Abstract
Background
Two ongoing phase II clinical trials (RENEW and SYNERGY) have been developed to test the efficacy of anti-LINGO-1 antibodies in acute optic neuritis and relapsing forms of multiple sclerosis, respectively. Across a range of experimental models, LINGO-1 has been found to inhibit neuron and oligodendrocyte survival, axon regeneration, and (re)myelination. The therapeutic effects of anti-LINGO-1 antibodies on optic nerve axonal loss and regeneration have not yet been investigated.
Objective
In this series of studies we investigate if LINGO-1 antibodies can prevent acute inflammatory axonal loss, and promote axonal regeneration after injury in rodent optic nerves.
Methods
The effects of anti-LINGO-1 antibody on optic nerve axonal damage were assessed using rodent myelin oligodendrocyte glycoprotein experimental autoimmune encephalomyelitis (EAE), and its effects on axonal regeneration were assessed in optic nerve crush injury models.
Results
In the optic nerve, anti-LINGO-1 antibody therapy was associated with improved optic nerve parallel diffusivity measures on MRI in mice with EAE and reduced axonal loss in rat EAE. Both anti-LINGO-1 antibody therapy and the genetic deletion of LINGO-1 reduced nerve crush-induced axonal degeneration and enhanced axonal regeneration.
Conclusion
These data demonstrate that LINGO-1 blockade is associated with axonal protection and regeneration in the injured optic nerve.
Keywords: Optic neuritis, optic nerve degeneration and regeneration, axons and retinal ganglion cells, LINGO-1, anti-LINGO-1 antibody, multiple sclerosis
Introduction
Axonal degeneration and neuronal loss often lead to progressive functional impairment and worsening disability in central nervous system (CNS) disorders such as multiple sclerosis (MS), Parkinson's disease, and glaucoma.1–8 Neuroprotective agents could offer a new therapeutic approach to complement the current standards of care by reducing the neuronal degeneration rate and/or preserving or restoring CNS neuronal function.8 Previous efforts at developing CNS regeneration therapeutics focused on neurotrophins and growth factors and were not clinically successful because of their lack of tissue specificity and attendant off-target tissue toxicity.8
Leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1 (LINGO-1) represents an attractive target for therapeutic intervention across a range of neurodegenerative CNS disorders.8 LINGO-1 is a potent negative regulator of oligodendrocyte differentiation, neuronal and axonal survival, and axonal regeneration.8–10 Unlike other known negative myelination regulators, such as γ-secretase11 or chemokine receptor CXCR2,12 LINGO-1 is selectively expressed in the CNS.9,10,13,14
LINGO-1 expression is up-regulated in animal models of spinal cord injury, MS, Parkinson's disease, and glaucoma.8 In human postmortem studies, LINGO-1 expression is increased in Parkinson's disease and MS brain tissue.8 In vitro, attenuation of LINGO-1 expression or function enhances oligodendrocyte differentiation, myelination, neuronal survival, and axonal regeneration, as well as functional recovery in animal models of CNS demyelination.8,15 Blocking or ablating LINGO-1 function in experimental autoimmune encephalomyelitis (EAE) using LINGO-1-null mice or an anti-LINGO-1 neutralizing antibody facilitates remyelination.15 In murine toxin-induced Parkinson's disease models, dopamine neuron survival was increased and behavioral abnormalities reduced in LINGO-1-null versus wild-type mice,16 and in a glaucoma disease model, LINGO-1 antagonism promoted retinal ganglion cell (RGC) survival.8
Optic neuritis (ON) occurs in MS17 and is characterized by acute inflammatory optic nerve demyelination, resulting in RGC loss, axonal degeneration, and, potentially, permanent visual impairment.17–19 Currently, no neuroprotective or neuroregenerative therapies are approved for treating ON associated with MS. Here we assessed whether pharmacologic antagonism of LINGO-1 function with monoclonal blocking antibodies had beneficial effects on either axon protection or regeneration in the optic nerve in vivo. The experiments used rodent EAE models of acute inflammatory axonal injury, and optic nerve crush injury models, to assess effects on axonal degeneration and axonal regeneration, respectively.
Materials and methods
Animal care
All animal handling and surgical procedures were approved by the Howard Florey Institute Animal Ethics Committee or Biogen's Animal Care and Use Committee.
Monoclonal antibody (mAb) preparations
Three different anti-LINGO-1 mAbs were used in the mouse EAE (3B5), rat EAE (BIIB033), and rat optic nerve crush studies (1A7); all were supplied by Biogen (Cambridge, MA, USA). As these studies paralleled the clinical development of anti-LINGO-1 antibodies, we were unable to access the same antibody preparations for all experiments. These mAbs target different LINGO-1 epitopes, but likely have similar blood-brain barrier penetration (∼0.1%).8 Human, rat, and mouse LINGO-1 share 99.5% identity.8 BIIB033 and 3B5 bind human and mouse LINGO-1 with the same affinity (unpublished data). 1A7 was administered by local and intravitreal delivery in these experiments because of its 100-fold lower affinity for rat LINGO-1 than BIIB033 and 3B5 (unpublished data); BIIB033 and 3B5 were given intraperitoneally (IP).
Assessing the effects of LINGO-1 antibody therapy on optic nerve axonal loss in murine EAE by diffusion-weighted imaging and histology
Optic nerve parallel and perpendicular diffusivity detected by diffusion magnetic resonance imaging (MRI) have been validated as measures of axonal degeneration and demyelination, respectively.20,21 We previously demonstrated that C57BL/6 mouse myelin oligodendrocyte glycoprotein peptide amino acid 35 to 55 (MOG35–55) EAE is associated with reduced optic nerve parallel diffusivity, with no change in perpendicular diffusivity.22 Importantly, we also confirmed that parallel diffusivity reduction was highly correlated with optic nerve axonal atrophy measured by histology, and that gross selective demyelination or oligodendrocyte loss in the optic nerve are not features of the pathology of this model at paraplegia onset. In the present study we used C57BL/6 MOG-EAE to measure the efficacy of anti-LINGO-1 antibody therapy on acute inflammatory optic nerve axonal injury in the absence of selective demyelination and oligodendrocyte loss.
EAE was induced in 8- to 12-week-old C57BL/6 male and female mice, using MOG35–55 (95% purity; Auspep Pty Ltd, Victoria, Australia) emulsified in complete Freund's adjuvant containing Mycobacterium tuberculosis H37Ra 4 mg/ml (Difco, Detroit, MI, USA), followed by intravenous injection of pertussis toxin 300 ng in phosphate-buffered saline (PBS) immediately afterwards and 3 days later.23,24
EAE severity was assessed daily using a standardized seven-step paraplegia scale, where 0 signifies no disease and 7 signifies death.25–27 Two separate cohorts of 14 mice each (n = 28 total) received, in a blinded fashion, 10 mg/kg IP anti-LINGO-1 mouse mAb 3B5 (n = 7) or a control mAb IP (n = 7). Another five mice were included as healthy controls for histological studies. Mice were treated four times, once every 3 days, starting on day 6 post EAE induction and before onset of symptoms.
Optic nerve diffusion tensor MRI scans were conducted under anesthesia on control-exposed and anti-LINGO-1-exposed mice at peak EAE disease severity on day 16 or 17 post induction. Mice were anesthetized with 2.5% isoflurane using an MR-compatible head holder and nose cone over the snout, and anesthesia maintained with 0.5–2.0% isoflurane.22 Animals euthanized because of disease severity before day 16 were not analyzed. The optic nerve regions of interest (ROI) comprised 10 voxels in the center of the optic nerves at the prechiasmal level. The optic nerve was imaged using a diffusion-weighted sequence with a spin-echo acquisition on a Bruker 4.7T MRI system (Bruker Corporation, Billerica, MA, USA). MRI images were acquired with the following parameters: repetition time of 1 s, echo time of 30 ms, Δ of 10 ms, number of excitations of eight, slice thickness 0.5 mm, field of view 2 × 2 cm2, and data matrix 256 × 128. We employed b-values of 0 s/mm2 (nondiffusion-weighted image) and 700 s/mm2 for one parallel and one perpendicular diffusion-sensitizing gradient directions.22
Immediately following MRI, mice were euthanized (100 mg/kg IP pentobarbital), transcardially perfused with 0.1 M PBS and 4% paraformaldehyde (PFA), and the optic nerves were removed. Optic nerves were postfixed in 4% PFA, 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, and processed for high-resolution light microscopy as previously described.28 Images were taken from 0.5-µm thick methylene blue stained whole prechiasmal cross-sectional optic nerve sections at 10× and 100× magnification and merged using Photoshop CS3e (Adobe Systems Inc, San Jose, CA, USA). Five prechiasmal optic nerve ROIs were chosen for analysis, four peripheral and one central, each measuring ∼3600 µm2. Analysis was conducted using Image-Pro Plus (Media Cybernetics Inc, Rockville, MD, USA) to assess axonal number, axonal area, and axoplasmal area of each identified axon, for each ROI as previously described.28 The peripheral and central nerve areas were assessed separately for each nerve.
Assessing the effects of LINGO-1-antibody therapy on optic nerve axonal loss in rat EAE
To induce EAE, 8- to 10-week-old female Brown Norway rats were anesthetized by isoflurane inhalation, and injected intradermally at the tail base with 200 µl of inoculum, containing 100 µg rMOG1–125 in saline emulsified (1:1) with complete Freund's adjuvant containing 400 µg heat-inactivated Mycobacterium tuberculosis. After the onset of clinical symptoms (15–16 days after induction), rats were exposed to 30 mg/kg/day methylprednisolone in 5 ml/kg of saline solution (0.9% sodium chloride) or saline solution alone (vehicle) intravenously for 3 consecutive days. Beginning on the second day, rats were given three weekly IP injections of either 6 mg/kg of anti-LINGO-1 mAb BIIB033 or control mAb. Thus, there were four different groups: (a) vehicle + control mAb; (b) methylprednisolone + control mAb; (c) vehicle + mAb BIIB033; or (d) methylprednisolone + mAb BIIB033.
One week after the last mAb dose, animals were euthanized and perfused with 4% PFA in PBS. Twenty-micrometer thick frozen sections of optic nerves were stained with anti-βIII tubulin antibody (BioLegend Inc, San Diego, CA, USA) and Alexa Fluor® 488 goat anti-mouse antibody (Life Technologies, Carlsbad, CA, USA (now ThermoFisher Scientific)) and 4',6- diamidino-2-phenylindole (DAPI) and visualized by fluorescence microscopy at 40× magnification. For axonal quantification, three consecutive sections per optic nerve per animal were analyzed, and three to five animals were counted per group.
Measuring axonal degeneration/regeneration following optic nerve crush injury
We used the optic nerve crush model to investigate the effects of anti-LINGO-1 antibody on axonal regeneration in the optic nerve.
Rat optic nerve crush surgery was performed in adult female Sprague Dawley rats as previously described.29 Immediately after optic nerve crush, anti-LINGO-1 mAb 1A7 (n = 4) or vehicle (n = 8) was injected into either the vitreous chamber of the eye or locally at the crush site. Fluorescein isothiocyanate-conjugated cholera subunit toxin B (FITC-CTB) was injected into the eye to anterograde label intact RGC axons. Fluoro-Gold™ (Fluorochrome, LLC, Denver, CO, USA) was applied at the crush site to retrograde label RGC somata.
Animals were sacrificed after 2 weeks and tissue processed for visualization and quantification. For expression of LINGO-1, 16-µm thick frozen sections of retina were incubated with mouse anti-LINGO-1 (1:200; Biogen) and visualized using fluorescent goat anti-mouse antibody (Alexa Fluor 568; Life Technologies). To evaluate optic nerve axonal morphology after crush, frozen sections of the optic nerve were processed to visualize FITC-CTB-labeled axons, and the number of axons that had regrown beyond the crush site was measured.
To confirm that LINGO-1 inhibition is associated with optic nerve axonal regeneration, optic nerve crush surgery was also conducted in 8- to 9-week-old LINGO-1-null mice (n = 5) and C57BL6/J mice (n = 4) using Dumont #5SF forceps (Fine Science Tools, Foster City, CA, USA) for 2 s approximately 1 mm behind the optic disc, as previously described.30
To evaluate regenerated axons, intravitreous injection of Alexa Fluor 488-conjugated CTB (2 µg/µl; Life Technologies) was performed 2 days before the experimental endpoint. Four weeks after surgery, animals were perfused with 4% PFA. Optic nerves were dissected out and postfixed in the same fixative overnight at 4℃. Tissues were cryoprotected in 30% sucrose overnight at 4℃, and embedded in optimal cutting temperature compound (Fisher Scientific, Pittsburgh, PA, USA). Optic nerves were cut longitudinally (8 µm) and imaged with a 20× objective using a slide scanner (VS120 (Olympus Life Sciences, Waltham, MA, USA)).
The numbers of CTB-labeled axons that crossed different distances from the crush site per section were determined in four sections per mouse to quantify regenerating RGC axons in crushed optic nerves.
Statistics
All data were analyzed using descriptive statistics. In mouse EAE, mortality and paraplegia curves are shown as percentage of total animals exposed, as analyzed using the log-rank sum test (GraphPad Prism version 4.01, La Jolla, CA, USA). All other comparisons between treatment groups were assessed using one-way analysis of variance or unpaired t tests. Data are available from the authors.
Results
A summary of the main findings for each experiment can be found in Table 1.
Table 1.
Summary of the main findings in each experimental model.
| Disease model | Outcome measure | Observation (anti‐LINGO‐1 treatment group vs control antibody) | Antibody |
|---|---|---|---|
| Mouse MOG‐EAE | EAE grade Diffusion-weighted MRI optic nerve Quantitative histology optic nerve (semi‐thin sections) | ↓Number of mice with complete hindlimb paralysis (p = 0.002) ↑Survival (p = 0.007) ↑Parallel diffusivity (p < 0.01) 13% ↑in individual axon area in central region of interest (p = 0.07) | mAb 3B5 |
| Rat MOG‐EAE | Counts of BIII tubulin axons in optic nerve | ↑BIII tubulin-positive axon numbers (p < 0.01) Axon survival not significantly changed by co‐administration of methylprednisolone | mAb BIIB033 |
| Rat optic nerve crush injury | Counts of anterograde-labeled intact RGC axons | ↑Axonal outgrowth (p < 0.05 at all distances measured) | mAb 1A7 |
| Mouse optic nerve crush | Counts of anterograde-labeled intact RGC axons | ↑Axonal outgrowth (p < 0.05 at all distances measured, comparison is for LINGO‐1 null mice vs WT) | Nil |
EAE: experimental autoimmune encephalomyelitis; LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1; mAb: monoclonal antibody; MOG‐EAE: myelin oligodendrocyte glycoprotein experimental autoimmune encephalomyelitis; MRI: magnetic resonance imaging; RGC: retinal ganglion cells; WT: wild-type.
LINGO-1 antagonism reduces morbidity/mortality and improves optic nerve MRI parallel diffusivity in MOG-EAE mice
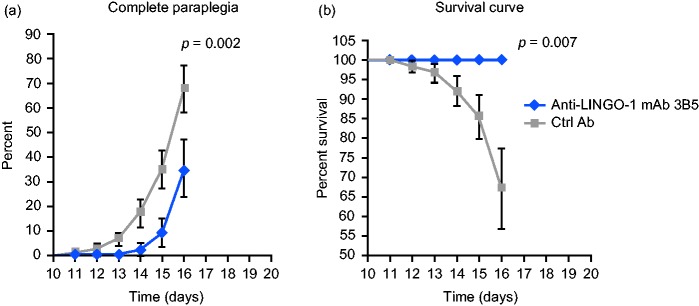
Mice that received control antibody developed acute monophasic EAE with ascending paralysis starting 9–10 days after immunization (Figure 1(a)). The proportion of mice reaching complete hindlimb paralysis (i.e. grade 3.0 disease severity)27 in the control antibody-exposed group (seven of 14 mice) was higher than in the anti-LINGO-1 mAb 3B5-exposed group (four of 14 mice; p = 0.002). Moreover, there was no EAE mortality in mAb 3B5-exposed mice, whereas five of 14 mice exposed to control had to be euthanized because of EAE severity (p = 0.007; Figure 1(b)).
Figure 1.
LINGO-1 blockade reduced morbidity and mortality of myelin oligodendrocyte glycoprotein experimental autoimmune encephalomyelitis (MOG-EAE) mice. (a) The percentage of MOG-EAE mice reaching complete hindlimb paralysis (grade 3 disease severity) exposed to anti-LINGO-1 monoclonal antibody (mAb) 3B5 or control antibody (ctrl Ab). (b) The percentage survival of MOG-EAE mice exposed to mAb 3B5 or ctrl Ab. Data shown as mean ± standard deviation (SD). Day 16 p values were determined using a log-rank test. LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1.
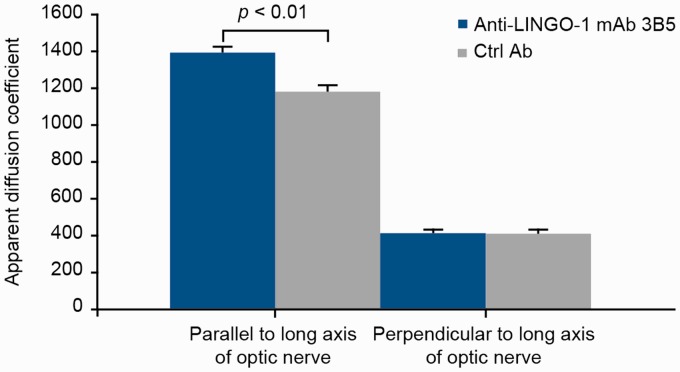
Diffusion-weighted MRI showed significantly higher apparent diffusion coefficient values parallel to the long axis (longitudinal, axial, or parallel diffusivity or λ‖) in mAb 3B5-exposed mice (mean ± SD, 1400 ± 27 µm2/s) versus control-exposed mice (1183 ± 36 µm2/s; p < 0.01; Figure 2). In contrast, there was no difference in apparent diffusion coefficient values perpendicular to the optic nerve long axis (radial or perpendicular diffusivity or λ⊥; 415 ± 19 µm2/s in mAb 3B5-exposed versus 403 ± 25 µm2/s in control mice).
Figure 2.
LINGO-1 blockade protected optic nerve axonal integrity of myelin oligodendrocyte glycoprotein experimental autoimmune encephalomyelitis (MOG-EAE) mice. Analysis of optic nerve axonal integrity by diffusion-weighted imaging in mouse MOG-EAE exposed to anti-LINGO-1 monoclonal antibody (mAb) 3B5 or control antibody (ctrl Ab). Imaging was performed on day 16 or 17 after induction of EAE. Data shown as mean ± SD. p value was determined using a t test. LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1.
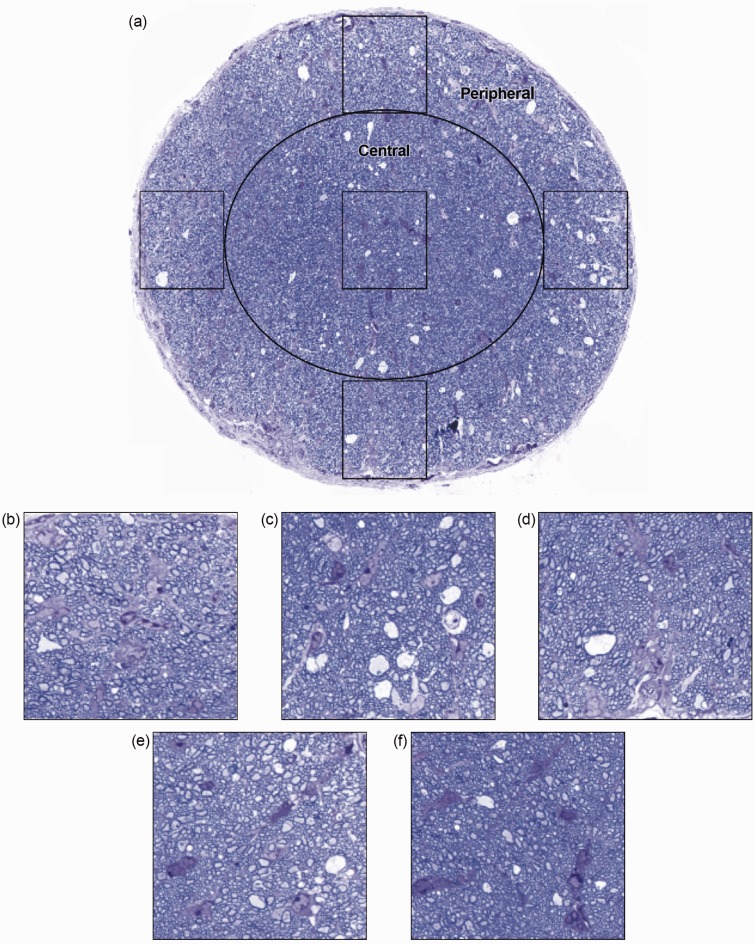
Histological analyses conducted immediately after MRI found no differences in total cross-sectional optic nerve area, number of axons in the central or peripheral ROI, or individual axonal area (a measure of axonal health) in the peripheral optic nerve ROI in control mice versus mAb 3B5-exposed mice (Table 2, Figure 3). Importantly, the individual axonal area of the central ROI in the optic nerve trended 13% lower in control-treated mice relative to anti-LINGO-1-treated mice (p = 0.07, Table 2).
Table 2.
Quantitative histology (mean ± SE) of the optic nerve in MOG‐EAE mice, 16–17 days after EAE induction.
| Histological analysis | Healthy | Control antibody | Anti‐LINGO‐1 mAb 3B5 | p valuea |
|---|---|---|---|---|
| Optic nerve area (µm2) | 85,600 ± 200 | 85,600 ± 2900 | 85,000 ± 3800 | 0.77 |
| Average central axon area (µm2) | 0.46 ± 0.01 | 0.26 ± 0.01 | 0.30 ± 0.01 | 0.07 |
| Peripheral axon area (µm2) | 0.57 ± 0.08 | 0.32 ± 0.01 | 0.33 ± 0.01 | 0.80 |
| Total central axon count | 19,500 ± 2500 | 18,300 ± 800 | 17,400 ± 1300 | 0.56 |
| Total peripheral axon count | 29,500 ± 7400 | 21,900 ± 1000 | 22,100 ± 1400 | 0.90 |
| Total central axoplasmal area (µm2) | 8000 ± 1600 | 4800 ± 300 | 5100 ± 500 | 0.63 |
| Total peripheral axoplasmal area (µm2) | 12,100 ± 1800 | 7100 ± 500 | 7300 ± 600 | 0.78 |
EAE: experimental autoimmune encephalomyelitis; LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1; mAb: monoclonal antibody; MOG‐EAE: myelin oligodendrocyte glycoprotein experimental autoimmune encephalomyelitis; SE, standard error.
p values for control versus anti‐LINGO‐1 mAb 3B5.
Figure 3.
Histological analysis conducted on the 0.5-µm transverse sections of the pre-chiasmal optic nerve in five regions of interest (a). Analysis indicated no significant differences in axon numbers between anti-LINGO-1- and control-treated mice in either the peripheral (b–e) or the central regions (f) of the optic nerve. Axon numbers between the two treatment conditions remained unchanged (Table 2). However, average axon area of the central portion of the optic nerve showed a strong trend toward greater reduction in the placebo-treated mice than the anti-LINGO-1-treated mice when compared with healthy control tissues (Table 2). LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1.
LINGO-1 antagonism protected the optic nerve from axonal degeneration in rat MOG-EAE
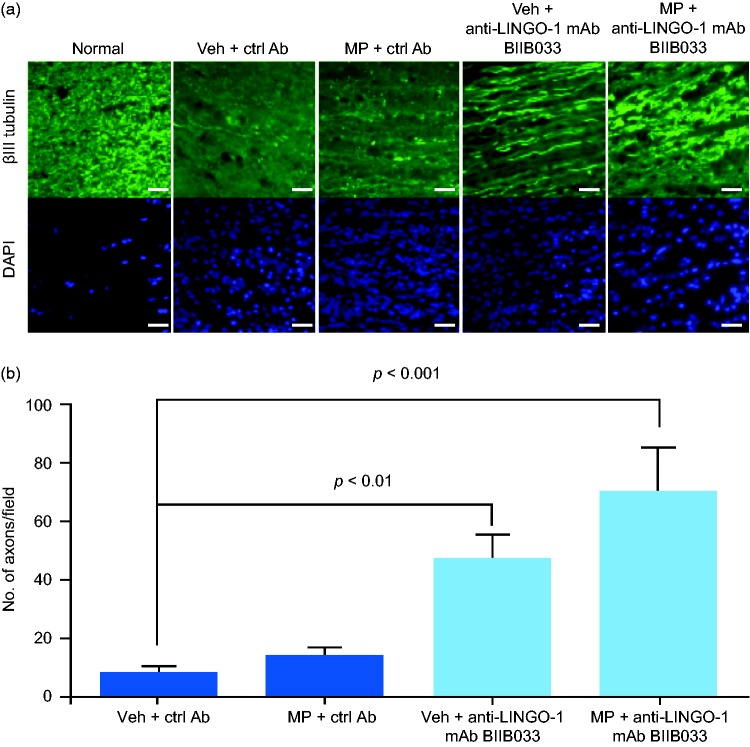
Axonal loss was detected in sections of rat optic nerves from the control group (vehicle + control antibody) by quantitating anti-βIII tubulin-stained axons (Figure 4). Inflammatory infiltration was observed in these areas by DAPI staining, confirming the presence of ON. No axonal protection occurred with daily exposure for 3 days with high-dose intravenous methylprednisolone. In contrast, anti-LINGO-1 mAb BIIB033 exposure alone (vehicle + BIIB033) was associated with a five-fold higher axonal number than exposure to control, indicating that mAb BIIB033 exposure reduced axonal loss. Rats exposed to a combination of methylprednisolone and mAb BIIB033 showed an eight-fold increase in axonal number versus control-exposed rats, indicating that high-dose corticosteroids did not interfere with the axonal protective effects of mAb BIIB033. There was no significant difference in axonal number between the group exposed to mAb BIIB033 alone and the group exposed to mAb BIIB033 + methylprednisolone.
Figure 4.
LINGO-1 blockade protected the optic nerve from axonal degeneration in MOG-EAE rats. (a) Immunohistochemical staining to visualize optic nerve axons in normal or MOG-EAE rats 28 days after the start of the indicated antibody treatments. Scale bar = 25 µm. (b) Quantification of axons from (a). Data in (b) shown as mean ± standard error of the mean (SEM). p values were determined using a one-way analysis of variance followed by Tukey post test. Ctrl Ab: control antibody; DAPI: 4',6-diamidino-2-phenylindole; LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1; mAb: monoclonal antibody; MOG-EAE: myelin oligodendrocyte glycoprotein experimental autoimmune encephalomyelitis; MP: methylprednisolone; Veh: vehicle.
LINGO-1 antagonism led to increased axonal regeneration following optic nerve crush
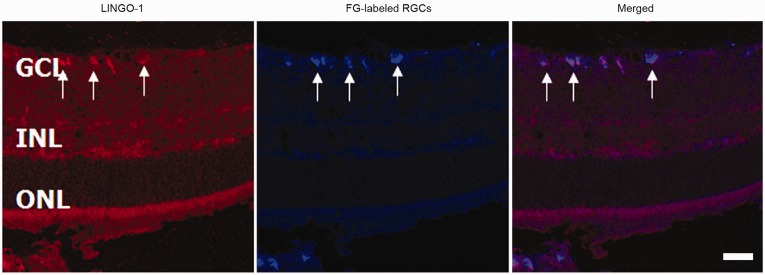
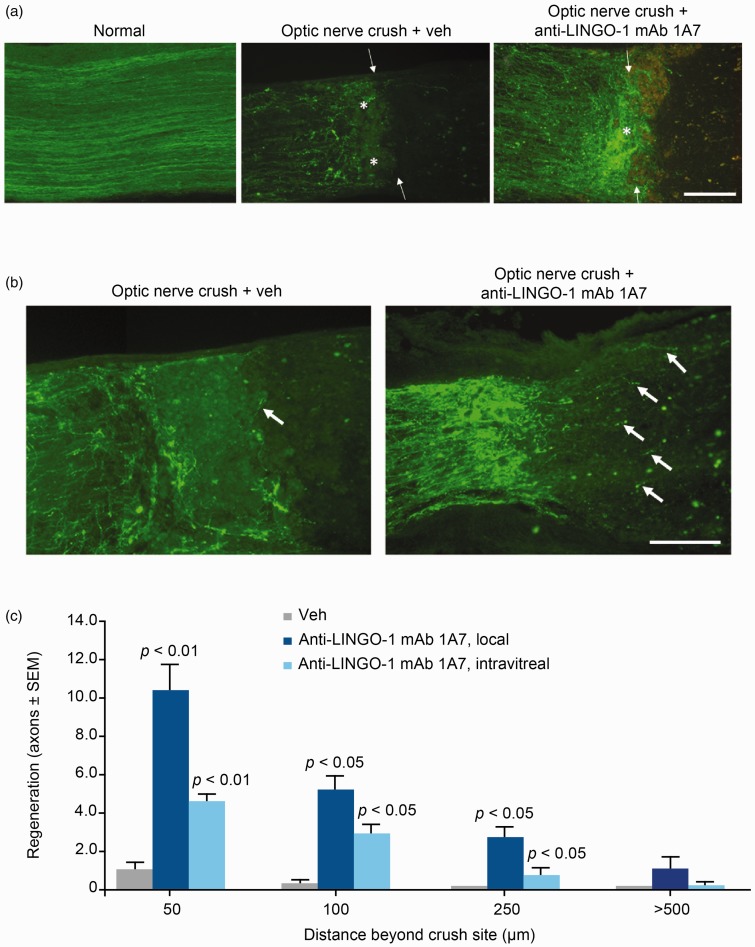
Optic nerve crush experiments were used to assess the effects of LINGO-1 blockade on optic nerve axon regeneration in rats. Immunohistochemical staining showed strong LINGO-1 expression in RGCs (Figure 5). In control rats, FITC-CTB labeling showed dramatic axonal degeneration and collapse of optic nerve fibers at the lesion site. In contrast, axonal degeneration was decreased in mAb 1A7-exposed rats, as evident from labeled axons in close proximity to the lesion site (Figure 6(a)). Additionally, mAb 1A7-exposed rats showed enhanced axonal outgrowth compared with control-exposed rats (Figure 6(b)). Quantification of regenerating axons showed that blocking LINGO-1 by local and, to a lesser extent, intravitreal injection of mAb 1A7 induced increased axonal regeneration relative to control exposure (Figure 6(c)).
Figure 5.
LINGO-1 is expressed in retinal ganglion cells (RGCs). Immunohistochemical staining of LINGO-1 in the retina 14 days after optic nerve crush. Arrows indicate RGCs. Scale bar = 100 µm. FG: Fluoro-Gold; GCL: ganglion cell layer; INL: inner nuclear layer; LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1; ONL: outer nuclear layer.
Figure 6.
Anti-LINGO-1 monoclonal antibody (mAb) 1A7 prevented optic nerve axonal degeneration and facilitated regeneration in a rat optic nerve crush model. (a) Images of optic nerves showing fluorescein isothiocyanate-conjugated cholera toxin subunit B (FITC-CTB)-labeled axons proximal to the crush site in normal or injured rats (14 days after optic nerve crush) exposed to mAb 1A7 or vehicle (veh). Arrows show the crush site, asterisks show axonal areas. Scale bar = 100 µm. (b) Images of optic nerves showing FITC-CTB-labeled axons beyond the crush site in injured rats exposed to mAb 1A7 or veh. Arrows show axonal outgrowth. Scale bar = 100 µm. (c) Quantification of regenerating axons per section at different distances beyond the crush sites in injured rats exposed to veh or mAb 1A7 through local or intravitreal delivery. Data in (c) shown as mean ± SEM. p values were determined using an unpaired t test. LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1.
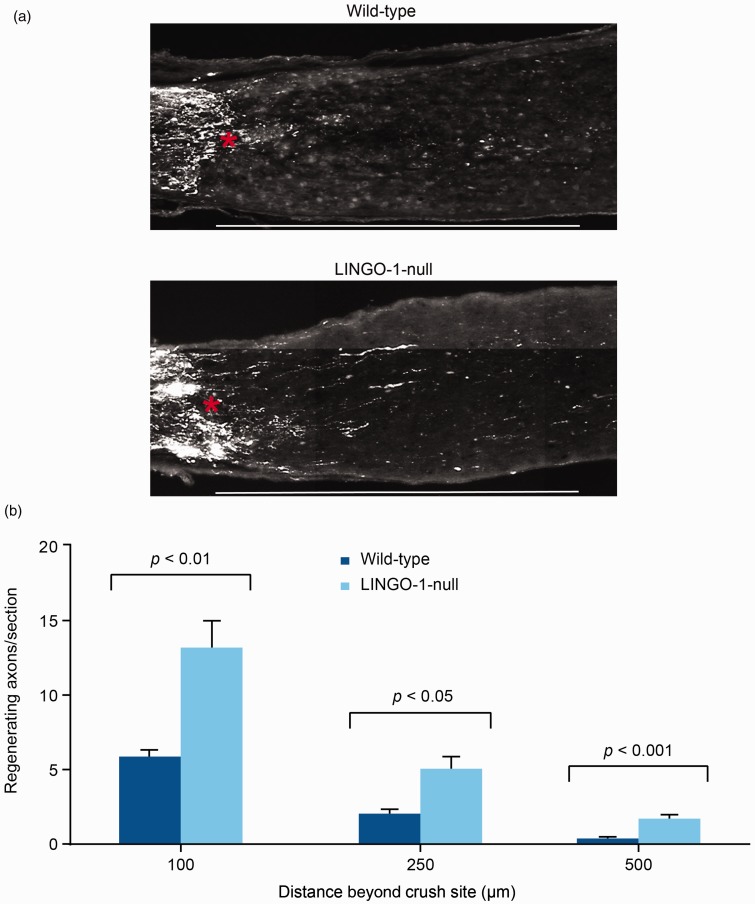
LINGO-1-null mice were used to confirm that absence of LINGO-1 also enhanced optic nerve axonal regeneration. As shown in Figure 7, there was a two-fold increase in CTB-labeled regenerating axons in LINGO-1-null mice compared with wild-type mice at 100, 250, and 500 µm distal to the crush site.
Figure 7.
LINGO-1 deletion enhanced optic nerve axon regeneration in a mouse optic nerve crush model. (a) Images of optic nerves showing Alexa Fluor 488-conjugated cholera toxin subunit B-labeled axons around the crush sites at 28 days after injury from wild-type and LINGO-1-null mice. Asterisk shows crush site. Scale bar = 500 µm. (b) Quantification of regenerating axons per section at different distances beyond the crush site. Data in (b) shown as mean ± SEM. p values were determined using an unpaired t test. LINGO-1: leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1.
Discussion
We have shown that blocking LINGO-1 function protects against optic nerve axonal degeneration in EAE, and promotes axonal regeneration following optic nerve crush injury.
Using the murine MOG-EAE model, we show that LINGO-1 blockade not only reduces disease severity and mortality, but also promotes improvements in optic nerve parallel diffusivity as measured by MRI. In previous studies, we confirmed that parallel diffusivity reductions in the mouse optic nerve following MOG-EAE likely reflect axonal degeneration,22,31 supported by histological evidence of reductions in axon numbers and area in semi-thin sections.22 We also demonstrated that changes in perpendicular diffusivity and selective demyelination are not features of this model. Collectively, our findings suggest that in the absence of gross selective demyelination, anti-LINGO-1 antibody therapy is likely to reduce axonal degeneration in the optic nerves of EAE mice. Importantly, although edema associated with inflammatory cell infiltration is known to affect MRI diffusivity measures, previous studies confirm this is not detectable in EAE mouse optic nerves despite evidence of marked inflammatory cell infiltration.22 Thus, it is unlikely that changes in inflammatory cell numbers are contributing to increased parallel diffusivity measures in the optic nerves of anti-LINGO-1-treated mice.
In this study we were unable to demonstrate conclusively by histology that axon pathology was decreased with anti-LINGO-1 therapy. We do report a strong trend to preserved individual axoplasmal area within the central optic nerve, in support of the MRI findings. These observations could be partly explained by the need to euthanize 35% of mice in the control group because of EAE severity, and their exclusion from histological analyses. Alternatively, the time point at which histology was performed may have been too early in the disease course to see an effect on axon numbers (as a strong trend toward increased central axon atrophy was documented). The technical challenges of en bloc mouse optic nerve dissection and manipulation for histological analysis also introduce variability. Larger and longer studies with the optic nerve assessed at later time points will be needed to fully characterize the neuroprotective effects of LINGO-1 blockade in mouse EAE. These studies will also determine if EAE-induced axonal atrophy is a reversible phenomenon or indicative of irreversible damage ultimately progressing to axonal loss.
Using an alternative model of CNS neuroinflammatory disease (rat MOG-EAE), we provide additional data to support an axonal protective effect of LINGO-1 blockade in the optic nerve, evidenced by increased numbers of βIII tubulin-positive axons relative to control mice. In this experimental model, we additionally investigated if administration of an anti-inflammatory adjunct therapy could improve axonal preservation following anti-LINGO-1 therapy, using high-dose intravenous methylprednisolone (the standard of care for humans with ON). Although the effect of anti-LINGO-1 therapy on axonal protection was preserved in the presence of methylprednisolone, a synergistic or additive effect was not observed. Furthermore, in the absence of anti-LINGO-1 therapy, methylprednisolone was not associated with a significant increase in βIII tubulin-positive axon numbers, highlighting the importance of developing therapies that are specifically targeted to axonal injury mechanisms in acute ON to maximize patient outcomes.
Finally, we show that both local and intravitreal administration of anti-LINGO-1 mAb 1A7 therapy can reduce optic nerve crush-induced axonal degeneration and facilitated axonal outgrowth beyond the crush site. We also confirm the deleterious role of LINGO-1 in axonal regeneration after nerve crush injury using LINGO-1-null mice. Hence, our studies collectively suggest that anti-LINGO-1 therapy could promote both axonal protection and regeneration in the optic nerves.
Importantly, given the supportive preclinical and human phase I data with mAb BIIB033,8,32 a phase II study program is ongoing. Preliminary results from the RENEW trial (presented at the 67th Annual Meeting of the American Academy of Neurology) showed some beneficial effect of anti-LINGO-1 antibody therapy in cases with acute unilateral ON.33 Our studies support the validity of these observations in the human disease.
Acknowledgments
Melissa M Gresle, Yaou Liu, Trevor J Kilpatrick, Dennis Kemper, Qi-Zhu Wu, Bing Hu, Qing-Ling Fu, Kwok-Fai So, Guoqing Sheng, Guanrong Huang, Blake Pepinsky, Helmut Butzkueven, and Sha Mi contributed to the concept and design of at least one set of experiments described, participated in the evaluation and interpretation of the data from those experiments, and were responsible for the statistical analyses. All authors contributed to the content of the manuscript and reviewed the drafts. All authors participated in reviewing and interpreting the data and the paper. All authors have read and approved the final version of this manuscript.
Biogen provided funding for medical writing support in the development of this paper; Malcolm JM Darkes from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, Becky Gardner from Excel Scientific Solutions assisted with the preparation of the manuscript, and Elizabeth Cassell from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper, and provided their final approval of all content.
Conflicts of interest.
Melissa M Gresle has received research support from Biogen and Novartis. Yaou Liu, Dennis Kemper, Qi-Zhu Wu, Bing Hu, Qing-Ling Fu, and Kwok-Fai So have nothing to declare. Trevor J Kilpatrick has received funding for travel to participate in academic meetings from Bayer Schering Pharma, Genzyme, Merck Serono, and Novartis; he receives research support from the Australian Research Council, Multiple Sclerosis Research Australia, the National Health and Medical Research Council of Australia, and the National Multiple Sclerosis Society, and the National Multiple Sclerosis Society. Guoqing Sheng, Guanrong Huang, Blake Pepinsky, and Sha Mi are full-time employees of Biogen and own stock/options in Biogen. Helmut Butzkueven has served on scientific advisory boards for and has received conference travel support from Biogen, Genzyme-Sanofi, and Novartis. He serves on steering committees for trials conducted by Biogen and Novartis. He has received research support from Biogen, Genzyme, Merck Serono, and Novartis in his capacity as honorary chair of the MSBase Foundation. He is on the editorial boards of Multiple Sclerosis International and Multiple Sclerosis and Related Disorders.
Funding
This work was supported by Biogen (Cambridge, MA, USA), which also funded writing and editorial support. Melissa M Gresle is the recipient of a Multiple Sclerosis Research Australia Fellowship. Helmut Butzkueven is the recipient of a National Health and Medical Research Council (NHMRC) Practitioner Fellowship (1080518), NHMRC Project Grants (1065157, 1083539), and an NHMRC Centre of Excellence Award (1001216).
References
- 1.Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol 2011; 93: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meissner WG, Frasier M, Gasser T, et al. Priorities in Parkinson's disease research. Nat Rev Drug Discov 2011; 10: 377–393. [DOI] [PubMed] [Google Scholar]
- 3.Schmier JK, Halpern MT, Jones ML. The economic implications of glaucoma: A literature review. Pharmacoeconomics 2007; 25: 287–308. [DOI] [PubMed] [Google Scholar]
- 4.Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: Genes, inflammation, and neurodegeneration. Neuron 2006; 52: 61–76. [DOI] [PubMed] [Google Scholar]
- 5.Schapira AH. Neurobiology and treatment of Parkinson's disease. Trends Pharmacol Sci 2009; 30: 41–47. [DOI] [PubMed] [Google Scholar]
- 6.Quigley HA, Nickells RW, Kerrigan LA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci 1995; 36: 774–786. [PubMed] [Google Scholar]
- 7.Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye (Lond) 2007; 21(Suppl 1): S11–S14. [DOI] [PubMed] [Google Scholar]
- 8.Mi S, Pepinsky RB, Cadavid D. Blocking LINGO-1 as a therapy to promote CNS repair: From concept to the clinic. CNS Drugs 2013; 27: 493–503. [DOI] [PubMed] [Google Scholar]
- 9.Mi S, Lee X, Shao Z, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci 2004; 7: 221–228. [DOI] [PubMed] [Google Scholar]
- 10.Mi S, Miller RH, Lee X, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci 2005; 8: 745–751. [DOI] [PubMed] [Google Scholar]
- 11.Watkins TA, Emery B, Mulinyawe S, et al. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 2008; 60: 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerstetter AE, Padovani-Claudio DA, Bai L, et al. Inhibition of CXCR2 signaling promotes recovery in models of multiple sclerosis. Exp Neurol 2009; 220: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T, Wen H, Brayton C, et al. Moderate reduction of gamma-secretase attenuates amyloid burden and limits mechanism-based liabilities. J Neurosci 2007; 27: 10849–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tecimer T, Dlott J, Chuntharapai A, et al. Expression of the chemokine receptor CXCR2 in normal and neoplastic neuroendocrine cells. Arch Pathol Lab Med 2000; 124: 520–525. [DOI] [PubMed] [Google Scholar]
- 15.Mi S, Hu B, Hahm K, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med 2007; 13: 1228–1233. [DOI] [PubMed] [Google Scholar]
- 16.Inoue H, Lin L, Lee X, et al. Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson's disease models. Proc Natl Acad Sci U S A 2007; 104: 14430–14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold AC. Evolving management of optic neuritis and multiple sclerosis. Am J Ophthalmol 2005; 139: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 18.Beck RW, Gal RL, Bhatti MT, et al. Visual function more than 10 years after optic neuritis: Experience of the optic neuritis treatment trial. Am J Ophthalmol 2004; 137: 77–83. [DOI] [PubMed] [Google Scholar]
- 19.Balcer LJ, Miller DH, Reingold SC, et al. Vision and vision-related outcome measures in multiple sclerosis. Brain 2015; 138: 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002; 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- 21.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 2002; 15: 435–455. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Butzkueven H, Gresle M, et al. MR diffusion changes correlate with ultra-structurally defined axonal degeneration in murine optic nerve. Neuroimage 2007; 37: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 23.Bittner S, Afzali AM, Wiendl H, et al. Myelin oligodendrocyte glycoprotein (MOG35–55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp 2014; 86: 51275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc 2006; 1: 1810–1819. [DOI] [PubMed] [Google Scholar]
- 25.Butzkueven H, Emery B, Cipriani T, et al. Endogenous leukemia inhibitory factor production limits autoimmune demyelination and oligodendrocyte loss. Glia 2006; 53: 696–703. [DOI] [PubMed] [Google Scholar]
- 26.Butzkueven H, Zhang JG, Soilu-Hanninen M, et al. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med 2002; 8: 613–619. [DOI] [PubMed] [Google Scholar]
- 27.Soilu-Hänninen M, Epa R, Shipham K, et al. Treatment of experimental autoimmune encephalomyelitis with antisense oligonucleotides against the low affinity neurotrophin receptor. J Neurosci Res 2000; 59: 712–721. [DOI] [PubMed] [Google Scholar]
- 28.Gresle MM, Alexandrou E, Wu Q, et al. Leukemia inhibitory factor protects axons in experimental autoimmune encephalomyelitis via an oligodendrocyte-independent mechanism. PLoS One 2012; 7: e47379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leon S, Yin Y, Nguyen J, et al. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci 2000; 20: 4615–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008; 322: 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gresle MM, Shaw G, Jarrott B, et al. Validation of a novel biomarker for acute axonal injury in experimental autoimmune encephalomyelitis. J Neurosci Res 2008; 86: 3548–3555. [DOI] [PubMed] [Google Scholar]
- 32.Tran JQ, Rana J, Barkhof F, et al. Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurol Neuroimmunol Neuroinflamm 2014; 1: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadavid D, Balcer B, Galetta S, et al. Evidence of remyelination with the anti-LINGO-1 monoclonal antibody BIIB033 in acute optic neuritis. Neurology 2015; 85: e44–e49. Abstract 008. [Google Scholar]