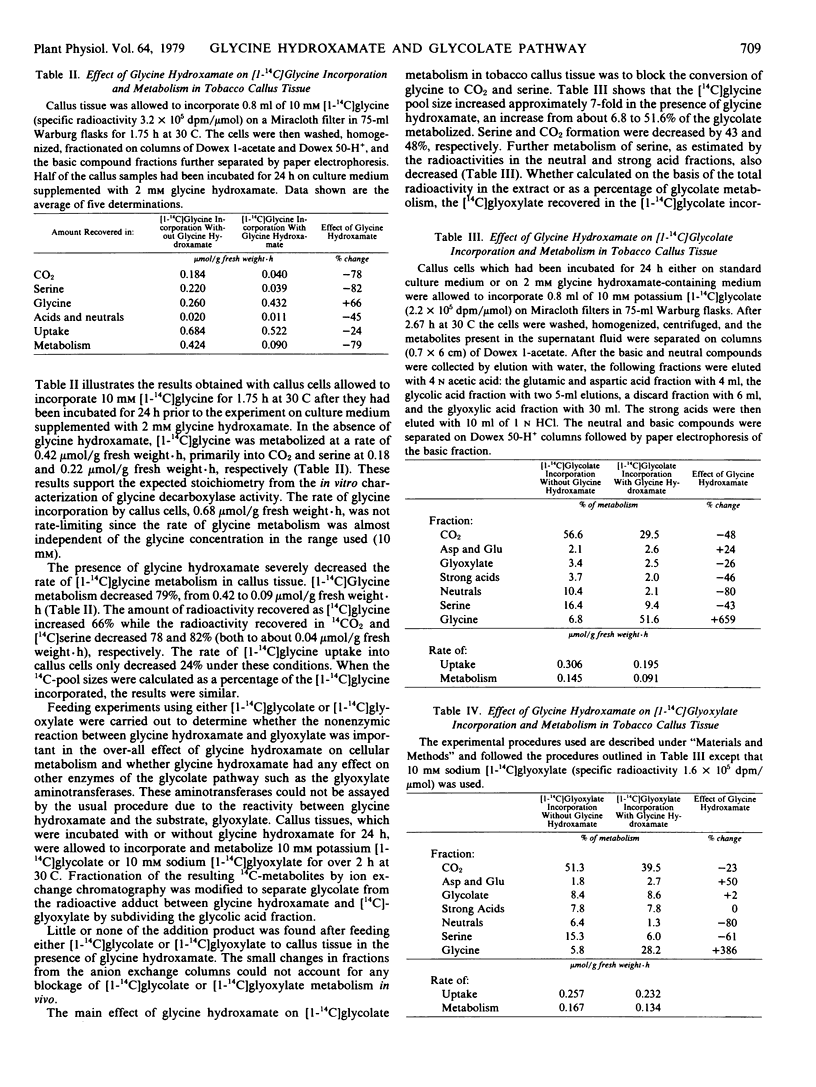
Abstract
Glycine hydroxamate is a competitive inhibitor of glycine decarboxylation and serine formation (referred to as glycine decarboxylase activity) in particulate preparations obtained from both callus and leaf tissue of tobacco. In preparations from tobacco callus tissues, the Ki for glycine hydroxamate was 0.24 ± 0.03 millimolar and the Km for glycine was 5.0 ± 0.5 millimolar. The inhibitor was chemically stable during assays of glycine decarboxylase activity, but reacted strongly when incubated with glyoxylate. Glycine hydroxamate blocked the conversion of glycine to serine and CO2in vivo when callus tissue incorporated and metabolized [1-14C]glycine, [1-14C]glycolate, or [1-14C]glyoxylate. The hydroxamate had no effect on glyoxylate aminotransferase activities in vivo, and the nonenzymic reaction between glycine hydroxamate and glyoxylate did not affect the flow of carbon in the glycolate pathway in vivo. Glycine hydroxamate is the first known reversible inhibitor of the photorespiratory conversion of glycine to serine and CO2.
Full text
PDF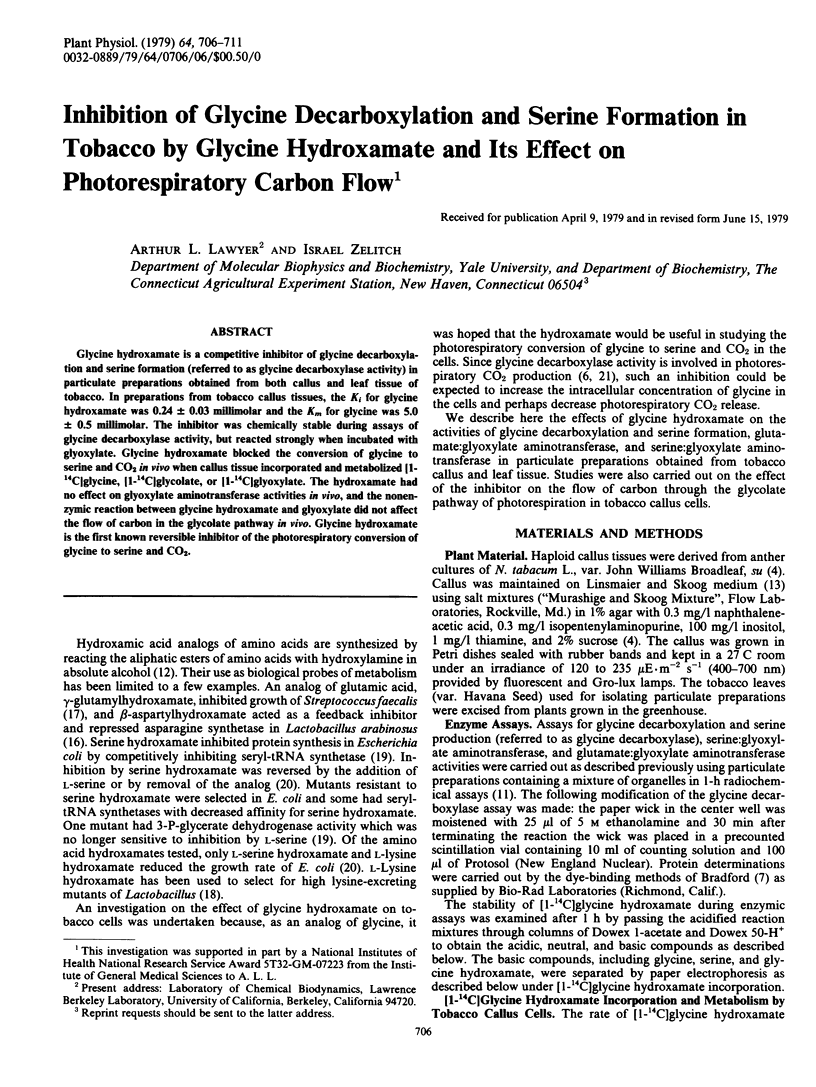




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H., GREEN D. E., HELE P., HIFT H., VON KORFF R. W., RAMAKRISHNAN C. V. The acetate activating enzyme system of heart muscle. J Biol Chem. 1953 Jul;203(1):35–45. [PubMed] [Google Scholar]
- Berlyn M. B., Zelitch I., Beaudette P. D. Photosynthetic characteristics of photoautotrophically grown tobacco callus cells. Plant Physiol. 1978 Apr;61(4):606–610. doi: 10.1104/pp.61.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn M. B., Zelitch I. Photoautotrophic growth and photosynthesis in tobacco callus cells. Plant Physiol. 1975 Dec;56(6):752–756. doi: 10.1104/pp.56.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Lawyer A. L., Zelitch I. Inhibition of glutamate:glyoxylate aminotransferase activity in tobacco leaves and callus by glycidate, an inhibitor of photorespiration. Plant Physiol. 1978 Feb;61(2):242–247. doi: 10.1104/pp.61.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Marker A. F., Whittingham C. P. The metabolism of glycine and glycollate by pea leaves in relation to photosynthesis. Biochim Biophys Acta. 1966 Jun 8;120(2):266–273. doi: 10.1016/0926-6585(66)90346-3. [DOI] [PubMed] [Google Scholar]
- Norton S. J., Chen Y. T. Beta-aspartylhydroxamic acid: its action as a feedback inhibitor and a repressor of asparagine synthetase in Lactobacillus arabinosus. Arch Biochem Biophys. 1969 Feb;129(2):560–566. doi: 10.1016/0003-9861(69)90215-x. [DOI] [PubMed] [Google Scholar]
- Plant J. A., Devlin H. B. Ileostomy and its management. Nurs Times. 1968 May 24;64(21):711–714. [PubMed] [Google Scholar]
- Sands D. C., Hankin L. Fortification of foods by fermentation with lysine-excreting mutants of lactobacilli. J Agric Food Chem. 1976 Nov-Dec;24(6):1104–1106. doi: 10.1021/jf60208a045. [DOI] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Biochemical bases for the antimetabolite action of L-serine hydroxamate. J Bacteriol. 1971 Jun;106(3):972–982. doi: 10.1128/jb.106.3.972-982.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Effect of serine hydroxamate on the growth of Escherichia coli. J Bacteriol. 1971 Jun;106(3):966–971. doi: 10.1128/jb.106.3.966-971.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



