Abstract
The unique geography of the Indian subcontinent has provided diverse natural environments for a variety of organisms. In this region, many ecological indices such as temperature and humidity vary predictably as a function of both latitude and altitude; these environmental parameters significantly affect fundamental dynamics of natural populations. Indian drosophilids are diverse in their geographic distribution and climate tolerance, possibly as a result of climatic adaptation. These associations with environmental parameters are further reflected in a large number of clines that have been reported for various fitness traits along these geographical ranges. This unique amalgamation of environmental variability and genetic diversity make the subcontinent an ecological laboratory for studying evolution in action. We assembled data collected over the last 20 years on the geographical clines for various phenotypic traits in several species of drosophilids and present a web-resource on Indian-Drosophila ( http://www.indian-drosophila.org/). The clinal data on ecologically relevant phenotypes of Indian drosophilids will be useful in addressing questions related to future challenges in biodiversity and ecosystems in this region.
Subject terms: Evolution, Ecology, Entomology
Background & Summary
Drosophilid flies are classical models for population genetic and evolutionary ecology studies1–3. Drosophilids as a broader taxonomic group are very diverse in subtropical India, with 25 genera and over 287 species identified to date4–6. As in several other taxonomic groups, the genus Drosophila is species-rich in India, presumably due to the extensive heterogeneity in ecological conditions. This environmental heterogeneity may have spurred diversification among populations and taxa, and many species in this genus are endemic to the Indian subcontinent6.
The Indian subcontinent ranges from 8.4 to 37.6 °N in southern Asia. It is mostly situated on the Indian plate and extends southward into the Indian ocean from the Himalayas. The region is characterized by a diverse geography with an extensive altitudinal range, vast central plains, and large river valleys in the northern foothills of Himalayas. The coefficient of variance of temperature and relative humidity changes predictively from the subtropical lower Himalayas to the tropical southern peninsula. The cumulative effects of the cold northern Himalayas, inland hot plains, and surrounding water bodies are considered as major drivers of the climatic gradients. Further details on these climatic variables can be found in Rajpurohit et al.7 and Rajpurohit and Nedved8.
Phenotypic and genetic clines in various drosophilid species on the Indian subcontinent have been well documented7,8. These studies have primarily examined latitudinal and/or altitudinal variation among populations in traits measured under common garden conditions. Some intriguing patterns have emerged, such as opposing clines for desiccation resistance and starvation resistance (reviewed in Rajpurohit and Nedved8). The environmental gradients present on the Indian subcontinent, in conjunction with the observed phenotypic and genetic clines, sets a platform for studies of local adaptation to natural variation in environmental conditions. These are also the ideal places to investigate adaptive dynamics of fitness-associated traits8. Natural selection acting along environmental gradients could result in the formation and maintenance of such clines8–10. Such patterns of association can also reflect aspects of demography11; if a clinal pattern is replicated in independent samples (e.g., populations sampled across gradients on multiple continents) the inference of selection is strengthened12.
However, studies of wild drosophilid populations in India are not as well-known as similar studies done on European, North American and Australian populations, presumably because of limited circulation or studies published in local journals. Studies from this region are not as well incorporated in the conceptual and empirical understanding of climatic adaptation. Therefore, we assembled data from previous studies on ecologically relevant fitness traits of the Indian drosophilids to generate a comprehensive, one-stop portal for the scientific community. The Indian Drosophila cline dataset contains data from ten Drosophila species from studies published between 1998 and 2013 and includes traits related to morphological variation, life-histories, stress resistance, behavioral differences and genetic markers. A majority of these studies used common garden experiments to test for latitudinal and/or altitudinal differentiation among populations, thus focusing on variation at the phenotypic level8. More recently, studies have also examined range shifts in species boundaries in the context of climate change scenarios13,14.
The compilation of historical datasets and species distributions in a central database repository will also be useful for assessing climate change related studies in this region.
To summarize existing studies on phenotypic and genetic clines of the Indian populations of Drosophila, and to track shifts in these clines over time, the ‘Indian-Drosophila’ portal (http://www.indian-drosophila.org/) was established. The ‘Indian-Drosophila’ web-resource contains: (1) latitudinal clinal data (i.e., trait variations along Indian latitudes), (2) altitudinal cline data (trait variations along Indian altitudinal ranges), and (3) details on species taxonomy and distribution. The web-resource will be continually updated and user-friendly interfaces will be incorporated as needed. In the present format of the resource a user-friendly application named DrosoCline has been incorporated for data visualization purposes.
Methods
Latitudinal and altitudinal traits in Indian drosophilids: Dataset selection
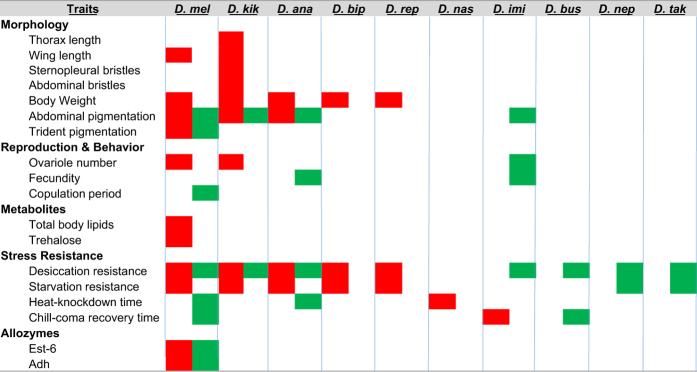
We began the development of this community resource with an exhaustive search of papers published between 1987 and 2015 that examined clinal variation in Indian populations of various drosophilid species. We selected papers and datasets which conformed to the following criteria: (1) at least three populations were included along the latitudinal/altitudinal transect; (2) traits were measured under common garden conditions, i.e., excluding studies where direct measurements were done on wild collected samples; and (3) if parallel studies of the same cline had been conducted, we selected the first, initial study only. With these criteria 16 papers were selected and incorporated to this web-resource. A point to note, most of the traits in this web-resource exhibited significant clines and were reported accordingly; there is the possibility of some bias, as work that failed to identify significant clines may not have been published. In the present clinal datasets five major categories of traits have been incorporated: (1) morphology, (2) reproduction and behavior, (3) metabolites, (4) stress resistance, and (5) allozymes (see Fig. 1).
Figure 1. Status of species and traits covered in this study showing latitudinal or altitudinal clines in India.
The red and green boxes represent the presence of latitudinal and altitudinal clines, respectively. D. mel: Drosophila melanogaster; D. kik: Drosophila kikkawai; D. ana: Drosophila ananassae; D. bip: Drosophila bipectinata; D. rep: Drosophila replete; D. nas: Drosophila nasuta; D. imi: Drosophila immigrans; D. bus: Drosophila buskii; D. nep: Drosophila nepalensis; D. tak: Drosophila takahashii.
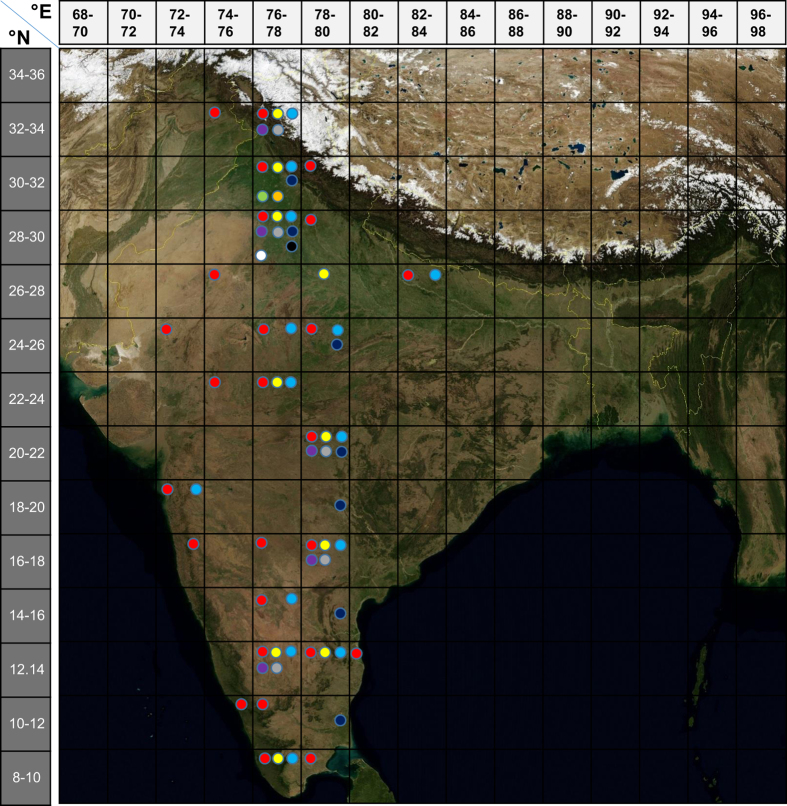
A total of 61 datasets have been incorporated in this resource from which 32 are latitudinal and 29 are altitudinal (see Data Citation 1). These datasets span ten different species, representing the most comprehensive range of studies in drosophilids. This is the only collection of datasets that incorporates both latitudinal and altitudinal clinal data from a single geographical region (the Indian subcontinent). The geographical distribution of populations from which these 61 clinal datasets are derived is between 9–33° N/ 75–83° E (see Fig. 2). This collection of datasets covers a total of 56 collection sites (the origin of sites of different Drosophila species populations are given in Fig. 2; also, see Data Citation 1). Latitude, longitude, and altitude of collection sites are provided in Data Citation 1. Climatic data for these sites can be accessed from the India Meteorological Department, Pune (http://www.imdpune.gov.in/).
Figure 2. Grid map depicting the distribution of various Drosophila species along the Indian latitudes.
The exact geographical coordinates and elevation (Latitude, Longitude, and Altitude) of collection sites are provided in Table S3. The ‘Indian-Drosophila’ web-resource consists of data on a range of traits showing altitudinal and /or latitudinal variations. Each color dot represents a species. A 2/2 latitude-longitude grid locates the origin of populations used in the various clinal studies. Red: D. melanogaster; Yellow: D. kikkawai; Light blue: D. ananassae; Purple: D. bipectinata; Gray: D. replete; Dark blue: D. nasuta; Light green: D. immigrans; Organe: D. buskii; Black: D. nepalensis; White: D. takahashii.
Traits values: Standardization
A total of 18 different traits under five major categories have been incorporated in this resource (Data Citation 1). The morphological traits include thorax length, wing length, sternopleural bristle number, body weight, trident pigmentation, and abdominal pigmentation15–18. Thorax length was measured from the posterior tip of the scutellum to the anterior margin of the thorax. Wing length measured from the point of attachment with thorax up to the tip of the third longitudinal vein. The thorax and wing measurements are expressed in mm×100. Trident pigmentation was scored by visual examination of dorsal side of the thorax using four phenotypic classes. Abdominal pigmentation was scored by the visual examination of lateral side of the abdominal tergites using discrete phenotypic classes. Body weight is expressed as mg×100.
The category for reproduction and behavior included ovariole number, fecundity and copulation duration19,20. Ovariole number was presented as average or sum of both ovary lobes. Fecundity was reported as the number of eggs laid per fly per day. Copulation duration was measured as the time from successful mounting to separation and is expressed in minutes.
The metabolites category included total lipid content as well as trehalose content21. For lipid content, the estimation method as described by Marron et al.22 was followed. Individual flies were dried at 60 °C for 48 h in smaller tubes and weighed. Di-ethyl ether was subsequently added to the samples and agitated at 37 °C for 24 h. Flies were then removed from the solvent and dried again at 60 °C for 24 h and reweighed. Lipid content was calculated by subtracting lipid free mass from dry mass. Trehalose content was estimated using commercially available kits (Megazyme trehalose assay kit). Flies were homogenized in groups and heated in a water bath set at 95 °C for 20 min. Subsequently, samples were centrifuged at 12,000 r.p.m. for 15 min and aliquots of the supernatant were digested with trehalase at 37 °C. The reaction absorbance was measured at 340 nm.
Stress resistance traits included desiccation resistance, starvation resistance, heat-knockdown time, and chill-coma recovery time23–30. Desiccation assays were performed in narrow size Drosophila culture vials where no food and water was provided. Flies were kept individually or in groups of ten with silica gel desiccant; flies were separated from the silica gel desiccant by a foam plug. To measure starvation resistance, flies were kept in narrow Drosophila culture vials in the absence of food. Water was made available with a cotton ball soaked in water or with 1–2% agar media in the vial. For heat-knockdown time measurements, individual flies were placed in glass tubes submerged in a water bath set at 39 ° C. To measure chill-coma recovery time, flies were placed in empty glass vials and buried in ice for a specific period of time. Flies were then brought to room temperature and the recovery time of each individual was recorded.
Adh and Est-6 allozymes were examined for allele frequency variation among populations using standard starch gel electrophoresis and staining methods. Each population was characterized by the frequency of one of the common electrophoretic alleles (for both ADH and Est-6, the frequency of the ‘fast’ mobility allele, F, was reported). For all the traits described above, the original publications give further methodological details. The original data with the accompanying reference can be downloaded using the DrosoCline application.
Data Records
A total of 61 datasets are made available through the Indian-Drosophila web-resource (http://www.indian-drosophila.org/). The datasets can be accessed through the DrosoCline application (https://indian-drosophila.shinyapps.io/DrosoCline/). A direct link for this application is provided in the Indian-Drosophila web-resource. DrosoCline allows the user to choose the type of geographical range (latitude or altitude), species, sex, and phenotype. The user can also download the data for the selected combinations by clicking the associated download tab. The downloaded data file (in.csv format) also contains information regarding the published references. This resource currently archives data on latitudinal and altitudinal variations for 18 ecologically relevant traits in 10 species, and more datasets will be incorporated as they become available. We propose to update the data portal via biannual literature surveys and direct author submission.
Technical Validation
The datasets reported in this collection are already published in peer-reviewed journals15–21,23–31. The data presented here in the paper and on the online resource have also been statistically analyzed in the associated published work. Data have been re-visualized and checked for possible typing errors in the various published papers. If any discrepancies were found they were corrected before placing them on the web-resource. Only the experiments that included control treatments and adequate replication were included in this analysis and the associated web-resource.
Usage Notes
Severe effects of global warming are projected for South Asia. The Regional Climatic Modeling System PRECIS (Providing Regional Climate for Impact Studies) developed by the Hadley Center predicts a 2–4 °C temperature rise in India as the 21st century progresses, with the northern parts of the India being more sensitive to this change32. A temperature profile for the past 100 years (1900–2000) on the Indian subcontinent is shown elsewhere (see Fig. 5 in Rajpurohit et al.7).
The global temperature rise may result in increasing physiological challenges that put organisms, taxa, and entire ecosystems at risk. Recent studies have clearly shown range expansion for many populations, and that such shifts are accompanied by changes in phenology and rapid adaptation to environmental change26,33,34. Studies on phenotypic differentiation across environmental gradients could be a valuable tool to understand fundamental aspects of the evolutionary response to climate change. In Drosophila species, genetic and phenotypic change across geographical transects could provide insight into the connectivity between climate change and evolutionary shifts. Along these lines, recent work on Indian altitudinal transects in the Western Himalayas has shed light on habitat shrinkage and expansion associated with climate change13,14. Ongoing studies from the Indian subcontinent will provide further opportunities for more precise studies merging patterns of phenotypic and genetic variation, species distributions, historical data, and climate change.
This unique amalgamation of taxonomic and genetic diversity, as well as the presence of robust clines for multiple traits across species, makes the Indian subcontinent an ecological laboratory for studying evolution in action. Pimm et al.35 proposed that information on species distribution and geographical variations over time could provide a basic framework to infer evolutionary responses associated with climate change35. The resource we develop here will help the scientific community track spatiotemporal changes in phenotype and associated genomic regions, and can also be used to examine associations between pattern shifts and temporal change in specific climatic variables.
Resources such as the Indian-Drosophila Database also allow us to make comparisons across populations residing on different continents. A resource for Australian drosophilids already exists36. A cross geographical comparison could be used to explore fundamental and unaddressed questions in Drosophila ecology and evolution.
Additional Information
How to cite this article: Rajpurohit, S. et al. A resource on latitudinal and altitudinal clines of ecologically relevant phenotypes of the Indian Drosophila. Sci. Data 4:170066 doi: 10.1038/sdata.2017.66 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The funding for this work was supported by the National Institutes of Health grant R01GM100366.
Footnotes
The authors declare no competing financial interests.
Data Citations
- Rajpurohit S., Zhao X., Schmidt P. S. 2016. Dryad Digital Repository. http://dx.doi.org/10.5061/dryad.8q7s6
References
- Parsons P. A. The Evolutionary Biology of Colonizing Species 1st edn (Cambridge University Press, 1983). [Google Scholar]
- Lemeunier F., David J. R., Tsacas L. & Ashburner M. The melanogaster species group (ed. Ashburner M., Carson H. L. & Thompson J. N. Jr) The Genetics and Biology of Drosophila Vol 3e, 147–256 (Academic Press, 1986). [Google Scholar]
- Powell J. R. Progress and prospects in evolutionary biology: The Drosophila model (Oxford University Press, 1997). [Google Scholar]
- Fartyal R. S. & Singh B. K. List of Drosophilid species so far described and recorded from India. Dros. Info. Service 84, 30–38 (2001). [Google Scholar]
- Gupta J. P. A monograph of Indian Drosophilidae. J. Sci. Res., (B.H.U.) 51, 1–252 (2005). [Google Scholar]
- Singh B. N. 2015. Species and genetic diversity in the genus Drosophila inhabiting the Indian subcontinent. J. Genet. 94, 351–361 (2015). [DOI] [PubMed] [Google Scholar]
- Rajpurohit S., Nedved O. & Gibbs A. G. Meta-analysis of geographical clines in desiccation tolerance of Indian drosophilids. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 164, 391–398 (2013). [DOI] [PubMed] [Google Scholar]
- Rajpurohit S. & Nedved O. Clinal variation in fitness related traits in tropical drosophilids of the Indian subcontinent. J. Therm. Biol. 38, 345–354 (2013). [Google Scholar]
- Endler J. A. Geographic Variation, Speciation, and Clines 2nd edn (Princeton University Press, 1997). [Google Scholar]
- Barton N. H. Clines in polygenic traits. Genet. Res. 74, 223–236 (1999). [DOI] [PubMed] [Google Scholar]
- Kao J. Y., Zubair A., Salomon M. P., Nuzhdin S. V. & Campo D. Population genomic analysis uncovers African and European admixture in Drosophila melanogaster populations from the south-eastern United States and Caribbean Islands. Mol. Ecol. 24, 1499–1509 (2015). [DOI] [PubMed] [Google Scholar]
- Adrion J. R., Hahn M. W. & Cooper B. S. Revisiting classic cline sin Drosophila melanogaster in the age of genomics. Trends Genet. 31, 434–444 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpurohit S., Parkash R. & Ramniwas S. Climatic changes and shifting species boundaries of drosophilids in the Western Himalaya. Acta Entomol. Sinica 51, 328–335 (2008a). [Google Scholar]
- Rajpurohit S., Parkash R., Singh S. & Ramniwas S. Climate change, boundary increase and elongation of a pre-existing cline: A case study in Drosophila ananassae. Entomol. Res. 38, 268–275 (2008b). [Google Scholar]
- Karan D. et al. Latitudinal clines for morphometrical traits in Drosophila kikkawai: a case study of natural populations from the Indian subcontinent. Genet. Res., Camb 71, 31–38 (1998). [DOI] [PubMed] [Google Scholar]
- Parkash R., Karan D., Kataria S. K. & Munjal A. K. Phenotypic variability of quantitative traits in Indian populations of Drosophila kikkawai. J. Zool. Syst. Evol. Research 37, 13–18 (1999a). [Google Scholar]
- Parkash R. & Munjal A. K. Phenotypic variability of thoracic pigmentation in Indian populations of Drosophila melanogaster. J. Zool. Syst. Evol. Research 37, 133–140 (1999b). [Google Scholar]
- Parkash R., Kalra B. & Sharma V. Impact of body melanization on contrasting levels of desiccation resistance in a circumtropical and a generalist Drosophila species. Evol. Ecol. 24, 207–225 (2010). [Google Scholar]
- Rajpurohit S., Parkash R., Ramniwas S. & Singh S. Variations in body melanization, ovariole number and fecundity in highland and lowland populations of Drosophila melanogaster from the Indian subcontinent. Insect Sci. 15, 553–561 (2008). [Google Scholar]
- Singh S., Ramniwas S. & Parkash R. Fitness consequences of body melanization in Drosophila immigrans from montane habitats. Entomol. Res. 39, 182–191 (2009). [Google Scholar]
- Parkash R., Aggarwal D. D. & Kalra B. Coadapted changes in energy metabolites and body color phenotypes for resistance to starvation and desiccation in latitudinal populations of D. melanogaster. Evol. Ecol. 26, 149–169 (2012). [Google Scholar]
- Marron M. T., Markow T. A., Kain K. J. & Gibbs A. G. Effects of starvation and desiccation on energy metabolism in desert and mesic. Drosophila. J. Insect Physiol. 49, 261–270 (2003). [DOI] [PubMed] [Google Scholar]
- Parkash R. & Munjal A. K. Climatic selection of starvation and desiccation resistance in populations of some tropical drosophilids. J. Zoo. Syst. Evol. Research 37, 195–202 (1999). [Google Scholar]
- Parkash R., Tyagi P. K., Sharma I. & Rajpurohit S. Adaptations to environmental stress in altitudinal populations of two Drosophila species. Physiol. Entomol. 30, 353–361 (2005). [Google Scholar]
- Parkash R., Ramniwas S., Rajpurohit S. & Sharma V. Variations in body melanization impact desiccation resistance in Drosophila immigrans from western Himalayas. J. Zool. 276, 219–227 (2008). [Google Scholar]
- Rajpurohit S., Parkash R., Singh S. & Ramniwas S. Climate change, boundary increase and elongation of a pre-existing cline: A case study in Drosophila ananassae. Entomol. Res. 38, 268–275 (2008). [Google Scholar]
- Parkash R., Sharma V. & Kalra B. Correlated changes in thermotolerance traits and body color phenotypes in montane populations of Drosophila melanogaster. analysis of withing- and between-population variations. J. Zool. 280, 49–59 (2009). [Google Scholar]
- Parkash R., Sharma V. & Kalra B. Sexual dimorphism for water balance mechanisms in montane populations of Drosophila kikkawai. Biol. Lett. 6, 570–574 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkash R., Aggarwal D. D., Kalra B. & Ranga P. Divergence of water balance mechanisms in two melanic Drosophila species from the western Himalayas. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 158, 531–541 (2011). [DOI] [PubMed] [Google Scholar]
- Parkash R. & Ranga P. Divergence for tolerance to thermal-stress related traits in two Drosophila species of immigrans group. J. of Therm. Biol. 38, 396–406 (2013). [Google Scholar]
- Parkash R., Munjal A. K. & Karan D. Thermal adaptive significance of ADH and EST-6 allozymes in Indian geographical populations of Drosophila melanogaster. J. of Zool. Syst. Evol. Research 36, 147–152 (1998). [Google Scholar]
- Kumar K. R. et al. High resolution climate change scenarios for India for the 21st century. Current Science 90, 334–345 (2006). [Google Scholar]
- Pau S. et al. Predicting phenology by integrating ecology, evolution and climate science. Glob. Chang. Biol. 17, 3633–3643 (2011). [Google Scholar]
- Charmantier A. & Gienapp P. Climate change and timing of avian breeding and migration: evolutionary versus plastic changes. Evol. Appl. 7, 15–28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimm S. L. et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014). [DOI] [PubMed] [Google Scholar]
- Hangartner S. B., Hoffmann A. A., Smith A. & Griffin P. C. A collection of Australian Drosophila datasets on climate adaptation and species distributions. Sci. Data 2, 150067 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rajpurohit S., Zhao X., Schmidt P. S. 2016. Dryad Digital Repository. http://dx.doi.org/10.5061/dryad.8q7s6