Abstract
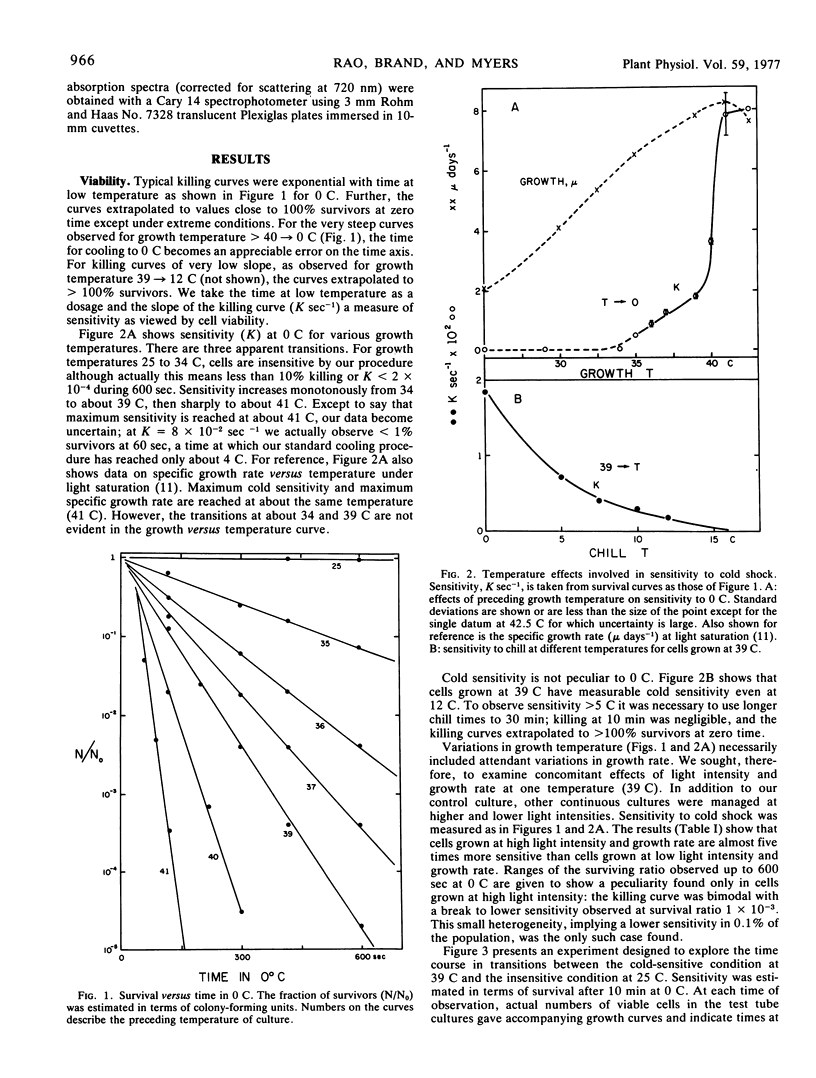
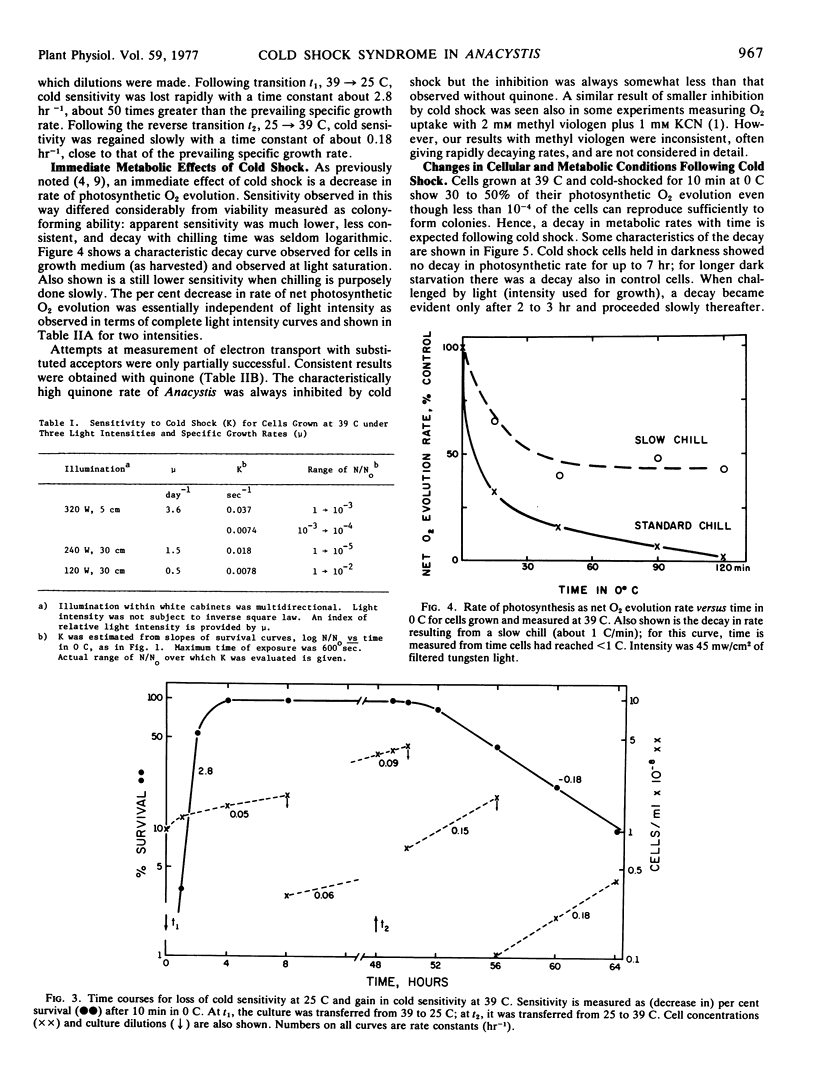
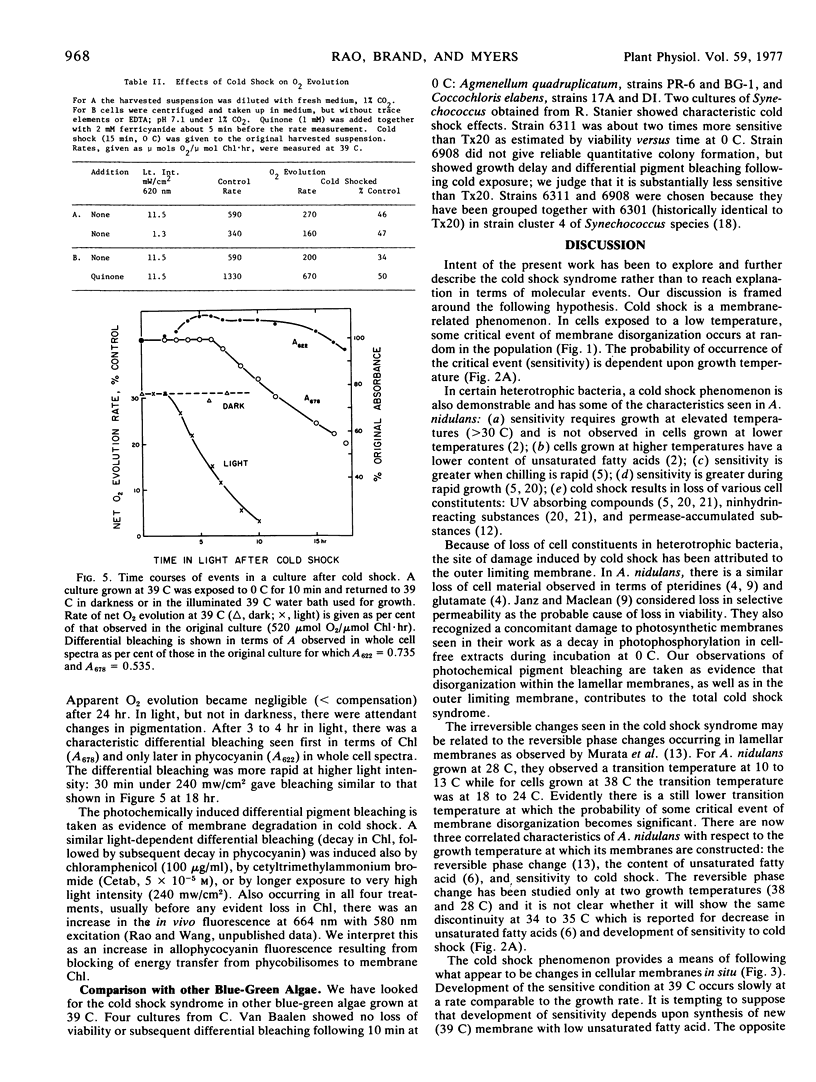
The phenomenon of cold shock in Anacystis nidulans has been explored further in terms of loss of viability and immediate and subsequent metabolic effects. Cold shock was observed also in two closely related strains in which unsaturated fatty acid contents are also known to be low and temperature-dependent. Loss of viability was maximum for cells grown at temperatures above 40 C (<10−4 survivors after 5 min at 0 C) but became negligibly small for cells grown below 34 C. Development of the cold-sensitive condition after transfer 25 → 39 C was slow and comparable to rate of growth; development of the insensitive condition after transfer 39 → 25 C was rapid, implying rapid in situ alteration. An immediate metabolic effect, observed as a decrease in rate of photosynthetic O2 evolution measured at growth temperature, was less severe than loss of viability. Continued light incubation under growth conditions led to slow decay in rate of O2 evolution accompanied by loss of membrane chlorophyll. The multiple effects which comprise the cold shock syndrome appear to be membrane-related phenomena and thereby provide an experimental probe of normal membrane function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farrell J., Rose A. H. Cold shock in a mesophilic and a psychrophilic pseudomonad. J Gen Microbiol. 1968 Mar;50(3):429–439. doi: 10.1099/00221287-50-3-429. [DOI] [PubMed] [Google Scholar]
- Forrest H. S., VAN Baalen C., Myers J. Occurrence of Pteridines in a Blue-Green Alga. Science. 1957 Apr 12;125(3250):699–700. doi: 10.1126/science.125.3250.699. [DOI] [PubMed] [Google Scholar]
- GORRILL R. H., McNEIL E. M. The effect of cold diluent on the viable count of Pseudomonas pyocyanea. J Gen Microbiol. 1960 Apr;22:437–442. doi: 10.1099/00221287-22-2-437. [DOI] [PubMed] [Google Scholar]
- Honeycutt R. C., Krogmann D. W. A light-dependent oxygen-reducing system from Anabaena variabilis. Biochim Biophys Acta. 1970 Mar 3;197(2):267–275. doi: 10.1016/0005-2728(70)90037-x. [DOI] [PubMed] [Google Scholar]
- Jansz E. R., Maclean F. I. Photosynthetic properties of extracts of Anacystis nidulans prepared by lysozyme digestion. Can J Microbiol. 1972 Nov;18(11):1727–1731. doi: 10.1139/m72-268. [DOI] [PubMed] [Google Scholar]
- Jansz E. R., Maclean F. I. The effect of cold shock on the blue-green alga Anacystis nidulans. Can J Microbiol. 1973 Mar;19(3):381–387. doi: 10.1139/m73-062. [DOI] [PubMed] [Google Scholar]
- Kenyon C. N. Fatty acid composition of unicellular strains of blue-green algae. J Bacteriol. 1972 Feb;109(2):827–834. doi: 10.1128/jb.109.2.827-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder I. G. Interrelated effects of cold shock and osmotic pressure on the permeability of the Escherichia coli membrane to permease accumulated substrates. J Bacteriol. 1972 Jul;111(1):211–219. doi: 10.1128/jb.111.1.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Relationships between the Transition of the Physical Phase of Membrane Lipids and Photosynthetic Parameters in Anacystis nidulans and Lettuce and Spinach Chloroplasts. Plant Physiol. 1975 Oct;56(4):508–517. doi: 10.1104/pp.56.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R. The photosynthetic unit in chlorella measured by repetitive short flashes. Plant Physiol. 1971 Sep;48(3):282–286. doi: 10.1104/pp.48.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. W., Harris R. V., James A. T. The lipid metabolism of blue-green algae. Biochem Biophys Res Commun. 1965 Jul 26;20(3):256–262. doi: 10.1016/0006-291x(65)90356-6. [DOI] [PubMed] [Google Scholar]
- Patterson C. O., Myers J. Photosynthetic Production of Hydrogen Peroxide by Anacystis nidulans. Plant Physiol. 1973 Jan;51(1):104–109. doi: 10.1104/pp.51.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Effect of chilling on Aerobacter aerogenes in aqueous suspension. J Gen Microbiol. 1962 Dec;29:719–730. doi: 10.1099/00221287-29-4-719. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., NESS A. G. Effect of chilling on bacteria in aqueous suspension. Nature. 1963 Feb 23;197:819–819. doi: 10.1038/197819a0. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


