2 pg/ml (P = 0.001). For all subjects, NFH levels decreased non-significantly from 178.3 pg/ml to 146.2 pg/ml (P = 0.067; Figure 2(c)). However, in the NEDA group NFH was significantly reduced post-rituximab (157.8 pg/ml pretreatment to 84.6 pg/ml after treatment, P = 0.046; Figure 2(c)), whereas the reduction was not significant in the non-NEDA group (185.1 pg/ml to 166.7 pg/ml, P = 0.446).
Figure 2.
Rituximab reduced levels of biomarkers of tissue damage and inflammation. Levels of (a) CSF MBP, (b) NFL, (c) NFH, and (d) CSF CXCL13 are shown before and after treatment with rituximab. Optimal responders meeting NEDA are shown with solid lines and non-NEDA responders with dotted lines. In NEDA subjects, MBP, NFL, and NFH levels were stable or decreased. CSF: cerebrospinal fluid; MBP: myelin basic protein; NFL: neurofilament light; NFH: neurofilament heavy; NEDA: no evidence of disease activity.
Given that rituximab depletes circulating B cells, B-cell biomarkers were compared pre versus post-treatment. After treatment, CXCL13 levels decreased in CSF from 17.5 pg/ml to 7.00 pg/ml (P < 0.001; Figure 2(d)) and in serum from 79.0 pg/ml to 42.6 pg/ml (P < 0.001). The CXCL13 index also decreased from 0.036 pg/ml to 0.026 pg/ml (P = 0.042; Figure 2(f)). After treatment, BAFF levels increased in CSF from 107.1 pg/ml to 114.4 pg/ml (P = 0.037) and in serum from 1116.3 to 2549.8 (P < 0.001), and the BAFF index fell (0.020–0.010, P < 0.001). As previously reported, the IgG index did not change significantly post-treatment.20
To understand better the changes in the putative biomarkers, they were compared with MRI and disability scores. CSF NFH did not correlate with NFL or MBP, whereas CSF NFL and MBP correlated with each other (r = 0.476, P = 0.019). NFL and MBP also correlated with the number of CELs (r = 0.473, P = 0.020; r = 0.710, P < 0.001, respectively), supporting the theory that each is affected by inflammatory lesions. Meanwhile, NFH correlated with disability measured by EDSS (r = 0.459, P = 0.024). The decrease in NFH in the NEDA cohort corresponded with EDSS improvement; EDSS improved in the NEDA group from 3.0 (2.0–6.0) to 2.0 (1.5–4.5) (P = 0.007) after treatment.
Discussion
An expanding number of DMT formulations for relapsing MS with different mechanisms of action has led to increased expectations of treatment benefits. Thus, the treatment target of ‘no evidence of disease activity’ has emerged.22 Choice of DMT for an individual patient remains largely empirical. In this study, we examined factors related to achieving NEDA on the addition of rituximab in a group of 30 relapsing patients with active MS even though they were taking beta-interferons or glatiramer acetate. Despite their sub-optimal response to standard therapies, one third of subjects who enrolled in our phase II study responded remarkably well to B-cell depletion and achieved the target of NEDA during the 6 months of follow-up.
Optimal responders who achieved NEDA had less disability and more evidence of inflammation at baseline. Better performance on most of the clinical measures was predictive of NEDA, including the nine-hole peg test, 25FTW, and EDSS, with each able to predict NEDA over 80% of the time. Baseline numbers of CELs were greater in optimal responders; seven of eight patients with four or more CELs at baseline achieved NEDA. Treatment with rituximab was associated with reduced putative biomarkers of tissue destruction of both oligodendrocyte and neuronal origin by decreasing MBP, NFL, and NFH levels in CSF, with the NEDA cohort generally displaying larger reductions. As in prior work, NFL and NFH were found not to correlate with each other; our results support another study indicating that NFL fluctuates more with disease activity compared to NFH, which better reflects the level of disability.23
Because rituximab depletes circulating B cells, we assessed factors related to B-cell activity. Previously, we reported that at 6 months after rituximab therapy, CSF B and T cells decreased by 95% and 50%, respectively, while IgG concentration, IgG index, and oligoclonal bands did not change.19 We measured CXCL13 because it is a strong chemoattractant for B cells and activated T cells. Elevated CSF CXCL13 has been predictive of an increased relapse rate and conversion from clinically isolated syndrome to MS.24 Using a cutoff of 22.9 pg/ml (mean + 3 standard deviations of non-inflammatory controls in our prior work),8 after treatment CSF CXCL13 returned to normal in 23 of the 24 patients with CSF available for analysis. Thus, we hypothesized that elevated CSF CXCL13 at baseline might predict response to B-cell depletion. Indeed, a higher CXCL13 index, to reflect intrathecal production of CXCL13, was predictive of achieving NEDA in this small cohort. A similar index for BAFF was not predictive of NEDA, although BAFF levels in blood and CSF increased after B-cell depletion. This suggests that the potential predictive value of a higher CXCL13 index was not an artifact of creation of these indices.
This clinical trial was begun prior to the knowledge that B-cell depletion would benefit relapsing MS, at a time when rituximab was approved only for non-Hodgkin’s lymphoma. Our original protocol criteria of ‘response’ were less stringent than NEDA.16 The definition of NEDA used here includes no measurable clinical activity or new lesions on MRI. An even more stringent definition of NEDA has been proposed to include no accelerated brain volume loss, but our study duration was insufficient to include this. We considered whether biomarkers of tissue destruction might further add to the definition of NEDA. We included analysis for NFL, NFH, MBP, and CXCL13. Of these, NFL might further refine our current definition of NEDA, because two of nine subjects with NEDA continued to have elevated CSF NFL after rituximab (a cutoff for normal levels of 900 ng/ml for NFL was used, based on normal mean + 3 standard deviations determined by Villar and colleagues).25
We report findings from a study with 30 subjects of whom 24 had pre and post-rituximab CSF and serum samples. We describe several potential predictors of an optimal response, and the subsequent reduction of biomarkers of tissue damage in association with NEDA. The predictive factors followed a theme that less disabled patients with more central nervous system inflammation were most responsive to rituximab. Baseline age and duration of disease did not differ statistically among responder groups although the NEDA group tended to be younger, female, and earlier into their disease. Notably, the much larger OLYMPUS trial of rituximab in primary progressive MS also found a better response to rituximab in younger patients, but did not observe a better response in female subjects. In our study all patients achieving NEDA were women, but this may have been a consequence of the low number of subjects.
An elevated CSF CXCL13 index to reflect intrathecally produced CXCL13 provided a provisional baseline biologic predictor of optimal response to rituximab. In addition, treatment with rituximab was associated with reduced tissue damage and inflammation proportionately more in the NEDA responders, suggesting a possible role of these putative biomarkers in future definitions of NEDA. Studies in a larger validation cohort are needed to support the use of these factors as biomarkers. We advocate that analyses such as these be included in future clinical trials of B-cell depleting agents in MS patients.
Acknowledgment
The scientific support of Dr John L Trotter (1943–2001) in the initiation of this clinical trial was invaluable.
Funding
This project was supported by the National Multiple Sclerosis Society USA (grant number RG 3292), NIH (grant number K24RR017100-02), the Washington University Institute of Clinical and Translational Sciences from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) (grant numbers UL1 TR000448, CO6 RR020092, and K23NS052430-01A1), and the Barnes-Jewish Hospital Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS, NIH, or the Barnes-Jewish Hospital Foundation. EA was supported by a Sylvia Lawry Physician Clinical Fellowship from the National MS Society and Gateway chapter (grant number FP 1772-A-1) and the Predoctoral Training Program at Washington University (grant number TR000448). LP was supported by the Harry Weaver Neuroscience Award from the National MS Society (grant number JF 2144A2/1) and the Fondazione Italiana Sclerosi Multipla (FISM) (grant number 2009/R/33). AHC was supported in part by the Manny and Rosalyn Rosenthal-Dr John L Trotter Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation.
Declaration of conflicting interests.
EA has received consulting honoraria from Teva Neurosciences, Biogen, Novartis, and Genzyme and research support from Teva Neurosciences, Novartis, Biogen, and Accorda.
LP has received research support from Biogen-Idec.
BJP has received speaking honoraria and consulting fees from Biogen-Idec, EMD Serono, Novartis, and Teva Neuroscience.
RTN has received speaking honoraria for consulting for Acorda, Alkermes, Bayer, Biogen, EMD Serono, Genentech, Genzyme, Novartis, and Questcor, and research support through Acorda.
AHC has received consulting honoraria from Abbvie, Biogen, EMD Serono, Genzyme, Genentech, Novartis, Roche, Mallinckrodt and Teva Neuroscience.
KT and RJM have nothing to disclose.
References
- 1.Browne P, Chandraratna D, Angood C, et al. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014; 83(11): 1022–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trojano M, Pellegrini F, Paolicelli D, et al. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol 2009; 66(4): 513–520. [DOI] [PubMed] [Google Scholar]
- 3.Giovannoni G, Goodman A. Neutralizing anti-IFN-beta antibodies: how much more evidence do we need to use them in practice? Neurology 2005; 65(1): 6–8. [DOI] [PubMed] [Google Scholar]
- 4.Salzer J, Svenningsson A, Sundstrom P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler 2010; 16(3): 287–292. [DOI] [PubMed] [Google Scholar]
- 5.Kuhle J, Leppert D, Petzold A, et al. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology 2011; 76(14): 1206–1213. [DOI] [PubMed] [Google Scholar]
- 6.Burman J, Zetterberg H, Fransson M, et al. Assessing tissue damage in multiple sclerosis: a biomarker approach. Acta Neurol Scand 2014; 130(2): 81–89. [DOI] [PubMed] [Google Scholar]
- 7.Lamers KJ, de Reus HP, Jongen PJ. Myelin basic protein in CSF as indicator of disease activity in multiple sclerosis. Mult Scler 1998; 4(3): 124–126. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez E, Piccio L, Mikesell RJ, et al. CXCL13 is a biomarker of inflammation in multiple sclerosis, neuromyelitis optica, and other neurological conditions. Mult Scler 2013; 19(9): 1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braitch M, Nunan R, Niepel G, et al. Increased osteopontin levels in the cerebrospinal fluid of patients with multiple sclerosis. Arch Neurol 2008; 65(5): 633–635. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury SA, Lin J, Sadiq SA. Specificity and correlation with disease activity of cerebrospinal fluid osteopontin levels in patients with multiple sclerosis. Arch Neurol 2008; 65(2): 232–235. [DOI] [PubMed] [Google Scholar]
- 11.Comabella M, Pericot I, Goertsches R, et al. Plasma osteopontin levels in multiple sclerosis. J Neuroimmunol 2005; 158(1–2): 231–239. [DOI] [PubMed] [Google Scholar]
- 12.Vogt MH, Floris S, Killestein J, et al. Osteopontin levels and increased disease activity in relapsing-remitting multiple sclerosis patients. J Neuroimmunol 2004; 155(1–2): 155–160. [DOI] [PubMed] [Google Scholar]
- 13.Vogt MH, Lopatinskaya L, Smits M, et al. Elevated osteopontin levels in active relapsing-remitting multiple sclerosis. Ann Neurol 2003; 53(6): 819–822. [DOI] [PubMed] [Google Scholar]
- 14.Amor S, van der Star BJ, Bosca I, et al. Neurofilament light antibodies in serum reflect response to natalizumab treatment in multiple sclerosis. Mult Scler 2014; 20(10): 1355--1362. [DOI] [PubMed]
- 15.Romme Christensen J, Ratzer R, Bornsen L, et al. Natalizumab in progressive MS: Results of an open-label, phase 2A, proof-of-concept trial. Neurology 2014; 82: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 16.Naismith RT, Piccio L, Lyons JA, et al. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology 2010; 74(23): 1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 2008; 63(3): 395–400. [DOI] [PubMed] [Google Scholar]
- 18.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008; 358(7): 676–688. [DOI] [PubMed] [Google Scholar]
- 19.Piccio L, Naismith RT, Trinkaus K, et al. Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch Neurol 2010; 67(6): 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cross AH, Stark JL, Lauber J, et al. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 2006; 180(1–2): 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross AH, Klein RS, Piccio L. Rituximab combination therapy in relapsing multiple sclerosis. Ther Adv Neurol Disord 2012; 5(6): 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009; 8(3): 254–260. [DOI] [PubMed] [Google Scholar]
- 23.Teunissen CE, Khalil M. Neurofilaments as biomarkers in multiple sclerosis. Mult Scler 2012; 18(5): 552–556. [DOI] [PubMed] [Google Scholar]
- 24.Khademi M, Kockum I, Andersson ML, et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler 2010; 17(3): 335–343. [DOI] [PubMed] [Google Scholar]
- 25.Villar LM, Picon C, Costa-Frossard L, et al. Cerebrospinal fluid immunological biomarkers associated with axonal damage in multiple sclerosis. Eur J Neurol: the official journal of the European Federation of Neurological Societies 2014; 22(8): 1169--1175. [DOI] [PubMed]