Abstract
The rate of phloem loading, its selectivity, and the disposition of labeled carbon were studied following application of 14C-labeled sugars to the free space of source leaves of sugar beet (Beta vulgaris L.). Buffered 10 mm solutions of 14C-labeled sucrose, fructose, stachyose, mannitol, 3-0-methyl glucose or l-glucose were applied to the abraded epidermis of source leaves held in the dark. Distribution of the labeled carbon from sugar taken up from the free space was studied by micro-densitometry of autoradiographs. Uptake of labeled sugar from the free space, partition between mesophyll and minor veins, metabolic conversions, export and respiration were followed during the 3-hr time course studies. Rates of sugar uptake into the minor veins, flux rates through the sieve element-companion cell complex membrane and concentration ratios between free space and the interior of the minor vein phloem cells were compared for the six sugars studied for evidence of active uptake. The composition of the free space solution in leaves photosynthesizing in 14CO2 was studied by vacuum infiltration of the source leaf air spaces and removal of the solution by centrifugation. Labeled compounds in this solution were compared to those in an aqueous ethanol extract of the same leaf pieces.
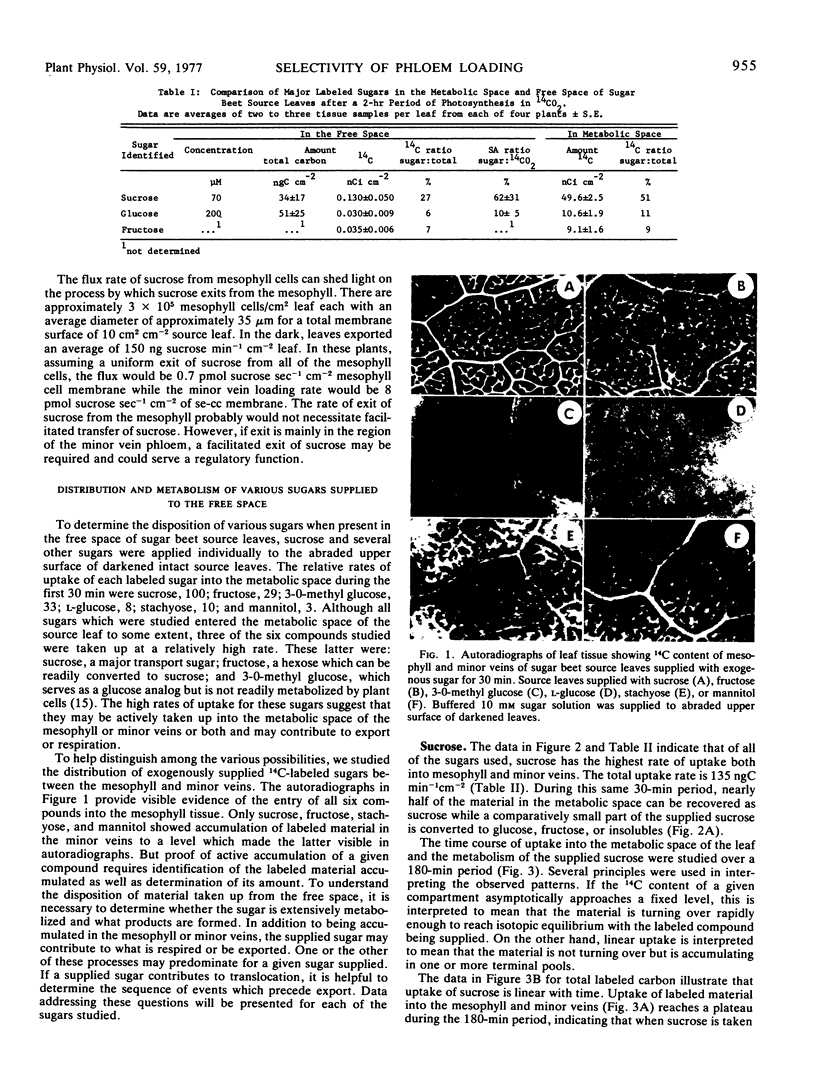
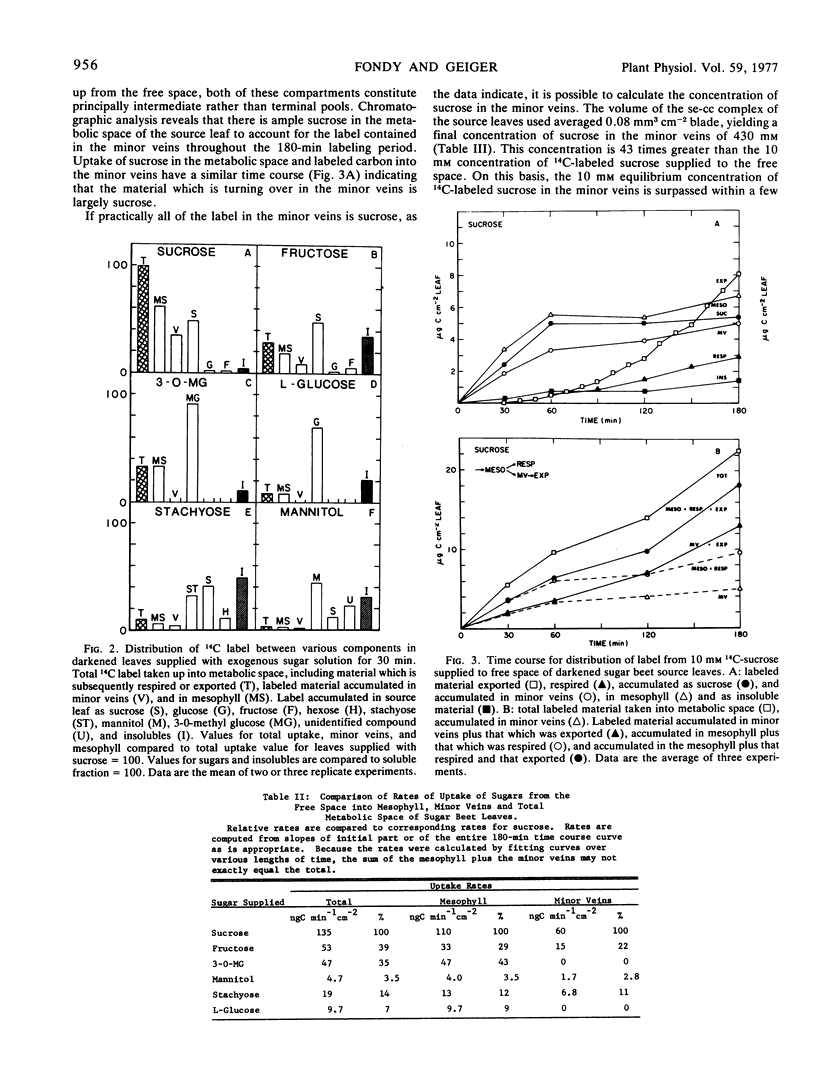
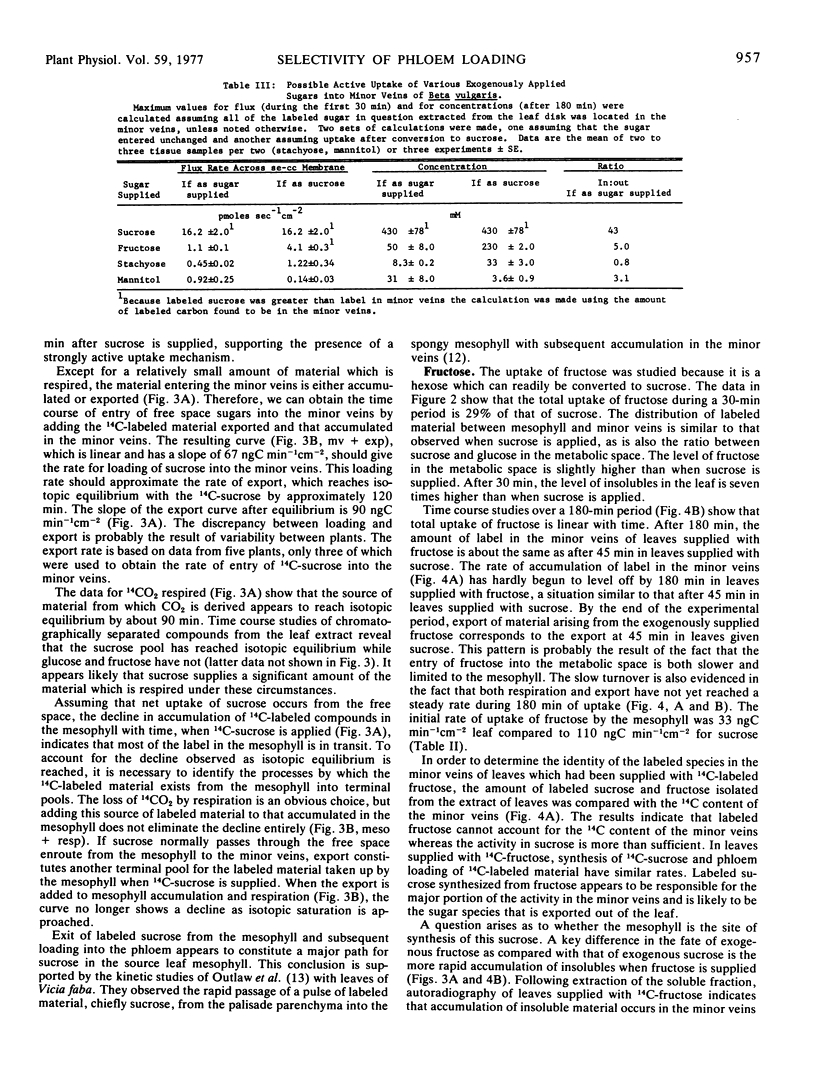
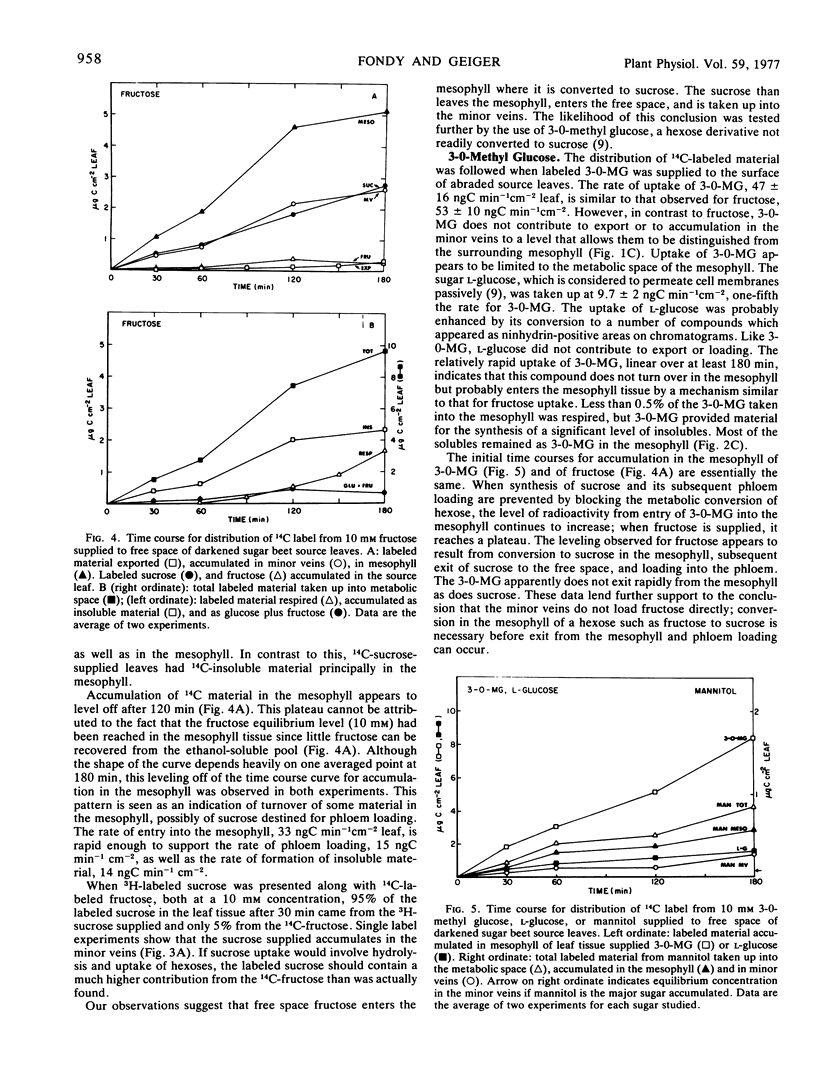
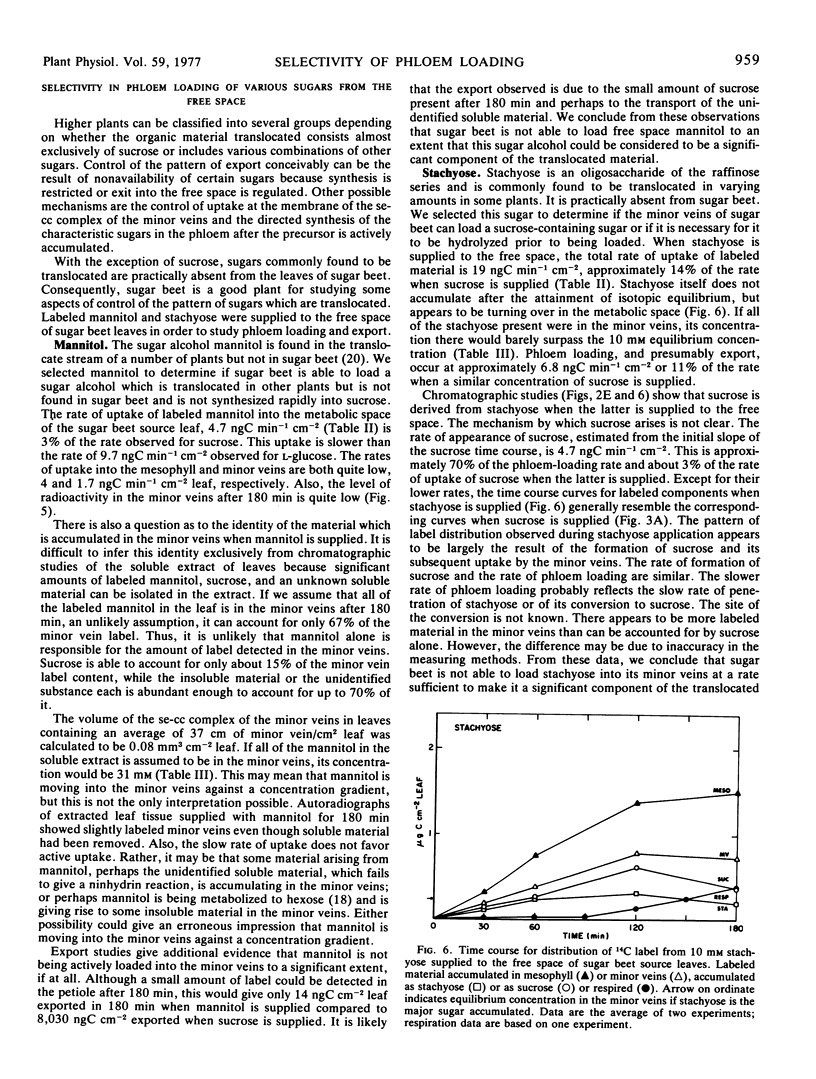
The results in sugar beet source leaves support the concept of direct, active uptake of sucrose from free space into minor veins. This is not the case for fructose, 3-0-methyl glucose, mannitol, or stachyose. The latter two sugars, which are translocated in some plants, are not loaded into the minor veins at a rate sufficient to make them a significant component of the material translocated. The rate of phloem loading is controlled in part by mesophyll metabolism, especially as it affects the availability of sucrose to the free space. Both the rate and selectivity of export are controlled by uptake from the free space into the sieve element-companion cell complex of the minor veins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cataldo D. A. Vein Loading: The Role of the Symplast in Intercellular Transport of Carbohydrate between the Mesophyll and Minor Veins of Tobacco Leaves. Plant Physiol. 1974 Jun;53(6):912–917. doi: 10.1104/pp.53.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Giaquinta R. T., Sovonick S. A., Fellows R. J. Solute distribution in sugar beet leaves in relation to Phloem loading and translocation. Plant Physiol. 1973 Dec;52(6):585–589. doi: 10.1104/pp.52.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Sovonick S. A., Shock T. L., Fellows R. J. Role of free space in translocation in sugar beet. Plant Physiol. 1974 Dec;54(6):892–898. doi: 10.1104/pp.54.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Evaluation of Selected Parameters in a Sugar Beet Translocation System. Plant Physiol. 1965 Sep;40(5):942–947. doi: 10.1104/pp.40.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Sucrose Translocation in the Sugar Beet. Plant Physiol. 1965 Jul;40(4):685–690. doi: 10.1104/pp.40.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Fisher D. B., Christy A. L. Compartmentation in Vicia faba Leaves: II. Kinetics of C-Sucrose Redistribution among Individual Tissues following Pulse Labeling. Plant Physiol. 1975 Apr;55(4):704–711. doi: 10.1104/pp.55.4.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Fisher D. B. Compartmentation in Vicia faba Leaves: I. Kinetics of C in the Tissues following Pulse Labeling. Plant Physiol. 1975 Apr;55(4):699–703. doi: 10.1104/pp.55.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold L., Eshhar Z. Transport of 3-o-Methylglucose Into and Out of Storage Cells of Daucus carota. Plant Physiol. 1968 Jul;43(7):1023–1030. doi: 10.1104/pp.43.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASHKO M. E., RICE E. W. Determination of glucose by an improved enzymatic procedure. Clin Chem. 1961 Oct;7:542–545. [PubMed] [Google Scholar]